Adjuvanted Protein Vaccines Boost RNA-Based Vaccines for Broader and More Potent Immune Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
Production of SARS-CoV-2 Immunogens
2.2. Adjuvant Production
2.3. Animal Experiments
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Pseudovirus-Neutralization Assays
2.6. Statistical Analyses
3. Results
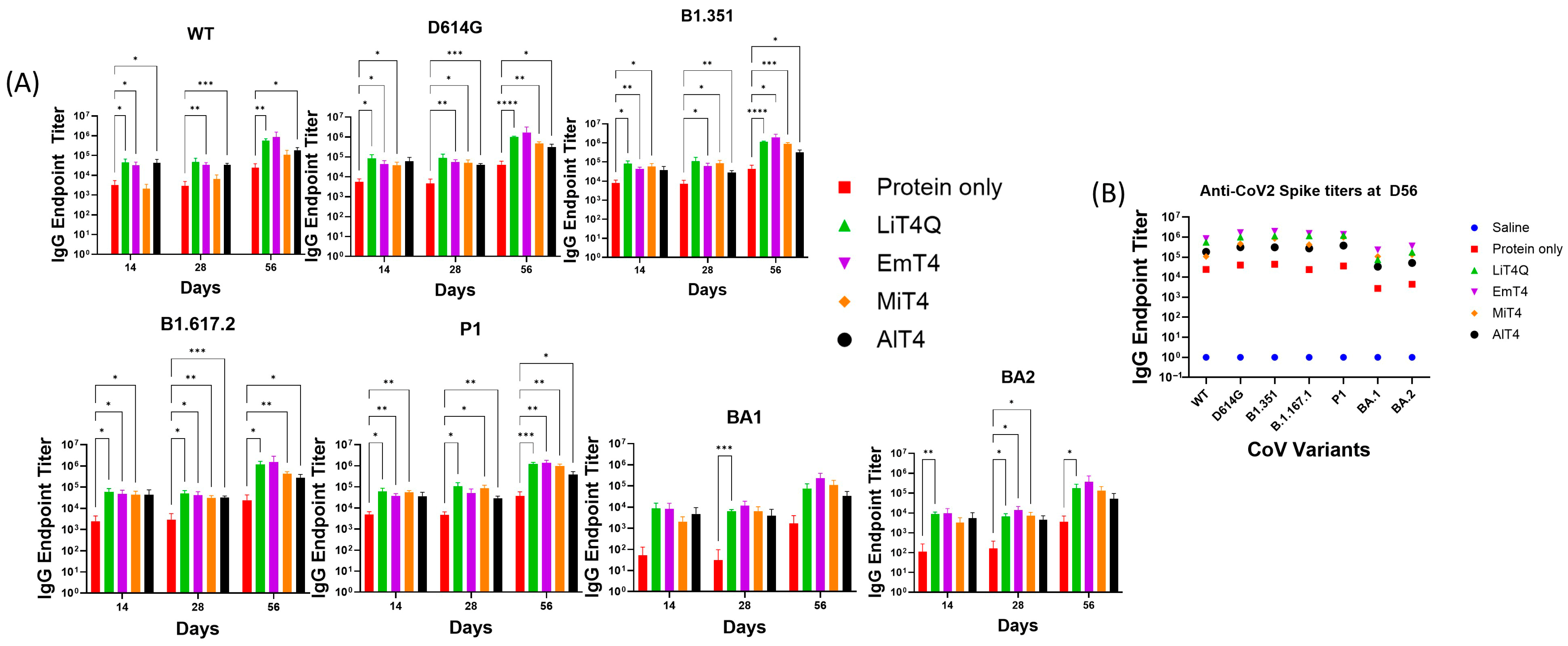
3.1. Adjuvants in Combination with Spike Protein Enhance the Breadth and Magnitude of the Immune Response
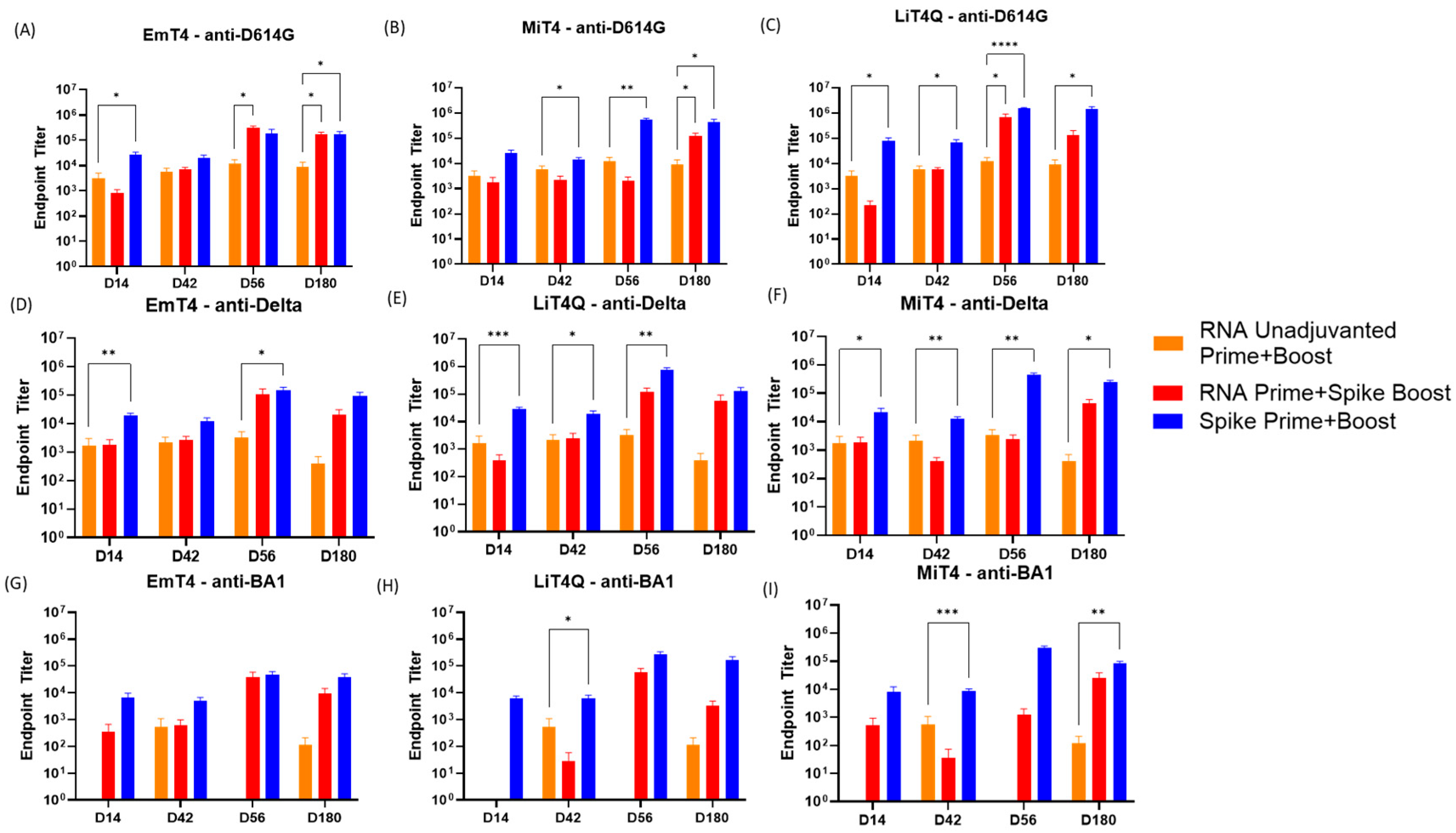
3.2. Key Adjuvants in Combination with mRNA and Peptides to Boost the Magnitude of the Immune Response
3.3. Key Adjuvants in Combination with RNA and Peptides to Boost the Breadth of the Immune Response
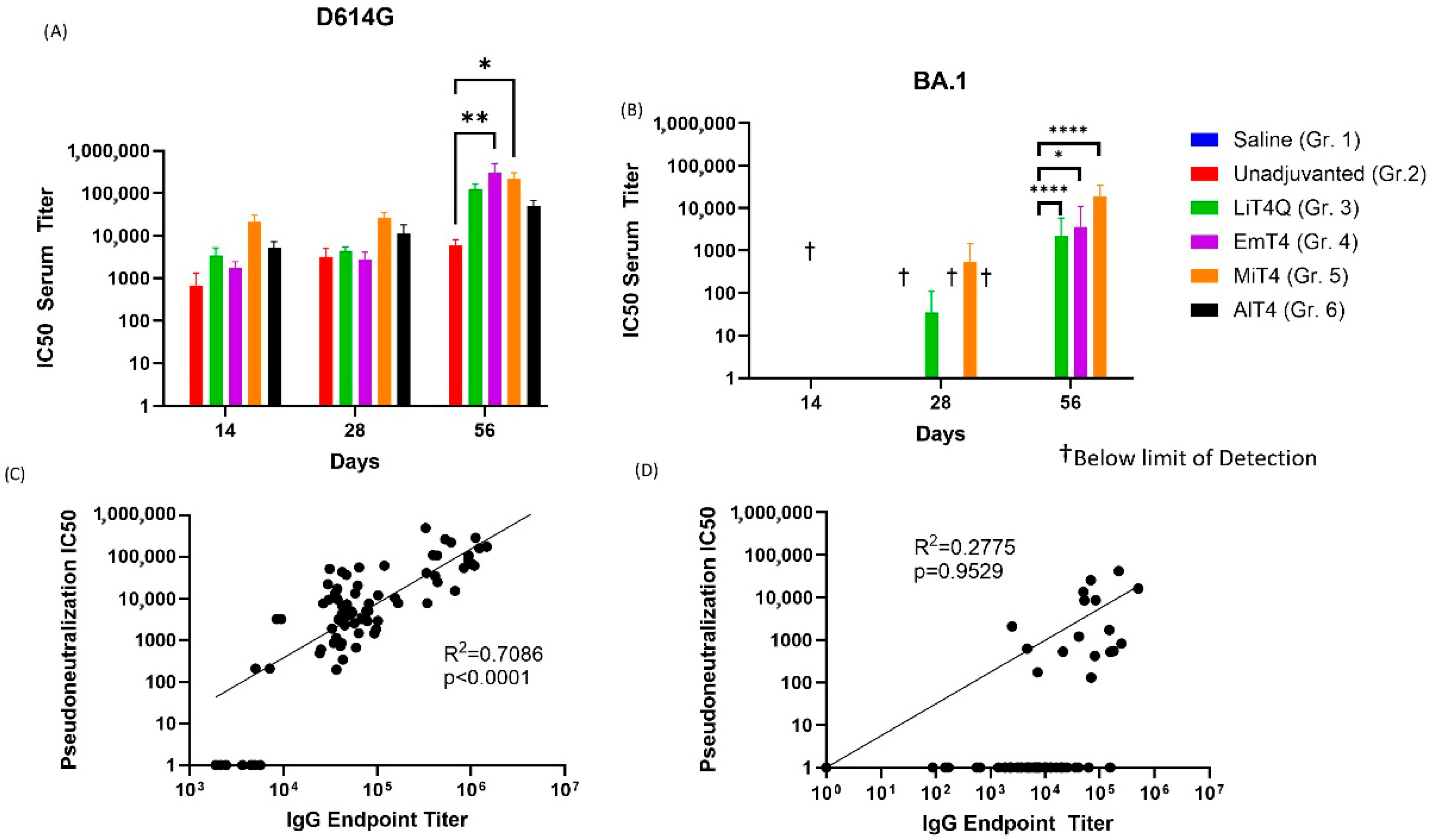
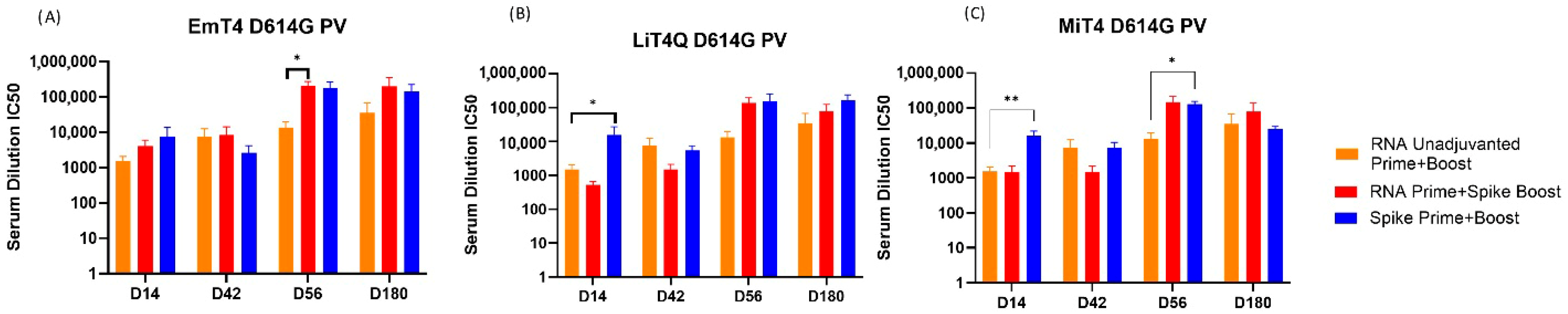
3.4. Pseudovirus Neutralization Capabilities of Prime + Boost Regimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO COVID Dashboard: World Health Organization. 2024. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 20 March 2025).
- Loembé, M.M.; Nkengasong, J.N. COVID-19 vaccine access in Africa: Global distribution, vaccine platforms, and challenges ahead. Immunity 2021, 54, 1353–1362. [Google Scholar] [CrossRef]
- Cueni, T. Lessons learned from COVID-19 to stop future pandemics. Lancet 2023, 401, 1340. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The dawn of mRNA vaccines: The COVID-19 case. J. Control. Release Off. J. Control. Release Soc. 2021, 333, 511–520. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Production, to January 31st 2022: Global Commission for Post-Pandemic Policy. 2022. Available online: https://globalcommissionforpostpandemicpolicy.org/covid-19-vaccine-production-to-january-31st-2022/ (accessed on 25 March 2025).
- Pollet, J.; Chen, W.-H.; Strych, U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv. Drug Deliv. Rev. 2021, 170, 71–82. [Google Scholar] [CrossRef]
- Heidary, M.; Kaviar, V.H.; Shirani, M.; Ghanavati, R.; Motahar, M.; Sholeh, M.; Ghahramanpour, H.; Khoshnood, S. A comprehensive review of the protein subunit vaccines against COVID-19. Front. Microbiol. 2022, 13, 927306. [Google Scholar] [CrossRef]
- Suryawanshi, Y.R. An overview of protein-based SARS-CoV-2 vaccines. Vaccine 2023, 41, 6174–6193. [Google Scholar] [CrossRef]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. npj Vaccines 2020, 5, 11. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles—From liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Korosec, C.S.; Farhang-Sardroodi, S.; Dick, D.W.; Gholami, S.; Ghaemi, M.S.; Moyles, I.R.; Craig, M.; Ooi, H.K.; Heffernan, J.M. Long-term durability of immune responses to the BNT162b2 and mRNA-1273 vaccines based on dosage, age and sex. Sci. Rep. 2022, 12, 21232. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Khandhar, A.P.; O’Connor, M.A.; Walls, A.C.; Hemann, E.A.; Murapa, P.; Archer, J.; Leventhal, S.; Fuller, J.T.; Lewis, T.B.; et al. An Alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci. Transl. Med. 2020, 12, eabc9396. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Baldwin, J.; Brar, D.; Salunke, D.B.; Petrovsky, N. Toll-like receptor (TLR) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics. Curr. Opin. Chem. Biol. 2022, 70, 102172. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”—Emulsion adjuvants rising to meet the pandemic challenges. npj Vaccines 2021, 6, 158. [Google Scholar] [CrossRef]
- Carter, D.; Fox, C.B.; Day, T.A.; Guderian, J.A.; Liang, H.; Rolf, T.; Vergara, J.; Sagawa, Z.K.; Ireton, G.; Orr, M.T.; et al. A structure-function approach to optimizing TLR4 ligands for human vaccines. Clin. Trans. Immunol. 2016, 5, e108. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, S.L.; Roeffen, W.; Singh, S.K.; Tiendrebeogo, R.W.; Christiansen, M.; Beebe, E.; Carter, D.; Fox, C.B.; Howard, R.F.; Reed, S.G.; et al. Synthetic TLR4 agonists enhance functional antibodies and CD4+ T-cell responses against the Plasmodium falciparum GMZ2.6C multi-stage vaccine antigen. Vaccine 2016, 34, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, B.; Joos, C.; Reed, S.G.; Coler, R.; Grubeck-Loebenstein, B. The stimulatory effect of the TLR4-mediated adjuvant glucopyranosyl lipid A is well preserved in old age. Biogerontology 2015, 17, 177–187. [Google Scholar] [CrossRef]
- Goff, P.H.; Hayashi, T.; Martinez-Gil, L.; Corr, M.; Crain, B.; Yao, S.; Cottam, H.B.; Chan, M.; Ramos, I.; Eggink, D.; et al. Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses. J. Virol. 2015, 89, 3221–3235. [Google Scholar] [CrossRef]
- Roman, F.; Burny, W.; Ceregido, M.A.; Laupèze, B.; Temmerman, S.T.; Warter, L.; Coccia, M. Adjuvant system AS01: From mode of action to effective vaccines. Expert Rev. Vaccines 2024, 23, 715–729. [Google Scholar] [CrossRef]
- Potchen, N.B.; Johnson, A.M.; Hager, K.; Graham, J.; Van, P.; Lyn-Kew, K.H.; Warrier, L.; Talavera, I.C.; Lund, J.M.; Kublin, J.G. Oral tolerance to systemic vaccination remains intact without RORγt expression in regulatory T cells. iScience 2023, 26, 108504. [Google Scholar] [CrossRef]
- Ryan, N.M.; Hess, J.A.; Robertson, E.J.; Tricoche, N.; Turner, C.; Davis, J.; Petrovsky, N.; Ferguson, M.; Rinaldi, W.J.; Wong, V.M.; et al. Adjuvanted Fusion Protein Vaccine Induces Durable Immunity to Onchocerca volvulus in Mice and Non-Human Primates. Vaccines 2023, 11, 1212. [Google Scholar] [CrossRef]
- Li, D.; Bian, L.; Cui, L.; Zhou, J.; Li, G.; Zhao, X.; Xing, L.; Cui, J.; Sun, B.; Jiang, C.; et al. Heterologous Prime-Boost Immunization Strategies Using Varicella-Zoster Virus gE mRNA Vaccine and Adjuvanted Protein Subunit Vaccine Triggered Superior Cell Immune Response in Middle-Aged Mice. Int. J. Nanomed. 2024, 19, 8029–8042. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Khandhar, A.P.; Guderian, J.; Granger, B.; Archer, J.; Archer, M.; Gage, E.; Fuerte-Stone, J.; Larson, E.; Lin, S.; et al. A nanostructured lipid carrier for delivery of a replicating viral RNA provides single, low-dose protection against Zika. Mol. Ther. 2018, 26, 2507–2522. [Google Scholar] [CrossRef]
- Larsen, S.E.; Berube, B.J.; Pecor, T.; Cross, E.; Brown, B.P.; Williams, B.D.; Johnson, E.; Qu, P.; Carter, L.; Wrenn, S.; et al. Qualification of ELISA and neutralization methodologies to measure SARS-CoV-2 humoral immunity using human clinical samples. J. Immunol. Methods 2021, 499, 113160. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.L.; Mantus, G.; Manning, K.E.; Ellis, M.; Patel, M.; Ciric, C.R.; Lu, A.; Turner, J.S.; O’hAlloran, J.A.; Presti, R.M.; et al. Loss of Pfizer (BNT162b2) Vaccine-Induced Antibody Responses against the SARS-CoV-2 Omicron Variant in Adolescents and Adults. J. Virol. 2022, 96, e0058222. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, P.S.; Lai, L.; Samaha, H.; Feng, Y.; Hu, M.; Hui, H.S.-Y.; Wali, B.; Ellis, M.; Davis-Gardner, M.E.; Huerta, C.; et al. Durability of immune responses to mRNA booster vaccination against COVID-19. J. Clin. Investig. 2023, 133, e167955. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.; Chung, N.P.Y.; Klasse, P.J.; Moutaftsi, M.; Carter, D.; Salazar, A.M.; Reed, S.G.; Sanders, R.W.; Moore, J.P.; Nixon, D.F. Clinical adjuvant combinations stimulate potent B-cell responses in vitro by activating dermal dendritic cells. PLoS ONE 2013, 8, e63785. [Google Scholar] [CrossRef]
- Wiley, S.R.; Raman, V.S.; Desbien, A.; Bailor, H.R.; Bhardwaj, R.; Shakri, A.R.; Reed, S.G.; Chitnis, C.E.; Carter, D. Targeting TLRs expands the antibody repertoire in response to a malaria vaccine. Sci. Transl. Med. 2011, 3, 93ra69. [Google Scholar] [CrossRef]
- Carter, D.; Reed, S.G. Role of adjuvants in modeling the immune response. Curr. Opin. HIV AIDS 2010, 5, 409–413. [Google Scholar] [CrossRef]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and heterologous COVID-19 booster vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef]
- Garg, I.; Sheikh, A.B.; Pal, S.; Shekhar, R. Mix-and-match COVID-19 vaccinations (heterologous boost): A review. Infect. Dis. Rep. 2022, 14, 537–546. [Google Scholar] [CrossRef]
- Pardo, I.; Maezato, A.M.; Callado, G.Y.; Gutfreund, M.C.; Hsieh, M.K.; Lin, V.; Kobayashi, T.; Salinas, J.L.; Subramanian, A.; Edmond, M.B.; et al. Effectiveness of heterologous and homologous COVID-19 vaccination among immunocompromised individuals: A systematic literature review and meta-analysis. Antimicrob. Steward. Healthc. Epidemiol. 2024, 4, e152. [Google Scholar] [CrossRef] [PubMed]
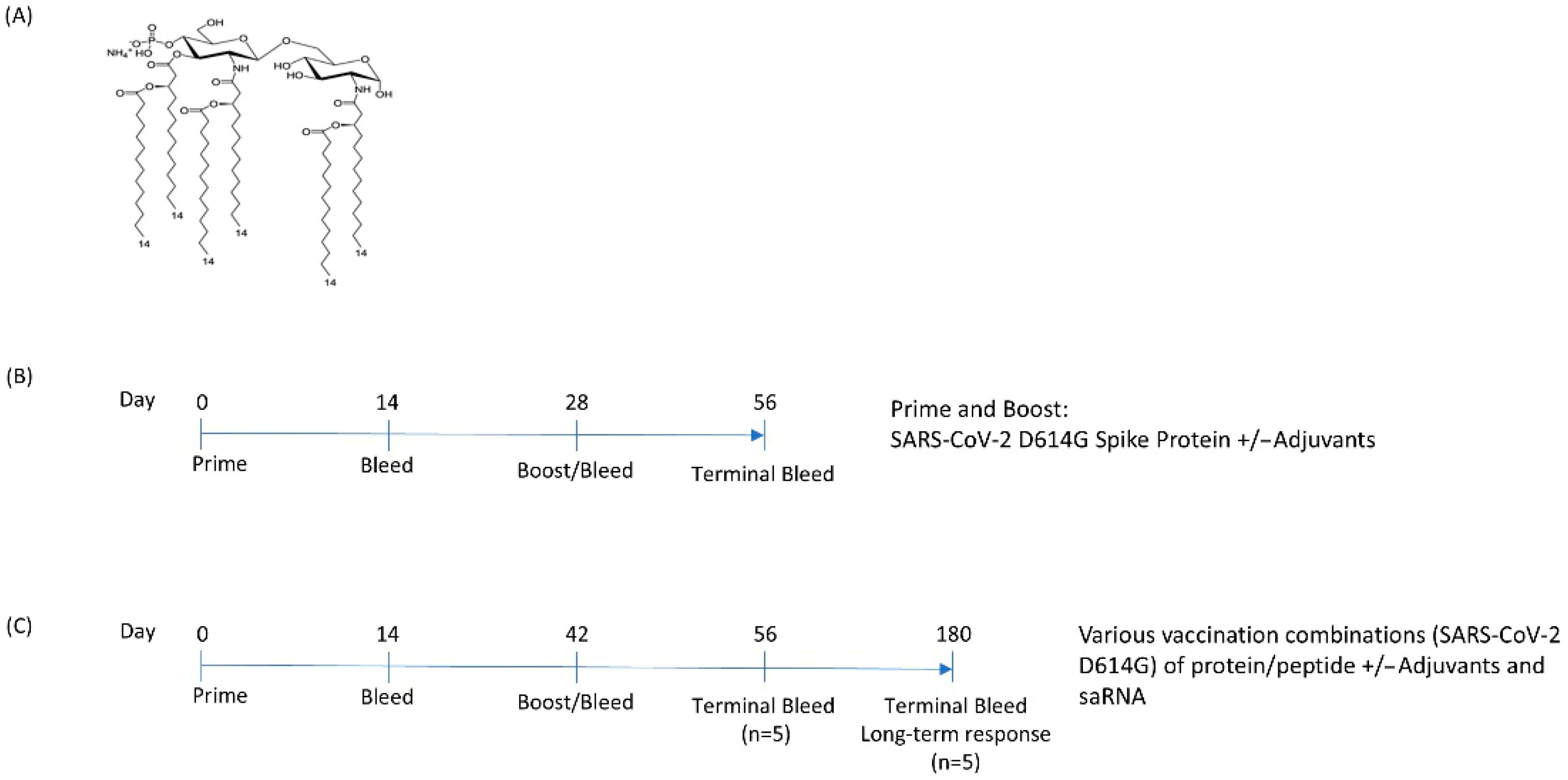
| Group | n | Prime (D0) | Boost (D28) | ||
|---|---|---|---|---|---|
| Antigen | Adjuvant | Antigen | Adjuvant | ||
| 1 | 5 | Saline | None | Saline | none |
| 2 | 5 | 5 µg D614G Spike Protein | None | 5 µg D614G Spike Protein | none |
| 3 | 5 | LiT4QTM | LiT4QTM | ||
| 4 | 5 | EmT4TM | EmT4TM | ||
| 5 | 5 | MiT4TM | MiT4TM | ||
| 6 | 5 | AlT4TM | AlT4TM | ||
| Isotype | Immunization Adjuvant | ||||||
|---|---|---|---|---|---|---|---|
| Control (PBS) | Protein Only | +LiT4QTM | +EmT4TM | +MiT4TM | +AlT4TM | ||
| IgG1 | Endpoint titer | 0 | 98,858 | 427,271 | 256,998 | 185,521 | 949,823 |
| Standard error | 0 | 21,270 | 78,937 | 31,741 | 50,874 | 144,934 | |
| p-value vs. Protein only | - | - | * 0.0327 | ** 0.0078 | 0.3681 | * 0.0157 | |
| IgG2a | Endpoint titer | 0 | 0 | 6047 | 6652 | 3670 | 0 |
| Standard error | 0 | 0 | 920.7 | 878.4 | 396.0 | 0 | |
| p-value vs. Protein only | - | - | † | † | † | † | |
| IgG3 | Endpoint titer | 0 | 468 | 14,660 | 5930 | 3153 | 117 |
| Standard error | 0 | 467 | 3850 | 2388 | 1323 | 116 | |
| p-value vs. Protein only | - | - | 0.0681 | 0.1326 | 0.3630 | 0.8888 | |
| Overall IgG | Endpoint titer | 0 | 41,407 | 1,022,269 | 1,708,718 | 469,938 | 320,410 |
| Standard error | 0 | 9521 | 37,003 | 669,855 | 48,279 | 52,482 | |
| p-value vs. Protein only | - | - | *** <0.0001 | 0.1714 | ** 0.0019 | * 0.0149 | |
| Group | n | Prime (D0) | Boost (D42) | ||
|---|---|---|---|---|---|
| Antigen | Adjuvant | Antigen | Adjuvant | ||
| 1a | 5 | Saline | None | None | EmT4TM |
| 1b | 5 | Saline | None | None | LiT4TM |
| 2 | 10 | 1 µg D614G saRNA | None | Saline | None |
| 3 | 10 | 1 µg D614G saRNA | None | 1 µg D614G saRNA | None |
| 4 | 10 | 1 µg D614G saRNA | None | 5 µg D614G Spike | EmT4TM |
| 5 | 10 | 1 µg D614G saRNA | None | 5 µg D614G Spike | LiT4QTM |
| 6 | 10 | 1 µg D614G saRNA | None | 5 µg D614G Spike | MiT4TM |
| 7 | 10 | 5 µg D614G Spike | EmT4TM | 5 µg D614G Spike | EmT4TM |
| 8 | 10 | 5 µg D614G Spike | LiT4QTM | 5 µg D614G Spike | LiT4QTM |
| 9 | 10 | 5 µg D614G Spike | MiT4TM | 5 µg D614G Spike | MiT4TM |
| 10 | 10 | 1 µg D614G saRNA | None | 5 µg D614G Peptides | EmT4TM |
| 11 | 10 | 5 µg D614G Peptides | EmT4TM | 5 µg D614G Peptides | EmT4TM |
| 12 | 10 | 1 µg D614G saRNA | LiT4QTM | 5 µg D614G Peptides | LiT4QTM |
| 13 | 10 | 5 µg D614G Peptides | LiT4QTM | 5 µg D614G Peptides | LiT4QTM |
| 14 | 10 | 1 µg D614G saRNA | MiT4TM | 5 µg D614G Peptides | MiT4TM |
| 15 | 10 | 5 µg D614G Peptides | MiT4TM | 5 µg D614G Peptides | MiT4TM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Davis, J.; Berube, B.; Duthie, M.; Gray, S.A.; Carter, D. Adjuvanted Protein Vaccines Boost RNA-Based Vaccines for Broader and More Potent Immune Responses. Vaccines 2025, 13, 797. https://doi.org/10.3390/vaccines13080797
Kim J, Davis J, Berube B, Duthie M, Gray SA, Carter D. Adjuvanted Protein Vaccines Boost RNA-Based Vaccines for Broader and More Potent Immune Responses. Vaccines. 2025; 13(8):797. https://doi.org/10.3390/vaccines13080797
Chicago/Turabian StyleKim, Jiho, Jenn Davis, Bryan Berube, Malcolm Duthie, Sean A. Gray, and Darrick Carter. 2025. "Adjuvanted Protein Vaccines Boost RNA-Based Vaccines for Broader and More Potent Immune Responses" Vaccines 13, no. 8: 797. https://doi.org/10.3390/vaccines13080797
APA StyleKim, J., Davis, J., Berube, B., Duthie, M., Gray, S. A., & Carter, D. (2025). Adjuvanted Protein Vaccines Boost RNA-Based Vaccines for Broader and More Potent Immune Responses. Vaccines, 13(8), 797. https://doi.org/10.3390/vaccines13080797








