The Platform Readiness Dashboard: A Tool for Evaluating Vaccine Platform Suitability for a Rapid Response to Epidemic and Pandemic Threats
Abstract
1. Introduction
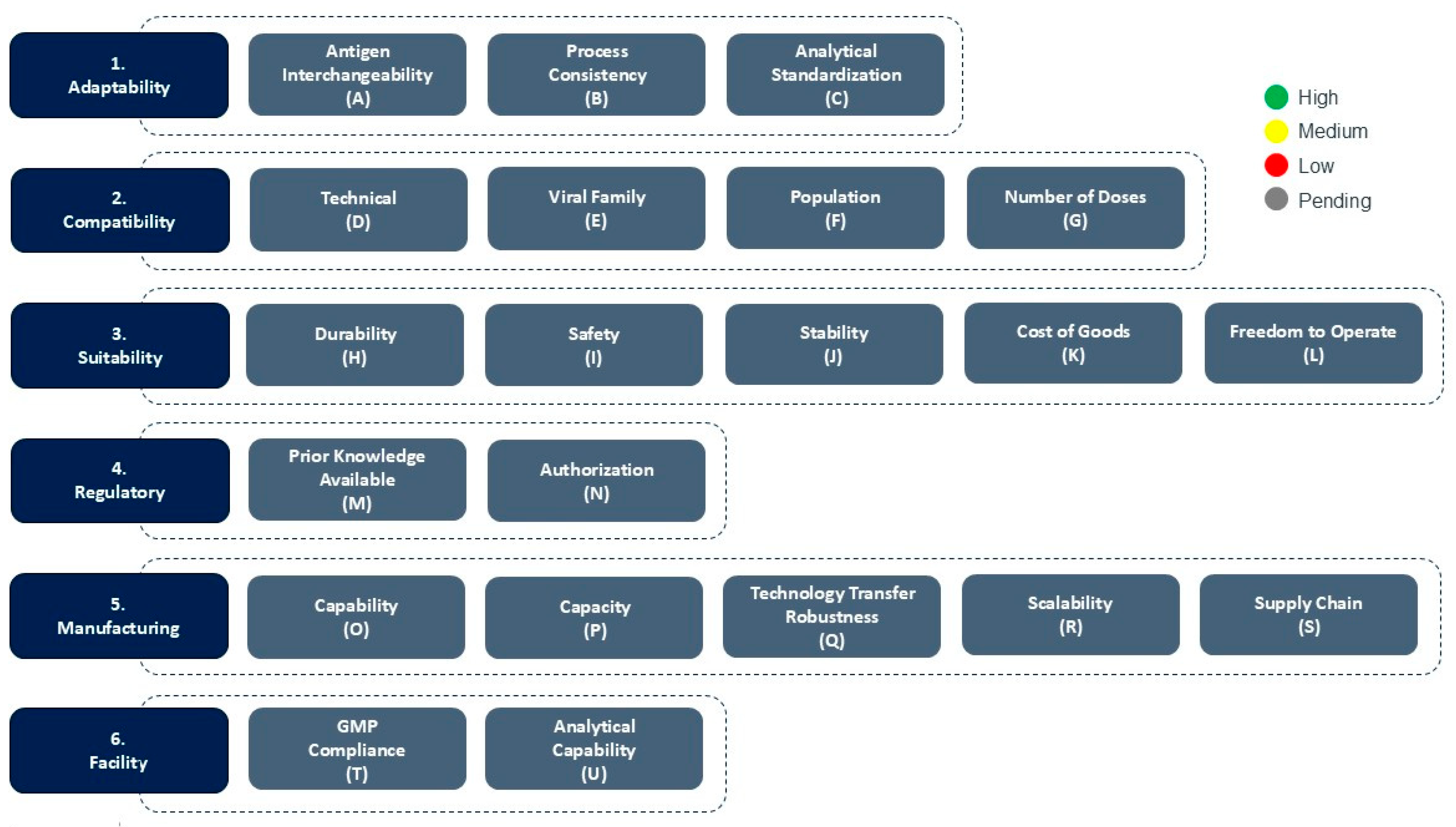
2. CEPI’s Platform Readiness Dashboard
2.1. Adaptability
- Process consistency refers to the ability to maintain the vaccine Drug Substance and Drug Product manufacturing processes, with no to minimal changes to the quality of the final vaccine product. Process consistency in a platform supports faster Process Development and Process Validation activities.
- Antigen interchangeability assesses how easily different antigen sequences can be incorporated into the platform without altering its core structure or performance, i.e., the structure/function-relationship of the vaccine.
- Analytical standardization ensures that the same validated assays and release criteria can be applied across different antigen constructs, except for antigen-specific assays such as Identity and Potency assays. Together, these attributes enable a platform to pivot quickly and reliably to new targets, making it highly suitable for outbreak response.
2.2. Compatability
- Technical refers to the technical opportunities (or limitations) of the platform, i.e., inherent constraints in the platform’s design or function, e.g., if the platform is limited in the type of antigens that can be presented due to structural or biochemical constraints, which in turn affect how antigens are displayed to and interact with the immune system.
- Viral Family relates to whether proof of concept has been demonstrated with the viral family, from which the pandemic or epidemic pathogen originates. This assesses whether the platform has previously been utilized in a vaccine candidate or product with demonstrated efficacy against viruses that are taxonomically related to the outbreak pathogen. If such proof of concept exists, it increases confidence that the platform can be adapted to the pathogen of interest. It would also suggest that some of the groundwork such as antigen design or immune response modeling could be in place, thereby accelerating development timelines even further.
- Population refers to whether the platform has demonstrated proof of concept in the human population who is the primary vaccination target group for outbreak containment (e.g., specific age groups (adults, the elderly, children/adolescents), immunocompromised individuals or populations with different demographic backgrounds). Demonstrated efficacy and safety in the target group is essential as vaccine effectiveness and safety can vary among different populations.
- Number of Doses refers to the number of doses required to complete a full vaccination series. This factor is important because it directly affects the logistics of immunization campaigns; platforms which require fewer doses (ideally a single dose) are more compatible with outbreak response efforts such as compliance with vaccination regimens, while platforms that require multiple doses over an extended period may be less practical in emergency settings where rapid immunization is essential and also may impose supply chain limitations. Important to note however that subsequent to the initial scoring, a thorough assessment of down-selected platforms should follow taking into consideration potential background immunity in the target population, e.g., immunity afforded by vaccination and boosting against SARS-CoV-2 in the event of a coronavirus outbreak.
2.3. Suitability
- Durability of protection refers to how long the vaccine remains effective after a full vaccine regimen. A vaccine that provides a long-lasting immunity is highly advantageous, as it ensures sustained protection in the target populations and reduces the need for booster doses. Subsequent to initial scoring of the platform during a health emergency, it is vital to consider durability of protection in the context of the outbreak scenario. In outbreak scenarios where infectivity is high, onset of protection could potentially weigh heavier than the durability of protection and need to be addressed for each of the down-selected platforms.
- Safety refers to the platform’s safety profile, including data collected during clinical trials, as well as data collected through pharmacovigilance surveillance for marketed products using the same platform for a different pathogen. This involves evaluating the benefit/risk ratio for the platform, i.e., weighing the potential risks of side effects against the benefits of prevention of infection or infectious disease.
- Stability refers to whether the platform’s drug product stability has been demonstrated considering the specific environmental and logistical conditions of the outbreak region. This assessment includes evaluating how well the vaccine maintains its stability and potency particularly in the context of the geographical location where it is to be deployed, the available cold chain infrastructure in that region, as well as handling of the vaccine during transport and otherwise. A vaccine that remains stable during handling and storage without requiring ultra-cold storage is far more suitable to use in low-resource settings, while stability might not be a critical factor in high resource settings.
- Cost of Goods Sold (COGS) refers to the full cost of manufacturing of each dose. Lower production costs enable broader access to the platform, particularly in Low- and Middle-Income Countries (LMICs). Low COGS supports large scale immunization efforts during global outbreaks, and vaccine equity.
- Freedom to Operate refers to the vaccine platform’s licensing and intellectual property (IP) rights. This assesses whether the developer has the legal authority to develop, manufacture and distribute the vaccine without infringing on existing patents or requiring complex licensing agreements. A clear Freedom to Operate is essential for timely outbreak response.
2.4. Regulatory
- Prior Knowledge available refers to the extent of existing familiarity with the platform. This includes CMC data, preclinical and clinical data, and data submitted as part of previous regulatory submissions. When a platform has a well-established catalog of data, developers can rely on this prior knowledge on the platform to expedite vaccine development. This is particularly important in emergency settings, where time is critical and full data packages may not be available.
- Authorization refers to whether a vaccine developed using the same platform has been licensed by a competent regulatory authority. This is a strong indicator of the platform’s maturity. If a vaccine using the platform has received licensure from a WHO listed regulatory authority, it has been demonstrated that the platform has met expected standards on safety, efficacy and quality. This would ideally significantly reduce the regulatory review burden for future vaccines of the same platform as many of the core components of the platform may already be accepted.
2.5. Manufacturing
- Capability and Capacity refer to whether there are already facilities, equipment and trained personnel in place and ready to produce the required number of doses of the vaccine to supply the target population. Platforms with established manufacturing infrastructure, especially those that are geographically distributed, are likely to supply doses much more quickly in health emergencies. This readiness is essential for reducing the time between outbreak and delivery of vaccine to affected populations.
- Technology Transfer Robustness assesses how easily and reliably the manufacturing process can be transferred to other production sites. This is essential for scaling out production across multiple sites globally, particularly when responding to outbreaks in different regions. This requires a robust manufacturing process, i.e., one that is well-characterized, reproducible and tolerant to minor variations in equipment or material. It should be noted that technology transfer of a platform to a new site will require generation of data to demonstrate comparability of product quality and subsequent regulatory authorization. Understanding and aligning the many regulatory expectations around jurisdictions to the greatest extent possible will be needed.
- Scalability evaluates whether the platform can be efficiently scaled not just from R&D or pilot scale to commercial scale, but also from commercial volumes to extraordinary levels required in the event of global outbreaks. Platforms that have already demonstrated successful scale up to industrial levels, and can be further expanded through, e.g., modular models, are far better positioned to meet the demands of major outbreaks, such as the one experienced during the COVID-19 pandemic.
- Supply chain refers to the availability of raw materials and consumables required for vaccine production, including adjuvants or raw materials for adjuvant production if applicable. Preparatory activities such as establishing the logistics of sourcing, storing and transporting of the materials to the manufacturing sites, as well as having relevant supply agreements in place, are key to enable rapid manufacturing of the vaccines. Platforms that rely on highly specialized or single source materials are more vulnerable to supply chain challenges, while platforms that use widely available and interchangeable components might be more resilient and better suited for rapid response vaccine manufacturing.
2.6. Facility
- GMP Compliance refers to whether the intended manufacturing site (s) are compliant with Good Manufacturing Practices (GMP) standards. GMP compliance is a regulatory requirement that ensures that the facility follows to strict quality and safety standards. GMP compliance is essential as only GMP compliant facilities are authorized to produce vaccines for human use.
- Analytical capability refer to the ability to perform the necessary quality control testing and batch release procedures. This includes testing for potency, purity, sterility, identity, amongst other tests. In an outbreak situation, any delay in testing and release of vaccine products can significantly slow down the response efforts. Therefore, platforms with a standardized analytical panel supported by facilities in-house or closely integrated analytical laboratories, including at official national and regional Control and Batch Release Laboratories, are better positioned for outbreak response.
| Category | Consideration | Scoring Guidance |
|---|---|---|
| Adaptability | Antigen Interchangeability | Is the antigen used in the platform interchangeable? Red—no or minimal part of structure is intact and replacing the antigen is expected to have a major effect on structure/function relation; Yellow—part of the structure is intact and replacing the antigen is expected to have a minor effect on structure/function relation; Green—most of the structure is intact and replacing the antigen is expected to have no effect on structure/function relation. |
| Process Consistency | For different vaccines manufactured using the same platform, are processes potentially similar for the platform and the non-platform modules? Red—major changes are expected for non-platform and platform modules; Yellow—Minor or major changes are expected for non-platform modules and minor changes are expected platform modules; Green—No changes expected for platform modules, and minor changes for non-platform modules. | |
| Analytical Standardization | Can assays that are already validated be used without further development (excluding identity and potency)? Red—major assay development activities are required; Yellow—some assay development activities are required, revalidation of some assays is possible; Green—no assay development is required, revalidation of all assays is possible. | |
| Compatibility | Technical | Is the platform technically capable of presenting the antigen? Red—no; Green—yes. |
| Viral Family | Has the platform been used for a pathogen in the same viral family? Red—no; Green—yes. | |
| Population | Has the platform been used successfully in the target population previously? Red—no; Yellow—yes, for general population; Green—yes, for general and special populations. | |
| Number of Doses | How many doses are needed for protection? Red—3 or more doses; Yellow—2 doses; Green—1 dose. | |
| Suitability | Durability | What is the known or expected vaccine durability of protection? Red—≤6 months; Yellow—6 to 12 months; Green—≥12 months. |
| Safety | Do vaccines manufactured using the platform have a positive risk-benefit outcome, demonstrating acceptable safety? Red—there have been significant adverse events with no known mitigation strategy at a frequency that would outweigh the benefit; Yellow—there have been significant adverse events with no known mitigation strategy but the benefit outweighs the risks; Green—there are no known significant adverse events and the benefits outweigh the risks. | |
| Stability | Are the vaccines manufactured using the platform sufficiently stable, during handling and storage? Red—the vaccine requires long- and short-term storage at −60 °C or colder; Yellow—the vaccine requires long-term storage at −60 °C −20 °C or colder and remains stable at 2–8 °C or above, including under relevant handling conditions, for 6 months prior to administration; Green—the vaccine remains stable during long-term storage at 2–8 °C or above, and during relevant handling conditions prior to administration. | |
| Cost of Goods | Is the cost of vaccines adequate to support equitable access? Red—no, it is ≥$50/dose; Yellow—maybe, it is >$10/dose and <$50/dose; Green—yes, it is ≤$10/dose. | |
| Freedom to Operate | Does the Sponsor have the IP rights to develop the vaccine using the identified platform? Red—no, the Sponsor does not have the freedom to operate; Yellow—no, the Sponsor does not have the freedom to operate, but there is a high likelihood of obtaining rights through negotiations with IP right holder; Green—yes, the Sponsor has the freedom to operate. | |
| Regulatory | Prior Knowledge | Is prior knowledge from the platform available to support regulatory authorization? Red—there is no prior knowledge of the platform available to support regulatory authorization; Yellow—there is limited prior knowledge of the platform available to support regulatory authorization; Green—significant prior knowledge is available to support regulatory authorization. |
| Authorization | Has the platform been authorized by a WHO Listed regulatory authority for use? Red—it has not been authorized for use in clinical trials or for marketing; Yellow—it has been authorized for use in clinical trials; Green—it has been authorized for marketing of one or more products. | |
| Manufacturing | Capability | Are there a commercial manufacturing site, equipment, and personnel available to make the vaccine? Red—no; Yellow—site, equipment or personnel are in development and will be available; Green—site equipment and personnel are available. |
| Capacity | Does the commercial manufacturing site have the capacity to manufacture the required number of doses to supply high-risk populations. Red—the capacity is only sufficient to support manufacturing for less than half of the high-risk populations; Yellow—the capacity is sufficient for manufacturing for high-risk populations in the region in which it is located; Green—the capacity is sufficient to support manufacturing for all high-risk populations globally. | |
| Technology Transfer Robustness | Is the process sufficiently robust to enable technology transfer? Red—no; Yellow– maybe, but it has not been successfully transferred yet; Green—yes, it has been successfully transferred to at least one additional facility. | |
| Scalability | Is the process readily scalable for ensuring manufacturing of the quantity needed for global supply, if needed Red—no, process is not readily scalable Yellow—yes, increasing the scale will require significant development activities; Green—yes, increasing the scale will require no development activities. | |
| Supply Chain | Are raw materials and consumables for manufacturing of a sufficient number of doses for outbreak control accessible and secured? Red—raw materials and/or consumables are not accessible; Yellow—raw materials and/or consumables are accessible but not secured; Green—raw materials and/or consumables are accessible and secured. | |
| Facility | GMP Compliance | Has the manufacturing facility received GMP certification from relevant authorities. Red—The facility lacks GMP certification Green –the facility has been inspected by relevant authorities and has received GMP certification. |
| Analytical Capability | Is there sufficient analytical capacity to test and release the vaccine product? Red—no; Green—yes. |
3. Comparison of Exemplar Platforms for Outbreak Response
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saville, M.; Cramer, J.P.; Downham, M.; Hacker, A.; Lurie, N.; Van der Veken, L.; Whelan, M.; Hatchett, R. Delivering Pandemic Vaccines in 100 Days—What Will It Take? NEJM 2022, 387, e3. [Google Scholar] [CrossRef]
- Aatresh, A.; Lurie, N.; Hatchett, R. Chapter 13—Accelerating Vaccine Development: The 100 Days Mission. In Principles and Practice of Emergency Research Response, 1st ed.; Springer: Cham, Switzerland, 2024; pp. 299–313. [Google Scholar]
- Kim, K.; Sabet-Azad, R.; Patel, D.; Malin, G.; Askary, S.H.; Särnefält, A. Fast Tracking Vaccine Manufacturing: CEPI’s Rapid Response Framework for the 100 Days Mission. Vaccines 2025. submitted for publication. [Google Scholar]
- International Council for Harmonisation. Development and Manufacture of Drug Substances (Chemical Entities and Biological Entities) (Q11); International Council for Harmonisation: Geneva, Switzerland, 2012. [Google Scholar]
- World Health Organization. Guidelines on the Quality, Safety and Efficacy of Ebola Vaccines. Annex 2, TRS No. 1011. 2018. Available online: https://www.who.int/publications/m/item/annex-2-trs1011-ebola (accessed on 1 June 2025).
- European Medicines Agency. Guideline on Data Requirements for Vaccine Platform Technology Master Files (vPTMF). EMA/CVMP/IWP/286631/2021. 2022. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-data-requirements-vaccine-platform-technology-master-files-vptmf_en.pdf (accessed on 1 June 2025).
- World Health Organization. Evaluation of the Quality, Safety and Efficacy of Messenger RNA Vaccines for the Prevention of Infectious Diseases: Regulatory Considerations. Annex 3. TRS No. 1039. 2022. Available online: https://www.who.int/publications/m/item/annex-3-mRNA-vaccines-trs-no-1039 (accessed on 1 June 2025).
- World Health Organization. Guidelines on the Nonclinical and Clinical Evaluation of Monoclonal Antibodies and Related Products Intended for the Prevention or Treatment of Infectious Diseases. TRS 1048, Annex 2. 2023. Available online: https://www.who.int/publications/m/item/guidelines-on-the-nonclinical-and-clinical-evaluation-of-monoclonal-antibodies-and-related-products-intended-for-the-prevention-or-treatment-of-infectious-diseases (accessed on 1 June 2025).
- European Medicines Agency. Draft Report on the Proposal for a Directive of the European Parliament and of the Council on the Union Code Relating to Medicinal Products for Human Use, and Repealing Directive 2001/183/EC and Directive 2009/35/EC. 2023. Available online: https://www.europarl.europa.eu/doceo/document/ENVI-PR-753470_EN.pdf (accessed on 1 June 2025).
- International Council for Harmonisation. Analytical Procedure Development (Q14); International Council for Harmonisation: Geneva, Switzerland, 2023. [Google Scholar]
- International Council for Harmonisation. Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin (Q5A(R2)); International Council for Harmonisation: Geneva, Switzerland, 2023. [Google Scholar]
- Food and Drug Administration. Guidance Document Platform Technology Designation Program for Drug Development. FDA-2024-D-1829; Food and Drug Administration: Silver Spring, MD, USA, 2024. [Google Scholar]
- The Global Health Network. Pandemic Preparedness & Response Playbook. 2024. Available online: https://tghncollections.pubpub.org/pandemic-preparedness-response-playbook (accessed on 1 June 2025).

| Source | Year | Title | Description | Reference |
|---|---|---|---|---|
| ICH | 2012 | Development and manufacture of drug substances (chemical entities and biological entities) (Q11). | “The approach of developing a production strategy for a new drug starting from manufacturing processes similar to those used by the same applicant to manufacture other drugs of the same type (e.g., as in the production of monoclonal antibodies using predefined host cell, cell culture, and purification processes, for which there already exists considerable experience).” | [4] |
| WHO | 2018 | Guidelines on the quality, safety and efficacy of Ebola Vaccines. | “Platform technology: a production technology in which different viral- vectored vaccines are produced by incorporating heterologous genes for different proteins into an identical viral vector backbone.” | [5] |
| EMA | 2022 | Guideline on data requirements for vaccine platform technology master files (vPTMF). | “A collection of technologies that have, in common, the use of a ‘backbone’ carrier or vector that is modified with a different active substance or set of active substances for each vaccine derived from the platform. This includes, but may not be limited to, protein-based platforms (virus-like particles), DNA vaccine platforms, mRNA-based platforms, replicons and other self-amplifying RNA and viral and bacterial vector vaccines. In practice, a vaccine platform is a manufacturing process that relies on a single vector or expression system (“backbone carrier”) and a standard process for inserting a gene or genes of interest into the system to generate different recombinant master seeds, master sequences or constructs, which are then used to produce a vaccine.” | [6] |
| WHO | 2022 | Evaluation of the quality, safety and efficacy of messenger RNA vaccines for the prevention of infectious diseases: regulatory considerations. | “Platform technology: a group of technologies used as a base upon which other applications, processes or technologies are developed. In the context of mRNA vaccines, a given manufacturer might have one or more platforms on which they will develop vaccines (or therapeutics) against various diseases (separate individual vaccines or a combination vaccine) or pathogen strains against the same disease (separate monovalent or mixed multivalent vaccines). The term could also be applied to a particular drug-delivery system (such as LNPs containing the mRNA) where identical lipids, concentrations, methods of preparation and purification and so on are used. Use of the term “platform technology” would be considered appropriate when: (a) the manufacturing methods are essentially unchanged (but may be optimized for each specific candidate vaccine); (b) the test methods (except for identity, potency and stability) and acceptance criteria are unchanged; (c) the immunomodulatory compounds or elements are unchanged; and (d) compliance with GMP is unchanged.” | [7] |
| WHO | 2023 | Guidelines on the nonclinical and clinical evaluation of monoclonal antibodies and related products intended for the prevention or treatment of infectious diseases. | “Platform technology: an existing technology, or group of technologies, that are applied to the development and/or production of similar mAb products by a manufacturer. A given manufacturer might have one or more platforms on which they will develop various mAbs. A platform would be considered when the elements of the manufacturing methods and/or processes, the mAb protein scaffold, and the compliance with good manufacturing practices are unchanged. The experience and knowledge gained, data generated (on manufacturing, control and stability), and the validation of unchanged methods can all be used as supportive data for the more rapid assessment and development of a new mAb product candidate that fits within the boundaries of the platform.” | [8] |
| EMA | 2023 | Draft report on the proposal for a directive of the European Parliament and of the Council on the Union code relating to medicinal products for human use, and repealing Directive 2001/83/EC and Directive 2009/35/EC | ‘Platform technology’ means a technology or collection of technologies used in the manufacturing process, quality control, or testing of medicinal products or their components that rely on prior knowledge and are established under the same underlying scientific principles.” | [9] |
| ICH | 2023 | Analytical Procedure Development (Q14) | “Platform Analytical Procedure: An analytical procedure that is suitable to test quality attributes of different products without significant change to its operational conditions, system suitability and reporting structure. This type of analytical procedure can be used to analyse molecules that are sufficiently alike with respect to the attributes that the platform analytical procedure is intended to measure.” | [10] |
| ICH | 2023 | Viral safety evaluation of biotechnology products derived from cell lines of human or animal origin (Q5A(R2)) | “Throughout this guideline, this term exclusively refers to validation of the process platform regarding viral clearance. In this context, platform validation is defined as the use of prior knowledge including in-house experience with viral reduction data from other products, to claim a reduction factor for a new similar product, according to current understanding.” | [11] |
| FDA | 2024 | Draft: Platform Technology Designation Program for Drug Development (FDA-2024-D-1829) | “Platform Technology: As defined in section 506K (h) (1) of the FD&C Act, a well-understood and reproducible technology, which can include a nucleic acid sequence, molecular structure, mechanism of action, delivery method, vector, or a combination of any such technologies that the Secretary determines to be appropriate, that the sponsor demonstrates (1) is incorporated in or used by a drug and is essential to the structure or function of such drug; (2) can be adapted for, incorporated into, or used by, more than one drug sharing common structural elements; and (3) facilitates the manufacture or development of more than one drug through a standardized production or manufacturing process or processes.” | [12] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabet-Azad, R.; Hoath, C.; Bézay, N.; Särnefält, A. The Platform Readiness Dashboard: A Tool for Evaluating Vaccine Platform Suitability for a Rapid Response to Epidemic and Pandemic Threats. Vaccines 2025, 13, 793. https://doi.org/10.3390/vaccines13080793
Sabet-Azad R, Hoath C, Bézay N, Särnefält A. The Platform Readiness Dashboard: A Tool for Evaluating Vaccine Platform Suitability for a Rapid Response to Epidemic and Pandemic Threats. Vaccines. 2025; 13(8):793. https://doi.org/10.3390/vaccines13080793
Chicago/Turabian StyleSabet-Azad, Ramin, Catherine Hoath, Nicole Bézay, and Anna Särnefält. 2025. "The Platform Readiness Dashboard: A Tool for Evaluating Vaccine Platform Suitability for a Rapid Response to Epidemic and Pandemic Threats" Vaccines 13, no. 8: 793. https://doi.org/10.3390/vaccines13080793
APA StyleSabet-Azad, R., Hoath, C., Bézay, N., & Särnefält, A. (2025). The Platform Readiness Dashboard: A Tool for Evaluating Vaccine Platform Suitability for a Rapid Response to Epidemic and Pandemic Threats. Vaccines, 13(8), 793. https://doi.org/10.3390/vaccines13080793






