Evaluation of Poxvirus-Specific Antibody Response in Monkey Poxvirus-Negative and -Positive Cohorts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
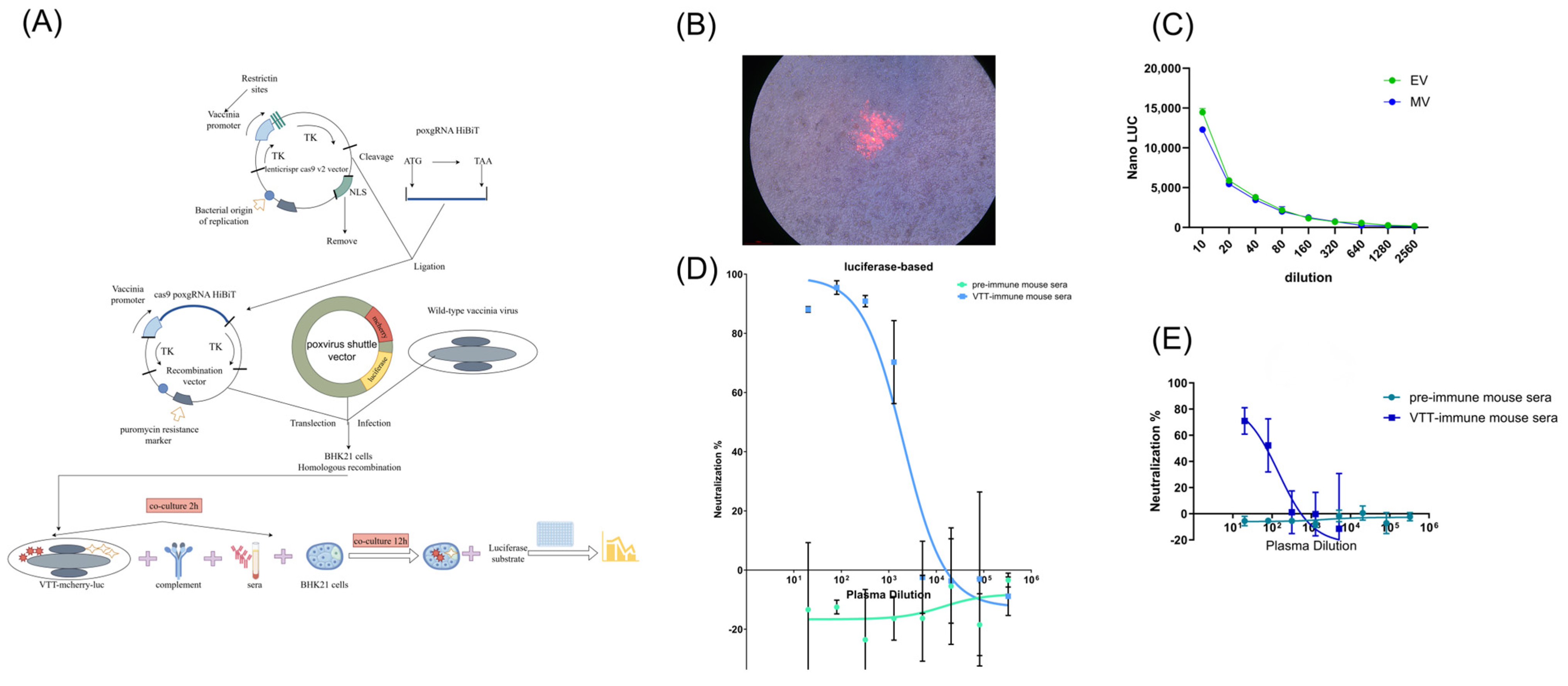
2.2. Construction of Recombinant VTT Virus Carrying Reporter Genes
2.3. Virus Titration
2.4. Neutralization Assays
2.5. ADCC Assay
2.6. Statistical Analysis
3. Results
3.1. Participant Information
3.2. Construction of Recombinant VTT Carrying mCherry and Luciferase Reporters
3.3. Validation of Luciferase-Based Neutralization Assay
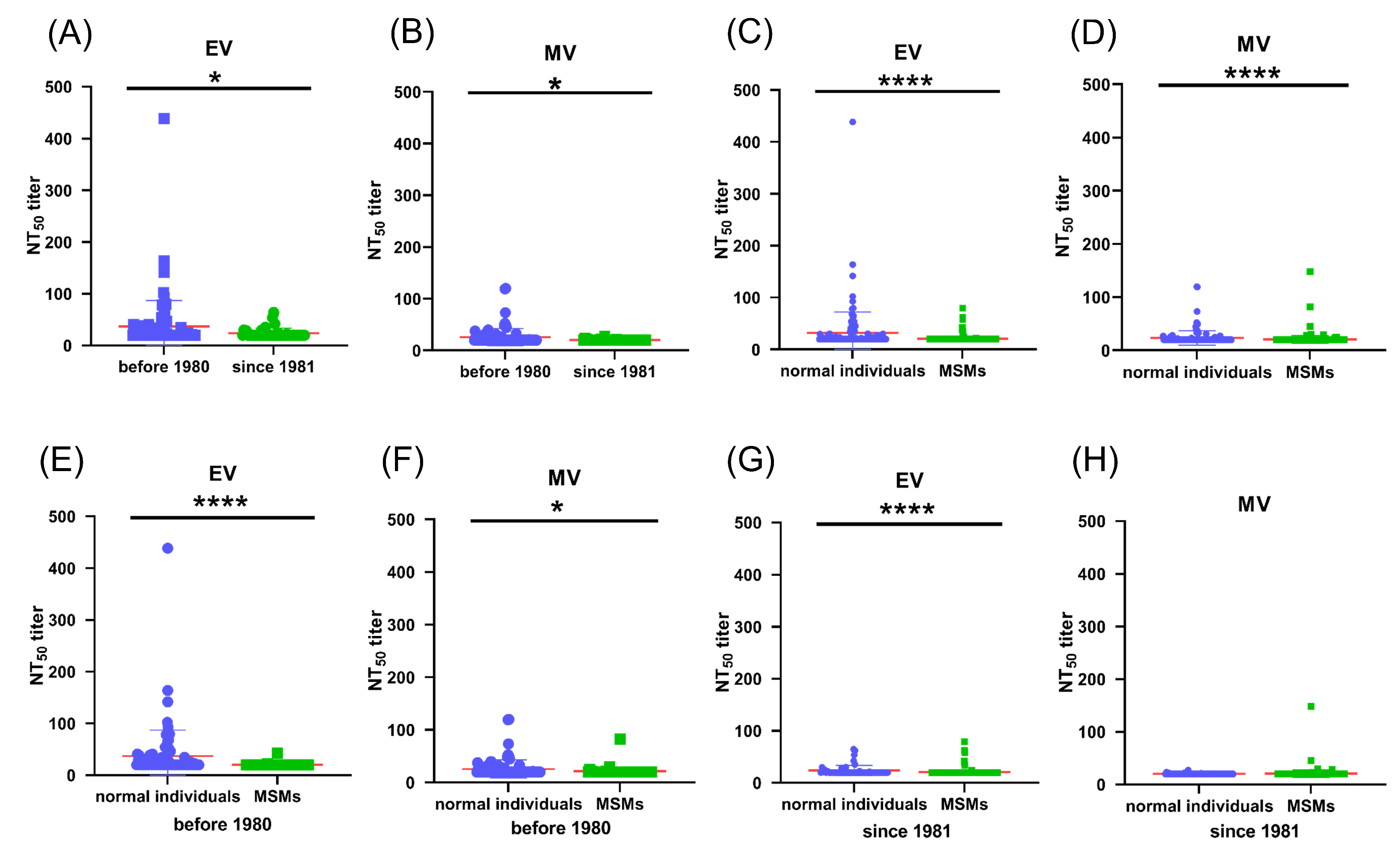
3.4. Pre-Existing Poxvirus-Specific Nabs Were Detected in MPXV-Negative Cohorts
3.5. Pre-Existing Poxvirus-Specific Nabs in MPXV-Negative MSM Individuals Are Lower than Those in Non-MSM Individuals
3.6. VTT-Neutralizing Antibodies Were Induced After MPXV Infection
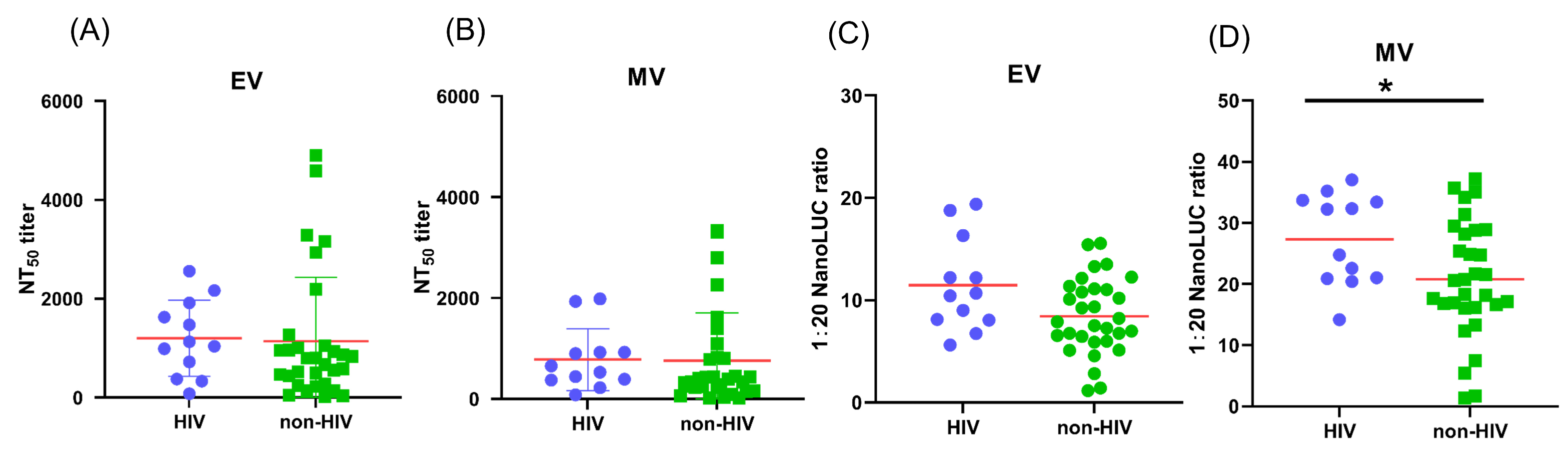
3.7. The Impact of HIV Infection on Antibody Response of MPXV-Infected Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCollum, A.M.; Damon, I.K. Human monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [CrossRef]
- Zhang, Q.; Tian, M.; Feng, Y.; Zhao, K.; Xu, J.; Liu, Y.; Shao, Y. Genomic sequence and virulence of clonal isolates of vaccinia virus Tiantan, the Chinese smallpox vaccine strain. PLoS ONE 2013, 8, e60557. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Wang, W. Monkeypox: Epidemiology, pathogenesis, treatment and prevention. Signal Transduct. Target. Ther. 2022, 7, 373. [Google Scholar] [CrossRef]
- Kumar, N.; Acharya, A.; Gendelman, H.E.; Byrareddy, S.N. The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun 2022, 131, 102855. [Google Scholar] [CrossRef]
- World Health Organization. Mpox Outbreak. 2024. Available online: https://www.who.int/emergencies/situations/mpox-outbreak (accessed on 20 July 2025).
- Li, M.; Guo, Y.; Deng, Y.; Gao, W.; Huang, B.; Yao, W.; Zhao, Y.; Zhang, Q.; Huang, M.; Liu, M.; et al. Long-lasting humoral and cellular memory immunity to vaccinia virus Tiantan provides pre-existing immunity against mpox virus in Chinese population. Cell Rep. 2024, 43, 13609. [Google Scholar] [CrossRef]
- Criscuolo, E.; Giuliani, B.; Ferrarese, R.; Ferrari, D.; Locatelli, M.; Clementi, M.; Mancini, N.; Clementi, N. Smallpox vaccination-elicited antibodies cross-neutralize 2022-Monkeypox virus Clade II. J. Med. Virol. 2023, 95, e28643. [Google Scholar] [CrossRef]
- Li, E.; Guo, X.; Hong, D.; Gong, Q.; Xie, W.; Li, T.; Wang, J.; Chuai, X.; Chiu, S. Duration of humoral immunity from smallpox vaccination and its cross-reaction with Mpox virus. Signal Transduct. Target. Ther. 2023, 8, 350. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Moss, B. Natural killer cells expanded in vivo or ex vivo with IL-15 overcomes the inherent susceptibility of CAST mice to lethal infection with orthopoxviruses. PLoS Pathog. 2020, 16, e1008505. [Google Scholar] [CrossRef]
- Zhang, A.; Stacey, H.D.; D’Agostino, M.R.; Tugg, Y.; Marzok, A.; Miller, M.S. Beyond neutralization: Fc-dependent antibody effector functions in SARS-CoV-2 infection. Nat. Rev. Immunol. 2022, 23, 381–396. [Google Scholar] [CrossRef]
- Sun, X.; Ma, H.; Wang, X.; Bao, Z.; Tang, S.; Yi, C.; Sun, B. Broadly neutralizing antibodies to combat influenza virus infection. Antivir. Res. 2024, 221, 105785. [Google Scholar] [CrossRef]
- Richard, J.; Prévost, J.; Alsahafi, N.; Ding, S.; Finzi, A. Impact of HIV-1 Envelope Conformation on ADCC Responses. Trends Microbiol. 2018, 26, 253–265. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Huang, P.; Ren, J.; Zhang, P.; Tian, H.; Tan, W. Non-replicating Vaccinia Virus TianTan Strain (NTV) Translation Arrest of Viral Late Protein Synthesis Associated With Anti-viral Host Factor SAMD9. Front. Cell. Infect. Microbiol. 2020, 10, 116. [Google Scholar] [CrossRef]
- Reading, P.C.; Smith, G.L. A kinetic analysis of immune mediators in the lungs of mice infected with vaccinia virus and comparison with intradermal infection. J. Gen. Virol. 2003, 84, 1973–1983. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Li, S.; Zhou, Y.; Zhang, Y.; Pei, R.; Chen, X.; Wang, Y. Immunization of mice with vaccinia virus Tiantan strain yields antibodies cross-reactive with protective antigens of monkeypox virus. Virol. Sin. 2023, 38, 162–164. [Google Scholar] [CrossRef]
- Earl, P.L.; Americo, J.L.; Moss, B. Development and use of a vaccinia virus neutralization assay based on flow cytometric detection of green fluorescent protein. J. Virol. 2003, 77, 10684–10688. [Google Scholar] [CrossRef]
- Li, W.; Handschumacher, R.E. Regulation of the nuclear factor of activated T cells in stably transfected Jurkat cell clones. Biochem. Biophys. Res. Commun. 1996, 219, 96–99. [Google Scholar] [CrossRef]
- Hsieh, Y.T.; Aggarwal, P.; Cirelli, D.; Gu, L.; Surowy, T.; Mozier, N.M. Characterization of FcγRIIIA effector cells used in in vitro ADCC bioassay: Comparison of primary NK cells with engineered NK-92 and Jurkat T cells. J. Immunol. Methods 2017, 441, 56–66. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Xu, Y.; Liu, J.; Li, X.; Chen, Y.; Chen, Y.; Xie, J.; Xiao, L.; Xiang, Z.; et al. Resistance of SARS-CoV-2 Omicron variant to convalescent and CoronaVac vaccine plasma. Emerg. Microbes Infect. 2022, 11, 424–427. [Google Scholar] [CrossRef]
- Thornhill, J.P.; Barkati, S.; Walmsley, S.; Rockstroh, J.; Antinori, A.; Harrison, L.B.; Palich, R.; Nori, A.; Reeves, I.; Habibi, M.S.; et al. Monkeypox Virus Infection in Humans across 16 Countries-April-June 2022. N. Engl. J. Med. 2022, 387, 679–691. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef]
- Mahase, E. Monkeypox: Gay and bisexual men with high exposure risk will be offered vaccine in England. BMJ 2022, 377, o1542. [Google Scholar] [CrossRef]
- Vivancos, R.; Anderson, C.; Blomquist, P.; Balasegaram, S.; Bell, A.; Bishop, L.; Brown, C.S.; Chow, Y.; Edeghere, O.; Florence, I.; et al. Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill.: Eur. Commun. Dis. Bull. 2022, 27, 2200422. [Google Scholar] [CrossRef]
- Macian, F. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 2005, 5, 472–484. [Google Scholar] [CrossRef]
- Mitjà, O.; Alemany, A.; Marks, M.; Lezama Mora, J.I.; Rodríguez-Aldama, J.C.; Torres Silva, M.S.; Corral Herrera, E.A.; Crabtree-Ramirez, B.; Blanco, J.L.; Girometti, N.; et al. Mpox in people with advanced HIV infection: A global case series. Lancet 2023, 401, 939–949. [Google Scholar] [CrossRef]
- Mackin, S.R.; Desai, P.; Whitener, B.M.; Karl, C.E.; Liu, M.; Baric, R.S.; Edwards, D.K.; Chicz, T.M.; McNamara, R.P.; Alter, G.; et al. Fc-γR-dependent antibody effector functions are required for vaccine-mediated protection against antigen-shifted variants of SARS-CoV-2. Nat. Microbiol. 2023, 8, 569–580. [Google Scholar] [CrossRef]
- Jegaskanda, S.; Vandenberg, K.; Laurie, K.L.; Loh, L.; Kramski, M.; Winnall, W.R.; Kedzierska, K.; Rockman, S.; Kent, S.J. Cross-Reactive Influenza-Specific Antibody-Dependent Cellular Cytotoxicity in Intravenous Immunoglobulin as a Potential Therapeutic Against Emerging Influenza Viruses. J. Infect. Dis. 2014, 210, 1811–1822. [Google Scholar] [CrossRef]
- Freyn, A.W.; Atyeo, C.; Earl, P.L.; Americo, J.L.; Chuang, G.Y.; Natarajan, H.; Frey, T.R.; Gall, J.G.; Moliva, J.I.; Hunegnaw, R.; et al. An mpox virus mRNA-lipid nanoparticle vaccine confers protection against lethal orthopoxviral challenge. Sci. Transl. Med. 2023, 15, eadg3540. [Google Scholar] [CrossRef]
- Gorman, M.J.; Patel, N.; Guebre-Xabier, M.; Zhu, A.L.; Atyeo, C.; Pullen, K.M.; Loos, C.; Goez-Gazi, Y.; Carrion, R.; Tian, J.-H.; et al. Fab and Fc contribute to maximal protection against SARS-CoV-2 following NVX-CoV2373 subunit vaccine with Matrix-M vaccination. Cell Rep. Med. 2021, 2, 100405. [Google Scholar] [CrossRef]
- Møller-Larsen, A.; Haahr, S. Humoral and cell-mediated immune responses in humans before and after revaccination with vaccinia virus. Infect. Immun. 1978, 19, 34–39. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Y.; Jiang, L.; Luo, L.; Wang, Y.; Wang, H.; Han, X.; Zhao, J.; Gu, G.; Fang, M.; et al. Mpox multi-antigen mRNA vaccine candidates by a simplified manufacturing strategy afford efficient protection against lethal orthopoxvirus challenge. Emerg. Microbes Infect. 2023, 12, 2204151. [Google Scholar] [CrossRef]
- Xia, A.; Wang, X.; He, J.; Wu, W.; Jiang, W.; Xue, S.; Zhang, Q.; Gao, Y.; Han, Y.; Li, Y.; et al. Cross-reactive antibody response to Monkeypox virus surface proteins in a small proportion of individuals with and without Chinese smallpox vaccination history. BMC Biol. 2023, 21, 205. [Google Scholar] [CrossRef]
- Edghill-Smith, Y.; Golding, H.; Manischewitz, J.; King, L.R.; Scott, D.; Bray, M.; Nalca, A.; Hooper, J.W.; Whitehouse, C.A.; Schmitz, J.E.; et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat. Med. 2005, 11, 740–747. [Google Scholar] [CrossRef]

| Variables (n [%] or Median [QR]) | MPXV-Uninfected Cohort (N = 142) | MSM 1 Cohort (N = 386) | MPXV-Infected Cohort (N = 43) |
|---|---|---|---|
| Sex | |||
| Female | 86 (60.56%) | 0 (0%) | 0 (0%) |
| Male | 56 (39.44%) | 386 (100%) | 43 (100%) |
| Age (yr) | 47 (20–79) | 32 (17–64) | 32 (22–59) |
| Birth year | |||
| After 1980 | 54 (38.03%) | 331 (85.75%) | 39 (90.70%) |
| In or Before 1980 | 88 (61.97%) | 55 (14.25%) | 4 (9.3%) |
| HIV infection | |||
| HIV | NA 2 | 0 (0%) | 12 (27.91%) |
| Non-HIV | NA 2 | 386 (100%) | 31 (72.09%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, N.; Ai, L.; Ma, Y.; Hua, C.; Shen, Q.; Wang, C.; Li, T.; Wang, Y.; Li, Y.; Yang, Y.; et al. Evaluation of Poxvirus-Specific Antibody Response in Monkey Poxvirus-Negative and -Positive Cohorts. Vaccines 2025, 13, 795. https://doi.org/10.3390/vaccines13080795
Jia N, Ai L, Ma Y, Hua C, Shen Q, Wang C, Li T, Wang Y, Li Y, Yang Y, et al. Evaluation of Poxvirus-Specific Antibody Response in Monkey Poxvirus-Negative and -Positive Cohorts. Vaccines. 2025; 13(8):795. https://doi.org/10.3390/vaccines13080795
Chicago/Turabian StyleJia, Nannan, Lin Ai, Yunping Ma, Chen Hua, Qi Shen, Chen Wang, Teng Li, Yingdan Wang, Yunyi Li, Yin Yang, and et al. 2025. "Evaluation of Poxvirus-Specific Antibody Response in Monkey Poxvirus-Negative and -Positive Cohorts" Vaccines 13, no. 8: 795. https://doi.org/10.3390/vaccines13080795
APA StyleJia, N., Ai, L., Ma, Y., Hua, C., Shen, Q., Wang, C., Li, T., Wang, Y., Li, Y., Yang, Y., Zhou, C., Chen, M., Wu, H., Chen, X., Lu, L., Zhou, Y., Huang, J., & Wu, F. (2025). Evaluation of Poxvirus-Specific Antibody Response in Monkey Poxvirus-Negative and -Positive Cohorts. Vaccines, 13(8), 795. https://doi.org/10.3390/vaccines13080795







