Protective Efficacy Induced by Virus-like Particles Expressing Dense Granule Protein 5 of Toxoplasma gondii
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Ethics Statement
2.2. Experimental Animals and Parasite Strains
2.3. Construction and VLP Production
2.4. VLP Characterization
2.5. GRA5 VLP Immunization and Challenge Infection in Mice
2.6. Sample Collection
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Antibody-Secreting Cell (ASC) Response
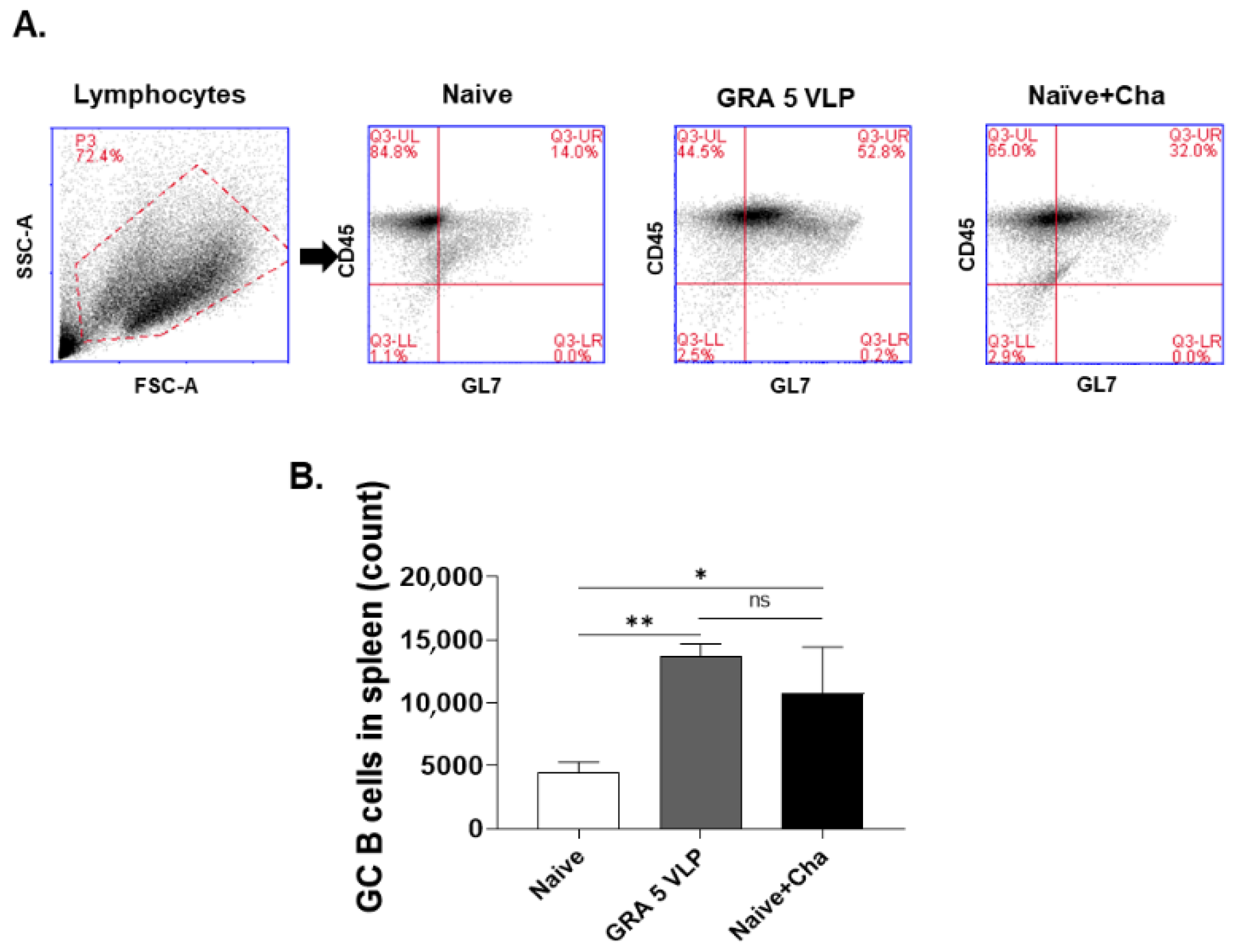
2.9. Germinal Center (GC) B Cell Response
2.10. Protective Efficacy
2.11. Statistical Analysis
3. Results
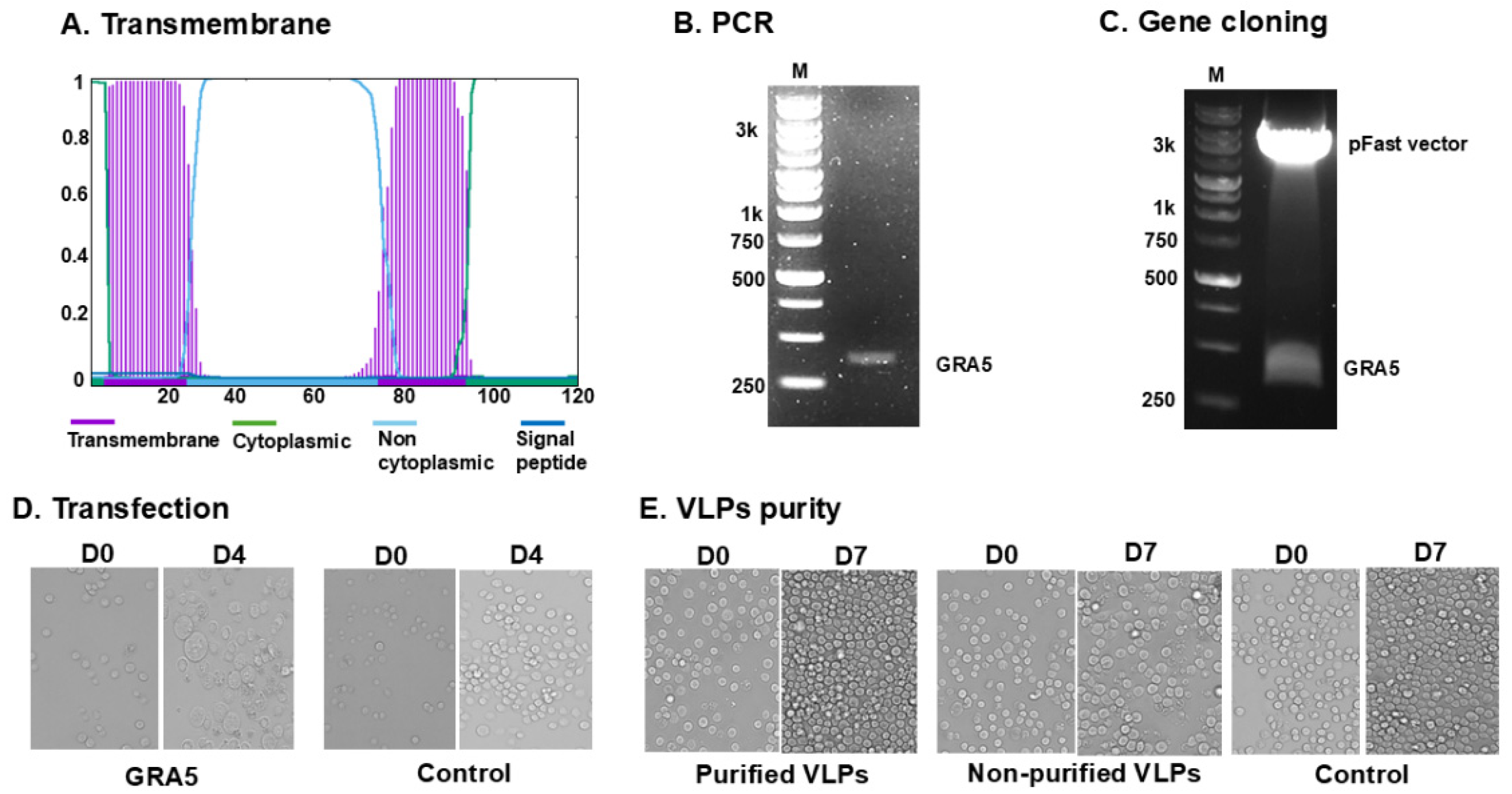
3.1. GRA5 Construct and VLP Generation
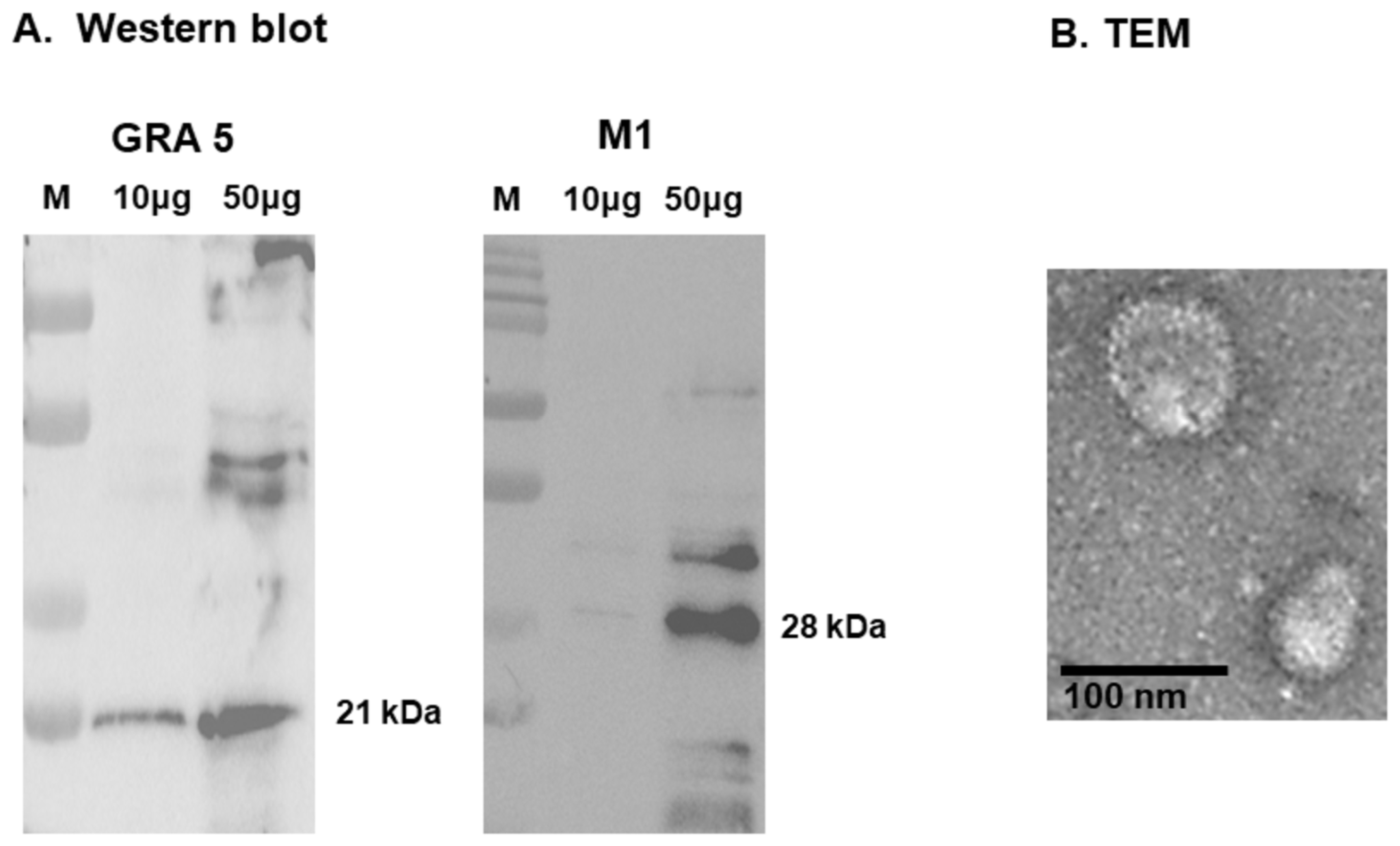
3.2. Antigen Expression and Structural Validation of GRA5 VLPs
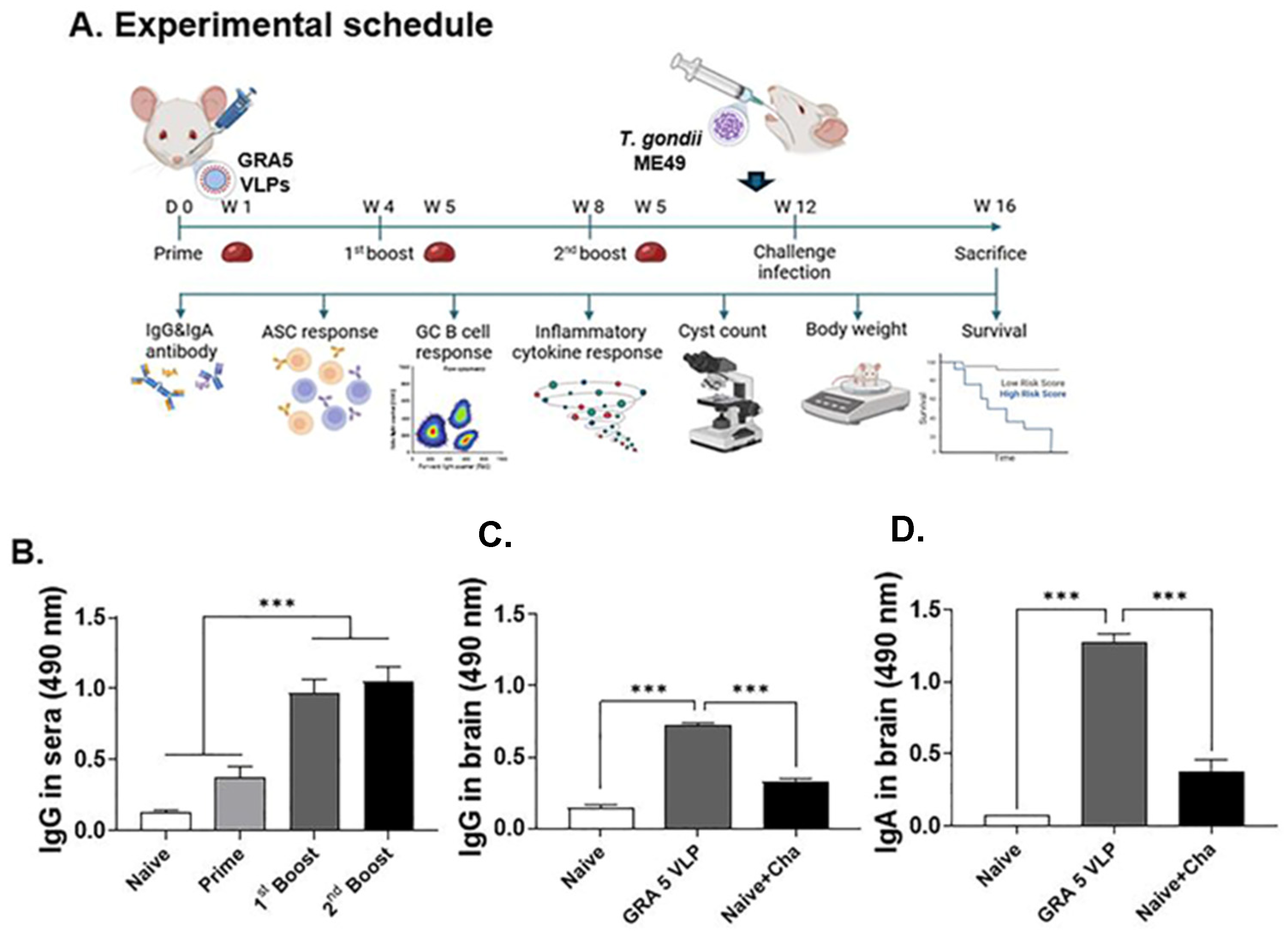
3.3. T. gondii-Specific IgG Antibody Responses in Serum and Brain
3.4. T. gondii-Specific IgG and IgA Responses in Spleen Tissue and ASC
3.5. T. gondii-Specific ASC (IgG, IgA) Responses
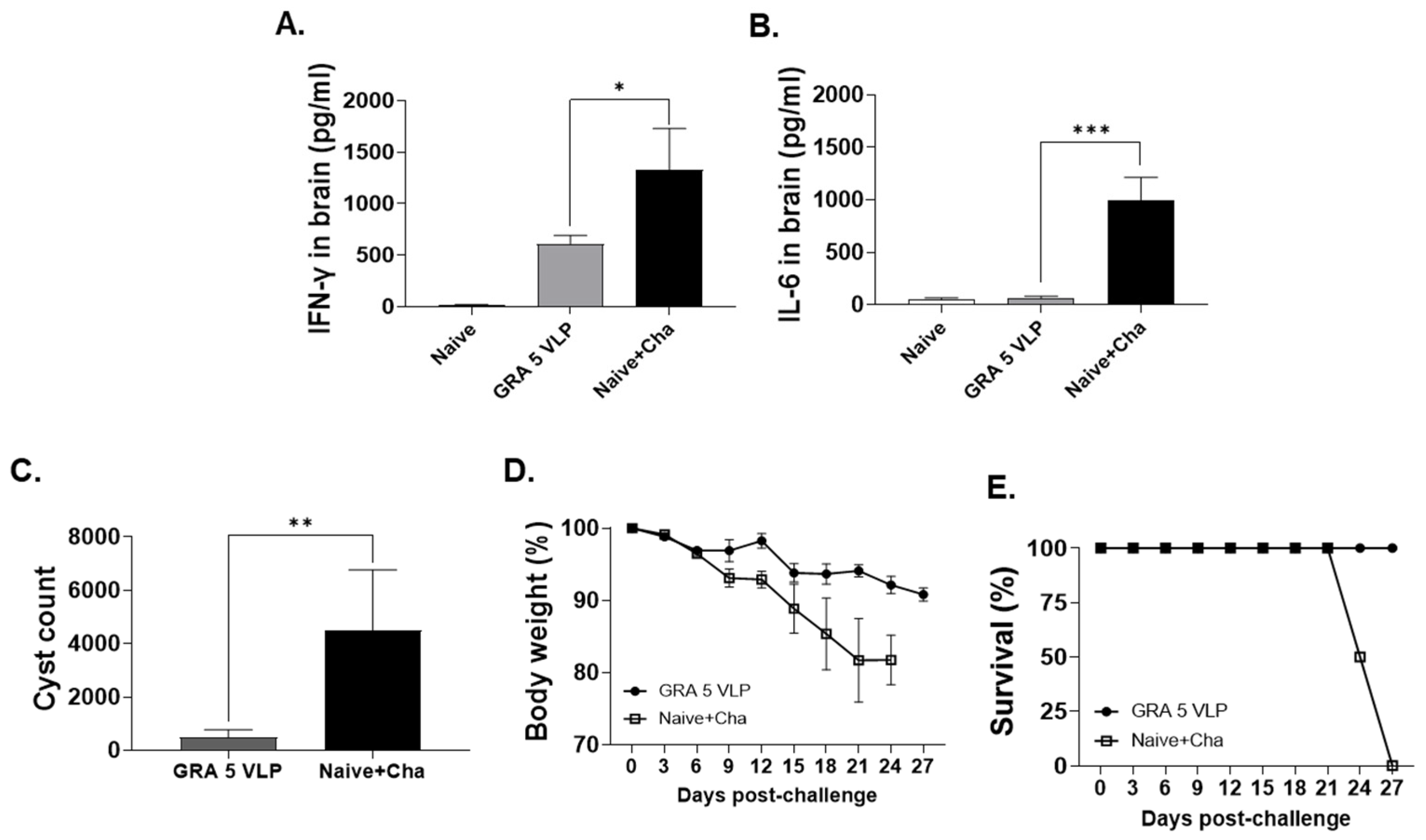
3.6. Protective Efficacy of GRA5 VLPs Against T. gondii ME49 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, K.; Bahia-Oliveira, L.; Dixon, B.; Dumètre, A.; de Wit, L.A.; VanWormer, E.; Villena, I. Environmental transmission of Toxoplasma gondii: Oocysts in water, soil and food. Food Waterborne Parasitol. 2019, 15, e00049. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.S.; Shabbir, N. Congenital toxoplasmosis. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Diebler, C.; Dusser, A.; Dulac, O. Congenital toxoplasmosis: Clinical and neuroradiological evaluation of the cerebral lesions. Neuroradiology 1985, 27, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hutson, S.L.; Wheeler, K.M.; McLone, D.; Frim, D.; Penn, R.; Swisher, C.N.; Heydemann, P.T.; Boyer, K.M.; Noble, A.G.; Rabiah, P. Patterns of hydrocephalus caused by congenital Toxoplasma gondii infection associate with parasite genetics. Clin. Infect. Dis. 2015, 61, 1831–1834. [Google Scholar] [CrossRef] [PubMed]
- Hopper, A.T.; Brockman, A.; Wise, A.; Gould, J.; Barks, J.; Radke, J.B.; Sibley, L.D.; Zou, Y.; Thomas, S. Discovery of selective Toxoplasma gondii dihydrofolate reductase inhibitors for the treatment of toxoplasmosis. J. Med. Chem. 2019, 62, 1562–1576. [Google Scholar] [CrossRef] [PubMed]
- Ben-Harari, R.R.; Goodwin, E.; Casoy, J. Adverse event profile of pyrimethamine-based therapy in toxoplasmosis: A systematic review. Drugs RD 2017, 17, 523–544. [Google Scholar] [CrossRef] [PubMed]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of toxoplasmosis: Historical perspective, animal models, and current clinical practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef] [PubMed]
- Aspinall, T.V.; Joynson, D.H.; Guy, E.; Hyde, J.E.; Sims, P.F. The molecular basis of sulfonamide resistance in Toxoplasma gondii and implications for the clinical management of toxoplasmosis. J. Infect. Dis. 2002, 185, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Alday, P.H.; Doggett, J.S. Drugs in development for toxoplasmosis: Advances, challenges, and current status. Drug Des. Dev. Ther. 2017, 11, 273–293. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Sugi, T.; Weiss, L.M.; Kato, K. Identification of compounds that suppress Toxoplasma gondii tachyzoites and bradyzoites. PLoS ONE 2017, 12, e0178203. [Google Scholar] [CrossRef] [PubMed]
- Innes, E.A.; Bartley, P.M.; Maley, S.; Katzer, F.; Buxton, D. Veterinary vaccines against Toxoplasma gondii. Memórias Do Inst. Oswaldo Cruz 2009, 104, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.-B.; Quan, F.-S. Advances in Toxoplasma gondii vaccines: Current strategies and challenges for vaccine development. Vaccines 2021, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Fox, B.A.; Guevara, R.B.; Rommereim, L.M.; Falla, A.; Bellini, V.; Pètre, G.; Rak, C.; Cantillana, V.; Dubremetz, J.-F.; Cesbron-Delauw, M.-F. Toxoplasma gondii parasitophorous vacuole membrane-associated dense granule proteins orchestrate chronic infection and GRA12 underpins resistance to host gamma interferon. MBio 2019, 10, e00589-19. [Google Scholar] [CrossRef] [PubMed]
- Ching, X.T.; Fong, M.Y.; Lau, Y.L. Evaluation of immunoprotection conferred by the subunit vaccines of GRA2 and GRA5 against acute toxoplasmosis in BALB/c mice. Front. Microbiol. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Ching, X.T.; Fong, M.Y.; Lau, Y.L. Evaluation of the protective effect of deoxyribonucleic acid vaccines encoding granule antigen 2 and 5 against acute Toxoplasmosis in BALB/c Mice. Am. J. Trop. Med. Hyg. 2017, 96, 1441. [Google Scholar] [CrossRef] [PubMed]
- Eom, G.-D.; Chu, K.-B.; Kang, H.-J.; Kim, M.-J.; Yoon, K.-W.; Mao, J.; Lee, S.-H.; Ahmed, M.A.; Moon, E.-K.; Quan, F.-S. Protective mucosal and systemic immunity induced by virus-like particles expressing Toxoplasma gondii cyst wall protein. PLoS ONE 2023, 18, e0283928. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Chu, K.B.; Lee, S.H.; Kim, M.J.; Park, H.; Jin, H.; Moon, E.K.; Quan, F.S. Toxoplasma gondii virus-like particle vaccination alleviates inflammatory response in the brain upon T gondii infection. Parasite Immunol. 2020, 42, e12716. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Chu, K.-B.; Kang, H.-J.; Quan, F.-S. Virus-like particles containing multiple antigenic proteins of Toxoplasma gondii induce memory T cell and B cell responses. PLoS ONE 2019, 14, e0220865. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.-B.; Kang, H.-J.; Yoon, K.-W.; Lee, H.-A.; Moon, E.-K.; Han, B.-K.; Quan, F.-S. Influenza virus-like particle (VLP) vaccines expressing the SARS-CoV-2 S glycoprotein, S1, or S2 domains. Vaccines 2021, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, A.-R.; Lee, D.-H.; Rubino, I.; Choi, H.-J.; Quan, F.-S. Protection induced by virus-like particles containing Toxoplasma gondii microneme protein 8 against highly virulent RH strain of Toxoplasma gondii infection. PLoS ONE 2017, 12, e0175644. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Lee, S.-H.; Kim, M.-J.; Chu, K.-B.; Lee, D.-H.; Chopra, M.; Choi, H.-J.; Park, H.; Jin, H.; Quan, F.-S. Influenza virus-like particles presenting both Toxoplasma gondii ROP4 and ROP13 Enhance Protection against T. gondii infection. Pharmaceutics 2019, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Mao, J.; Kim, M.-J.; Yoon, K.-W.; Eom, G.-D.; Chu, K.-B.; Moon, E.-K.; Quan, F.-S. The detection of Toxoplasma gondii ME49 infections in BALB/c mice using various techniques. Parasites Hosts Dis. 2023, 61, 418. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Chu, K.B.; Yoon, K.-W.; Kang, H.-J.; Lee, D.-H.; Moon, E.-K.; Quan, F.-S. Virus-like particles expressing microneme-associated antigen of Plasmodium berghei confer better protection than those expressing apical membrane antigen 1. Parasites Hosts Dis. 2024, 62, 193. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.-W.; Chu, K.-B.; Kang, H.-J.; Kim, M.-J.; Eom, G.-D.; Quan, F.-S. Orally administrated recombinant vaccinia virus displaying ROP4 induces protection against Toxoplasma gondii challenge infection. Vaccines 2022, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kang, H.-J.; Lee, D.-H.; Quan, F.-S. Protective immunity induced by incorporating multiple antigenic proteins of Toxoplasma gondii into influenza virus-like particles. Front. Immunol. 2019, 9, 3073. [Google Scholar] [CrossRef] [PubMed]
- Eom, G.-D.; Chu, K.B.; Mao, J.; Yoon, K.-W.; Kang, H.-J.; Moon, E.-K.; Kim, S.S.; Quan, F.-S. Heterologous immunization targeting the CST1 antigen confers better protection than ROP18 in mice. Nanomedicine 2024, 19, 2437–2446. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Eom, G.-D.; Yoon, K.-W.; Heo, S.I.; Kang, H.-J.; Chu, K.B.; Moon, E.-K.; Quan, F.-S. Protective humoral immunity induced by virus-like particles expressing Toxoplasma gondii CST1 or MIC8. Acta Trop. 2025, 261, 107501. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Ham, Y.S.; Gil, W.J.; Yang, C.-S. Exploring the potential of Toxoplasma gondii in drug development and as a delivery system. Exp. Mol. Med. 2024, 56, 289–300. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, S.I.; Kang, H.-J.; Mao, J.; Yang, Z.-S.; Ahmed, M.A.; Quan, F.-S. Protective Efficacy Induced by Virus-like Particles Expressing Dense Granule Protein 5 of Toxoplasma gondii. Vaccines 2025, 13, 787. https://doi.org/10.3390/vaccines13080787
Heo SI, Kang H-J, Mao J, Yang Z-S, Ahmed MA, Quan F-S. Protective Efficacy Induced by Virus-like Particles Expressing Dense Granule Protein 5 of Toxoplasma gondii. Vaccines. 2025; 13(8):787. https://doi.org/10.3390/vaccines13080787
Chicago/Turabian StyleHeo, Su In, Hae-Ji Kang, Jie Mao, Zhao-Shou Yang, Md Atique Ahmed, and Fu-Shi Quan. 2025. "Protective Efficacy Induced by Virus-like Particles Expressing Dense Granule Protein 5 of Toxoplasma gondii" Vaccines 13, no. 8: 787. https://doi.org/10.3390/vaccines13080787
APA StyleHeo, S. I., Kang, H.-J., Mao, J., Yang, Z.-S., Ahmed, M. A., & Quan, F.-S. (2025). Protective Efficacy Induced by Virus-like Particles Expressing Dense Granule Protein 5 of Toxoplasma gondii. Vaccines, 13(8), 787. https://doi.org/10.3390/vaccines13080787





