A Novel Approach to Estimate the Impact of PCV20 Immunization in Children by Incorporating Indirect Effects to Generate the Number Needed to Vaccinate
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Structure
2.2. Primary Analysis
2.3. Scenario Analyses
3. Results
3.1. Primary Analysis Results
3.2. Scenario Analysis
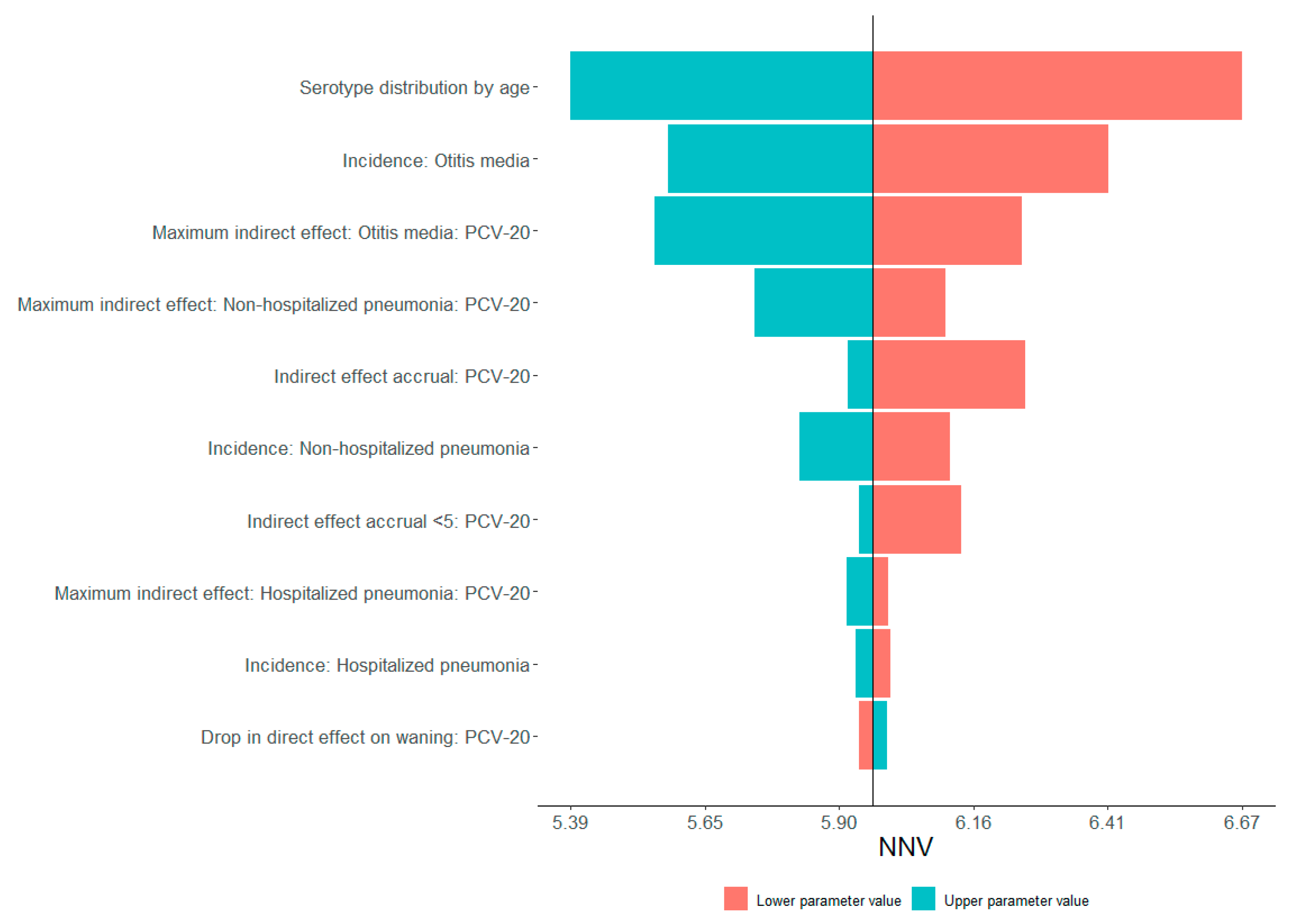
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musher, D.M. Infections caused by Streptococcus pneumoniae: Clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 1992, 14, 801–807. [Google Scholar] [CrossRef]
- Dao, T.H.; Rosch, J.W. JMM Profile: Streptococcus pneumoniae: Sugar-coated captain of the men of death. J. Med. Microbiol. 2021, 70, 001446. [Google Scholar] [CrossRef]
- Pilishvili, T.; Lexau, C.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Bennett, N.M.; Reingold, A.; Thomas, A.; Schaffner, W.; Craig, A.S.; et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 2010, 201, 32–41. [Google Scholar] [CrossRef]
- Moore, M.R.; Link-Gelles, R.; Schaffner, W.; Lynfield, R.; Holtzman, C.; Harrison, L.H.; Zansky, S.M.; Rosen, J.B.; Reingold, A.; Scherzinger, K.; et al. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: A matched case-control study. Lancet Respir. Med. 2016, 4, 399–406. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among children aged 6–18 years with immunocompromising conditions: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 521–524. [Google Scholar]
- Nuorti, J.P.; Whitney, C.G.; Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 2010, 59, 1–18. [Google Scholar] [PubMed]
- Whitney, C.G.; Pilishvili, T.; Farley, M.M.; Schaffner, W.; Craig, A.S.; Lynfield, R.; Nyquist, A.-C.; Gershman, K.A.; Vazquez, M.; Bennett, N.M.; et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: A matched case-control study. Lancet 2006, 368, 1495–1502. [Google Scholar] [CrossRef]
- Tomczyk, S.; Bennett, N.M.; Stoecker, C.; Gierke, R.; Moore, M.R.; Whitney, C.G.; Hadler, S.; Pilishvili, T.; Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 822–825. [Google Scholar] [PubMed]
- Simonsen, L.; Taylor, R.J.; Young-Xu, Y.; Haber, M.; May, L.; Klugman, K.P. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. MBio 2011, 2, e00309-10. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Wiemken, T.L.; Peyrani, P.; Arnold, F.W.; Kelley, R.; Mattingly, W.A.; Nakamatsu, R.; Pena, S.; Guinn, B.E.; Furmanek, S.P.; et al. Adults Hospitalized with Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin. Infect. Dis. 2017, 65, 1806–1812. [Google Scholar] [CrossRef]
- Centers for Disease and Control Prevention. Pneumococcal Disease: Antibiotic-Resistant Streptococcus Pneumoniae. Updated February 6 2024. Available online: https://www.cdc.gov/pneumococcal/php/drug-resistance/index.html (accessed on 3 July 2024).
- Centers for Disease and Control Prevention. ACIP Evidence to Recommendation User’s Guide. Available online: https://www.cdc.gov/acip/media/pdfs/2024/09/ACIP-EtR-Users-Guide_October-1-2020.pdf (accessed on 15 January 2025).
- Centers for Disease Control Prevention. CDC Recommendation for Hepatitis B Vaccination Among Adults with Diabetes: Grading of Scientific Evidence in Support of Key Recommendations; CDC: Atlanta, GA, USA, 2013.
- Centers for Disease and Control Prevention. Advisory Committee on Immunization Practices: Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) for Pneumococcal Vaccines for Adults Aged ≥65 Years. Available online: https://www.cdc.gov/acip/grade/pneumo-vac-adult.html (accessed on 15 January 2025).
- Stoecker, C. Economic Assessment of PCV21 in US Adults. Available online: https://www.cdc.gov/acip/downloads/slides-2024-06-26-28/02-Pneumococcal-Stoecker-508.pdf. (accessed on 15 January 2025).
- Kelly, H.; Attia, J.; Andrews, R.; Heller, R.F. The number needed to vaccinate (NNV) and population extensions of the NNV: Comparison of influenza and pneumococcal vaccine programmes for people aged 65 years and over. Vaccine 2004, 22, 2192–2198. [Google Scholar] [CrossRef]
- Hashim, A.; Dang, V.; Bolotin, S.; Crowcroft, N.S. How and why researchers use the number needed to vaccinate to inform decision making—A systematic review. Vaccine 2015, 33, 753–758. [Google Scholar] [CrossRef]
- McLaughlin, J.M.; Swerdlow, D.L.; Isturiz, R.E.; Jodar, L. Rethinking number-needed-to-vaccinate for pneumococcal conjugate vaccines in older adults: Current and future implications. Vaccine 2017, 35, 5360–5365. [Google Scholar] [CrossRef]
- Brisson, M.; Van de Velde, N.; De Wals, P.; Boily, M.-C. Estimating the number needed to vaccinate to prevent diseases and death related to human papillomavirus infection. Can. Med. Assoc. J. 2007, 177, 464–468. [Google Scholar] [CrossRef]
- Tuite, A.R.; Fisman, D.N. Number-needed-to-vaccinate calculations: Fallacies associated with exclusion of transmission. Vaccine 2013, 31, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Savulescu, C.; Krizova, P.; Valentiner-Branth, P.; Ladhani, S.; Rinta-Kokko, H.; Levy, C.; Mereckiene, J.; Knol, M.; Winje, B.A.; Ciruela, P.; et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine 2022, 40, 3963–3974. [Google Scholar] [CrossRef] [PubMed]
- Flem, E.; Mouawad, C.; Palmu, A.A.; Platt, H.; Johnson, K.D.; McIntosh, E.D.; Abadi, J.; Buchwald, U.K.; Feemster, K. Indirect protection in adults ≥18 years of age from pediatric pneumococcal vaccination: A review. Expert Rev. Vaccines 2024, 23, 997–1010. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Pondo, T.; Xing, W.; McGee, L.; Farley, M.; Schaffner, W.; Thomas, A.; Reingold, A.; Harrison, L.H.; Lynfield, R.; et al. Early impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions—United States. Clin. Infect. Dis. 2020, 70, 2484–2492. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Paran, Y.; Bishara, J.; Oren, I.; Chowers, M.; Tziba, Y.; Istomin, V.; Weinberger, M.; Miron, D.; Temper, V.; et al. Early impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneumococcal disease: A nationwide surveillance study. Vaccine 2015, 33, 1135–1142. [Google Scholar] [CrossRef]
- Harboe, Z.B.; Dalby, T.; Weinberger, D.M.; Benfield, T.; Mølbak, K.; Slotved, H.C.; Suppli, C.H.; Konradsen, H.B.; Valentiner-Branth, P. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin. Infect. Dis. 2014, 59, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control Prevention. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease--United States, 1998–2003. MMWR Morb. Mortal. Wkly. Rep. 2005, 54, 893–897. [Google Scholar]
- Centers for Disease Control and Prevention. Guidance for Health Economics Studies Presented to the Advisory Committee on Immunization Practices (ACIP), 2024 Update. Updated 18 December 2014. Available online: https://www.cdc.gov/acip/about/health-economics-studies.html (accessed on 15 January 2025).
- Huerta, J.L.; Ta, A.; Vinand, E.; Torres, G.I.; Wannaadisai, W.; Huang, L. PCV20 for the prevention of invasive pneumococcal disease in the Mexican pediatric population: A cost-effectiveness analysis. Hum. Vaccines Immunother. 2025, 21, 2475594. [Google Scholar] [CrossRef]
- Çakar, E.; Ta, A.; Peters, M.; Vinand, E.; Waterval-Overbeek, A.; Ilic, A.; Perdrizet, J. Economic Evaluation of Transitioning to the 20-Valent Pneumococcal Conjugate Vaccine in the Dutch Paediatric National Immunisation Programme. Infect. Dis. Ther. 2025, 14, 527–547. [Google Scholar] [CrossRef] [PubMed]
- Rey-Ares, L.; Ta, A.; Freigofaite, D.; Warren, S.; Mullen, M.M.; Carballo, C.; Huang, L. Cost-effectiveness analysis of the pediatric 20-valent pneumococcal conjugate vaccine compared with lower-valent alternatives in Argentina. Vaccine 2024, 42, 126043. [Google Scholar] [CrossRef]
- Ta, A.; Kühne, F.; Laurenz, M.; von Eiff, C.; Warren, S.; Perdrizet, J. Cost-effectiveness of PCV20 to prevent pneumococcal disease in the pediatric population: A German societal perspective analysis. Infect. Dis. Ther. 2024, 13, 1333–1358. [Google Scholar] [CrossRef] [PubMed]
- Rozenbaum, M.H.; Chilson, E.; Farkouh, R.; Huang, L.; Cane, A.; Arguedas, A.; Tort, M.J.; Snow, V.; Averin, A.; Weycker, D.; et al. Cost-Effectiveness of 20-Valent Pneumococcal Conjugate Vaccine Among US Children with Underlying Medical Conditions. Infect. Dis. Ther. 2024, 13, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Rozenbaum, M.H.; Huang, L.; Cane, A.; Arguedas, A.; Chapman, R.; Dillon-Murphy, D.; Tort, M.J.; Snow, V.; Chilson, E.; Farkouh, R. Cost-effectiveness and impact on infections and associated antimicrobial resistance of 20-valent pneumococcal conjugate vaccine in US children previously immunized with PCV13. J. Med. Econ. 2024, 27, 644–652. [Google Scholar] [CrossRef]
- Fridh, A.-C.; Palmborg, A.; Ta, A.; Freigofaite, D.; Warren, S.; Perdrizet, J. An economic evaluation of pneumococcal conjugate vaccines, PCV20 versus PCV15, for the prevention of pneumococcal disease in the Swedish pediatric population. Hum. Vaccines Immunother. 2024, 20, 2400751. [Google Scholar] [CrossRef]
- Shinjoh, M.; Togo, K.; Hayamizu, T.; Yonemoto, N.; Morii, J.; Perdrizet, J.; Kamei, K. Cost-effectiveness analysis of 20-valent pneumococcal conjugate vaccine for routine pediatric vaccination programs in Japan. Expert Rev. Vaccines 2024, 23, 485–497. [Google Scholar] [CrossRef]
- Rey-Ares, L.; Averin, A.; Mac Mullen, M.; Hariharan, D.; Atwood, M.; Carballo, C.; Huang, L. Cost-effectiveness of 20-valent pneumococcal conjugate vaccine in Argentinean adults. Infect. Dis. Ther. 2024, 13, 1235–1251. [Google Scholar] [CrossRef]
- Kang, D.-W.; Choe, Y.J.; Lee, J.-Y.; Suk, I.-A.; Kim, Y.-S.; Kim, H.-Y.; Byun, B.-K.; Park, S.-K. Cost-effectiveness analysis of the 20-valent pneumococcal conjugate vaccine for the pediatric population in South Korea. Vaccine 2024, 42, 126000. [Google Scholar] [CrossRef] [PubMed]
- Lytle, D.; Grajales Beltrán, A.G.; Perdrizet, J.; Yahia, N.A.; Cane, A.; Yarnoff, B.; Chapman, R. Cost-effectiveness analysis of PCV20 to prevent pneumococcal disease in the Canadian pediatric population. Hum. Vaccines Immunother. 2023, 19, 2257426. [Google Scholar] [CrossRef]
- Warren, S.; Barmpouni, M.; Kossyvaki, V.; Gourzoulidis, G.; Perdrizet, J. Estimating the clinical and economic impact of switching from the 13-valent pneumococcal conjugate vaccine (PCV13) to higher-valent options in Greek infants. Vaccines 2023, 11, 1369. [Google Scholar] [CrossRef]
- Wilson, M.; Lucas, A.; Mendes, D.; Vyse, A.; Mikudina, B.; Czudek, C.; Ellsbury, G.F.; Perdrizet, J. Estimating the cost-effectiveness of switching to higher-valency pediatric pneumococcal conjugate vaccines in the United Kingdom. Vaccines 2023, 11, 1168. [Google Scholar] [CrossRef]
- Huang, L.; McDade, C.L.; Perdrizet, J.E.; Wilson, M.R.; Warren, S.A.; Nzenze, S.; Sewdas, R. Cost-effectiveness analysis of the South African infant national immunization program for the prevention of pneumococcal disease. Infect. Dis. Ther. 2023, 12, 933–950. [Google Scholar] [CrossRef]
- Hoshi, S.-L.; Shono, A.; Seposo, X.; Okubo, R.; Kondo, M. Cost-effectiveness analyses of 15- and 20-valent pneumococcal conjugate vaccines for Japanese elderly. Vaccine 2022, 40, 7057–7064. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-W.; Kim, C.-R.; Song, J.Y.; Park, S.-K. Cost-effectiveness of the 20-valent pneumococcal conjugate vaccine versus the 23-valent pneumococcal polysaccharide vaccine for older adults in South Korea. Vaccine 2024, 42, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Gourzoulidis, G.; Barmpouni, M.; Kossyvaki, V.; Vietri, J.; Tzanetakos, C. Health and economic outcomes of 20-valent pneumococcal conjugate vaccine compared to 15-valent pneumococcal conjugate vaccine strategies for adults in Greece. Front. Public Health 2023, 11, 1229524. [Google Scholar] [CrossRef]
- Mendes, D.; Averin, A.; Atwood, M.; Sato, R.; Vyse, A.; Campling, J.; Weycker, D.; Slack, M.; Ellsbury, G.; Mugwagwa, T. Cost-effectiveness of using a 20-valent pneumococcal conjugate vaccine to directly protect adults in England at elevated risk of pneumococcal disease. Expert Rev. Pharmacoeconomics Outcomes Res. 2022, 22, 1285–1295. [Google Scholar] [CrossRef]
- Wilson, M.; McDade, C.; Beby-Heijtel, A.T.; Waterval-Overbeek, A.; Sundaram, V.; Perdrizet, J. Assessing public health impact of four pediatric pneumococcal conjugate vaccination strategies in the Netherlands. Infect. Dis. Ther. 2023, 12, 1809–1821. [Google Scholar] [CrossRef]
- Senders, S.; Klein, N.P.; Tamimi, N.; Thompson, A.; Baugher, G.; Trammel, J.; Peng, Y.; Giardina, P.; Scully, I.L.; Pride, M.; et al. A phase three study of the safety and immunogenicity of a four-dose series of 20-valent pneumococcal conjugate vaccine in healthy infants. Pediatr. Infect. Dis. J. 2024, 43, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.; Claxton, K.; Sculpher, M. Decision Modelling for Health Economic Evaluation; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Lewnard, J.A.; Hong, V.; Bruxvoort, K.J.; Grant, L.R.; Jódar, L.; Cané, A.; Arguedas, A.; Pomichowski, M.E.; Gessner, B.D.; Tartof, S.Y. Burden of lower respiratory tract infections preventable by adult immunization with 15- and 20-valent pneumococcal conjugate vaccines in the United States. Clin. Infect. Dis. 2023, 77, 1340–1352. [Google Scholar] [CrossRef]
- de Boer, P.T.; van Werkhoven, C.H.; van Hoek, A.J.; Knol, M.J.; Sanders, E.A.M.; Wallinga, J.; de Melker, H.E.; Steens, A. Higher-valency pneumococcal conjugate vaccines in older adults, taking into account indirect effects from childhood vaccination: A cost-effectiveness study for the Netherlands. BMC Med. 2024, 22, 69. [Google Scholar] [CrossRef]
- Lo, S.W.; Gladstone, R.A.; Van Tonder, A.J.; Lees, J.A.; du Plessis, M.; Benisty, R.; Givon-Lavi, N.; Hawkins, P.A.; Cornick, J.E.; Kwambana-Adams, B.; et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: An international whole-genome sequencing study. Lancet Infect. Dis. 2019, 19, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Lewnard, J.A.; Hanage, W.P. Making sense of differences in pneumococcal serotype replacement. Lancet Infect. Dis. 2019, 19, e213–e220. [Google Scholar] [CrossRef] [PubMed]


| Outcome | Mean NNV (95% CI a) |
|---|---|
| IPD | 854.32 (773.62–952.85) |
| Hospitalized pneumonia | 105.72 (98.18–114.19) |
| Non-hospitalized pneumonia | 24.92 (23.18–26.87) |
| Any hospitalization b | 94.08 (87.89–100.82) |
| OM | 8.55 (7.80–9.37) |
| Total disease c | 5.96 (5.60–6.36) |
| Antibiotic-resistant cases | 7.08 (6.64–7.55) |
| Antibiotic prescription | 10.67 (10.02–11.38) |
| Death | 2488.07 (2290.50–2726.50) |
| 1-Year Time Horizon | 10-Year Time Horizon | 25-Year Time Horizon | |||||||
|---|---|---|---|---|---|---|---|---|---|
| With Indirect Effects | W/o Indirect Effects | <18 with Indirect Effects | With Indirect Effects | W/o Indirect Effects | <18 with Indirect Effects | With Indirect Effects | W/o Indirect Effects | <18 with Indirect Effects | |
| IPD | 28,788 | 28,788 | 28,788 | 1212 | 19,180 | 11,016 | 854 | 15,726 | 9040 |
| Any hospitalization a | 1534 | 1534 | 1534 | 124 | 980 | 455 | 94 | 791 | 370 |
| Hospitalized pneumonia | 1620 | 1620 | 1620 | 138 | 1033 | 475 | 106 | 833 | 386 |
| Non-hospitalized pneumonia | 1996 | 1996 | 1996 | 31 | 614 | 70 | 25 | 428 | 56 |
| OM | 57 | 57 | 57 | 11 | 31 | 11 | 9 | 25 | 9 |
| Total disease b | 53 | 53 | 53 | 7 | 29 | 9 | 6 | 23 | 7 |
| Resistant cases | 63 | 63 | 63 | 9 | 34 | 11 | 7 | 26 | 9 |
| Antibiotic prescriptions | 95 | 95 | 95 | 13 | 51 | 16 | 11 | 40 | 13 |
| Death | 104,207 | 104,207 | 104,207 | 3666 | 92,703 | 40,974 | 2488 | 72,054 | 32,853 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozenbaum, M.H.; Tort, M.J.; Capitano, B.; Chapman, R.; Dillon-Murphy, D.; Althouse, B.M.; Cane, A. A Novel Approach to Estimate the Impact of PCV20 Immunization in Children by Incorporating Indirect Effects to Generate the Number Needed to Vaccinate. Vaccines 2025, 13, 805. https://doi.org/10.3390/vaccines13080805
Rozenbaum MH, Tort MJ, Capitano B, Chapman R, Dillon-Murphy D, Althouse BM, Cane A. A Novel Approach to Estimate the Impact of PCV20 Immunization in Children by Incorporating Indirect Effects to Generate the Number Needed to Vaccinate. Vaccines. 2025; 13(8):805. https://doi.org/10.3390/vaccines13080805
Chicago/Turabian StyleRozenbaum, Mark H., Maria J. Tort, Blair Capitano, Ruth Chapman, Desmond Dillon-Murphy, Benjamin M. Althouse, and Alejandro Cane. 2025. "A Novel Approach to Estimate the Impact of PCV20 Immunization in Children by Incorporating Indirect Effects to Generate the Number Needed to Vaccinate" Vaccines 13, no. 8: 805. https://doi.org/10.3390/vaccines13080805
APA StyleRozenbaum, M. H., Tort, M. J., Capitano, B., Chapman, R., Dillon-Murphy, D., Althouse, B. M., & Cane, A. (2025). A Novel Approach to Estimate the Impact of PCV20 Immunization in Children by Incorporating Indirect Effects to Generate the Number Needed to Vaccinate. Vaccines, 13(8), 805. https://doi.org/10.3390/vaccines13080805






