XBB.1.5 COVID-19 mRNA Vaccines Induce Inadequate Mucosal Immunity in Patients with Inflammatory Bowel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Recruitment
2.2. Study Procedures
2.3. Quantification of Mucosal Anti-Receptor-Binding Domain Antibodies
2.4. Study Outcomes and Statistical Analysis
3. Results
3.1. Patient Characteristics
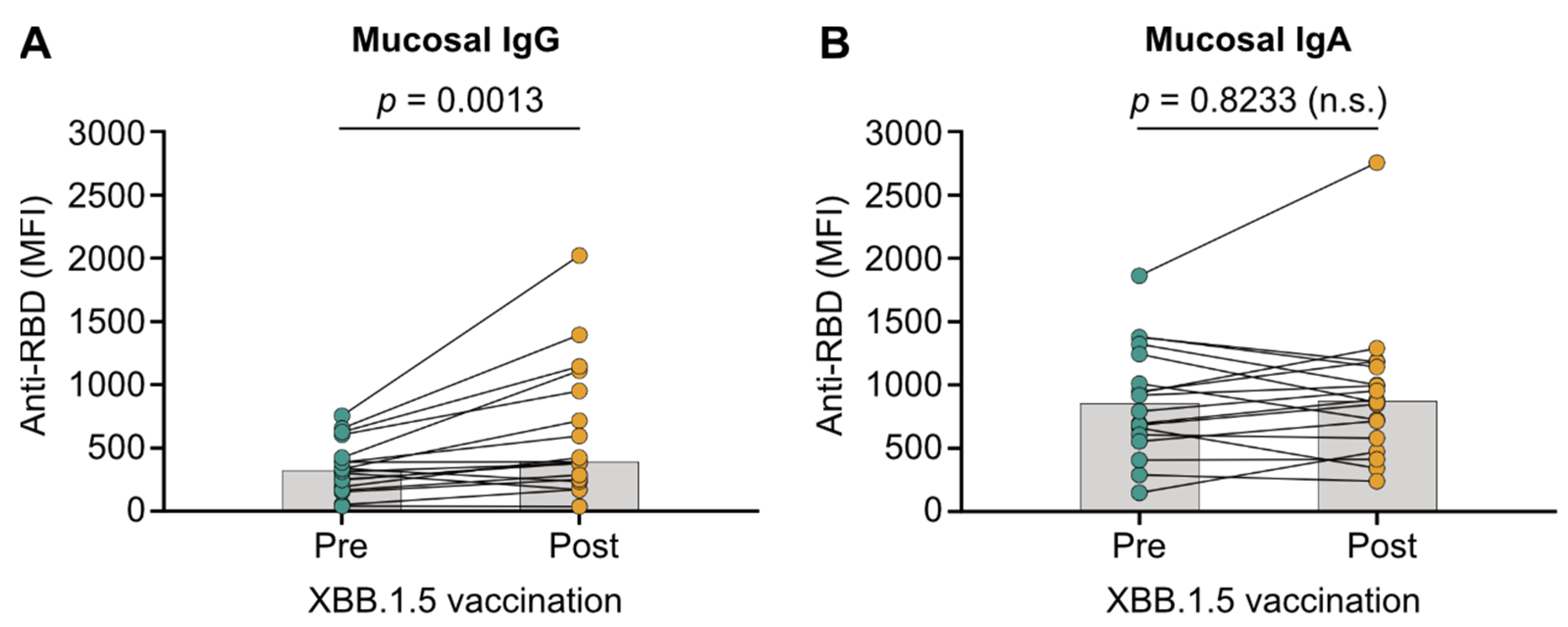
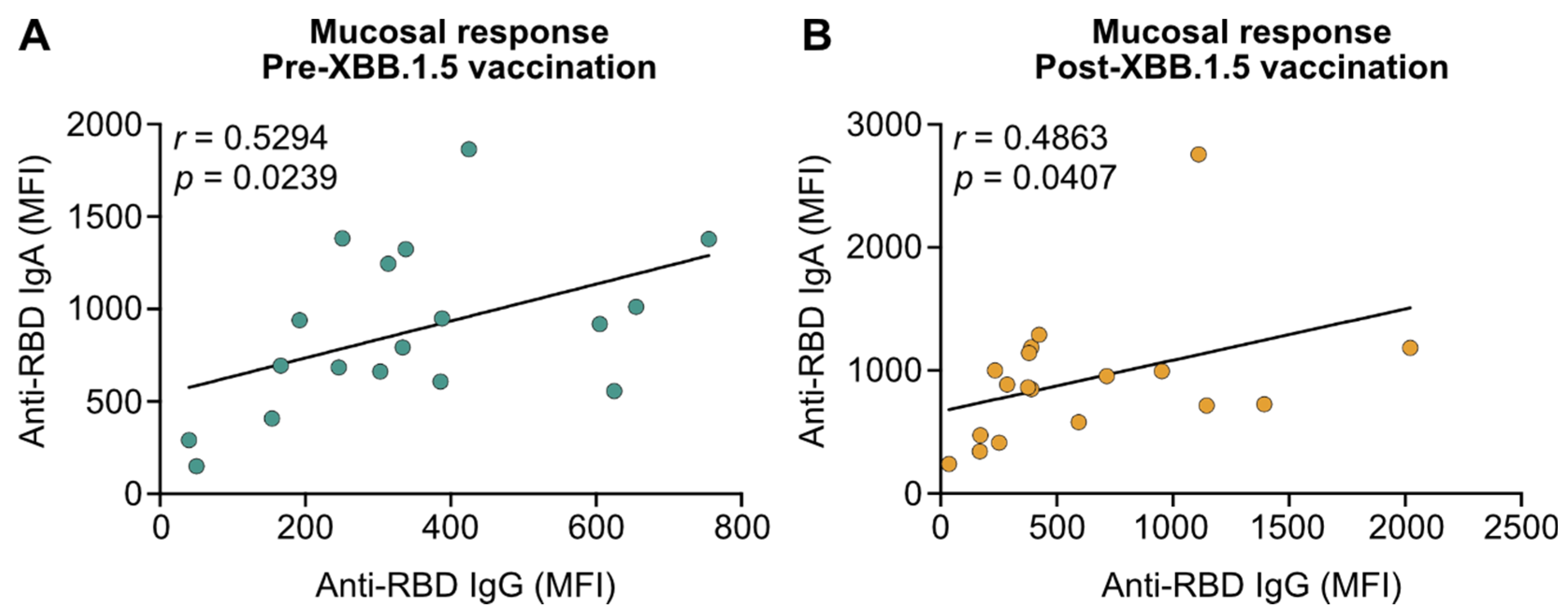
3.2. XBB.1.5 mRNA Vaccines Do Not Boost Mucosal IgA in Patients with IBD
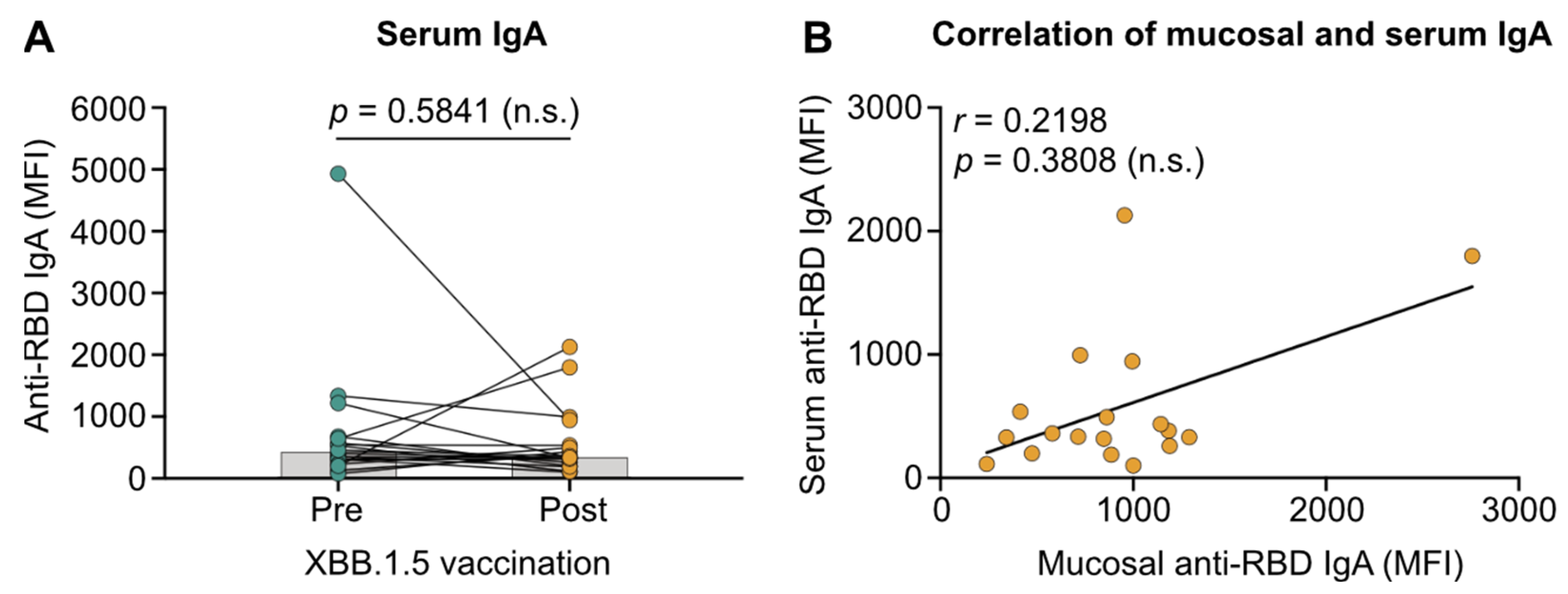
3.3. Migration of Vaccine-Induced Systemic IgA to the Respiratory Mucosa Is Absent in Patients with IBD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Krammer, F. mRNA Vaccines for Infectious Diseases—Advances, Challenges and Opportunities. Nat. Rev. Drug Discov. 2024, 23, 838–861. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-Y.; Du, Y.; Xu, Y.; Paritala, S.; Donahue, M.; Maloney, P. Durability of XBB.1.5 Vaccines against Omicron Subvariants. N. Engl. J. Med. 2024, 390, 2124–2127. [Google Scholar] [CrossRef] [PubMed]
- Davis-Gardner, M.E.; Lai, L.; Wali, B.; Samaha, H.; Solis, D.; Lee, M.; Porter-Morrison, A.; Hentenaar, I.T.; Yamamoto, F.; Godbole, S.; et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA Bivalent Booster. N. Engl. J. Med. 2023, 388, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guo, Y.; Bowen, A.; Mellis, I.A.; Valdez, R.; Gherasim, C.; Gordon, A.; Liu, L.; Ho, D.D. XBB.1.5 Monovalent mRNA Vaccine Booster Elicits Robust Neutralizing Antibodies against XBB Subvariants and JN.1. Cell Host Microbe 2024, 32, 315–321.e3. [Google Scholar] [CrossRef] [PubMed]
- Meslé, M.M.I.; Brown, J.; Mook, P.; Katz, M.A.; Hagan, J.; Pastore, R.; Benka, B.; Redlberger-Fritz, M.; Bossuyt, N.; Stouten, V.; et al. Estimated Number of Lives Directly Saved by COVID-19 Vaccination Programmes in the WHO European Region from December, 2020, to March, 2023: A Retrospective Surveillance Study. Lancet Respir. Med. 2024, 12, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Català, M.; Mercadé-Besora, N.; Kolde, R.; Trinh, N.T.H.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.Y.; Delmestri, A.; et al. The Effectiveness of COVID-19 Vaccines to Prevent Long COVID Symptoms: Staggered Cohort Study of Data from the UK, Spain, and Estonia. Lancet Respir. Med. 2024, 12, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Effect of COVID-19 Vaccination on Long COVID: Systematic Review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.Y.B.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of COVID-19 Vaccines in Immunocompromised Patients: Systematic Review and Meta-Analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef] [PubMed]
- Barnes, E.; Goodyear, C.S.; Willicombe, M.; Gaskell, C.; Siebert, S.; De Silva, T.I.; Murray, S.M.; Rea, D.; Snowden, J.A.; Carroll, M.; et al. SARS-CoV-2-Specific Immune Responses and Clinical Outcomes after COVID-19 Vaccination in Patients with Immune-Suppressive Disease. Nat. Med. 2023, 29, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Brand, S. Crohn’s Disease: Th1, Th17 or Both? The Change of a Paradigm: New Immunological and Genetic Insights Implicate Th17 Cells in the Pathogenesis of Crohn’s Disease. Gut 2009, 58, 1152–1167. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Artis, D.; Becker, C. The Intestinal Barrier: A Pivotal Role in Health, Inflammation, and Cancer. Lancet Gastroenterol. Hepatol. 2025, 10, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Goodhand, J.R.; Bewshea, C.; Nice, R.; Chee, D.; Lin, S.; Chanchlani, N.; Butterworth, J.; Cooney, R.; Croft, N.M.; et al. Anti-SARS-CoV-2 Antibody Responses Are Attenuated in Patients with IBD Treated with Infliximab. Gut 2021, 70, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Edelman-Klapper, H.; Zittan, E.; Bar-Gil Shitrit, A.; Rabinowitz, K.M.; Goren, I.; Avni-Biron, I.; Ollech, J.E.; Lichtenstein, L.; Banai-Eran, H.; Yanai, H.; et al. Lower Serologic Response to COVID-19 mRNA Vaccine in Patients With Inflammatory Bowel Diseases Treated With Anti-TNFα. Gastroenterology 2022, 162, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Kennedy, N.A.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Castro Seoane, R.; Liu, Z.; Nice, R.; Bewshea, C.; D’Mello, A.; et al. COVID-19 Vaccine-Induced Antibody Responses in Immunosuppressed Patients with Inflammatory Bowel Disease (VIP): A Multicentre, Prospective, Case-Control Study. Lancet Gastroenterol. Hepatol. 2022, 7, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Liu, Z.; Muñoz Sandoval, D.; Reynolds, C.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Castro Seoane, R.; Anand, N.; Nice, R.; et al. COVID-19 Vaccine-Induced Antibody and T-Cell Responses in Immunosuppressed Patients with Inflammatory Bowel Disease after the Third Vaccine Dose (VIP): A Multicentre, Prospective, Case-Control Study. Lancet Gastroenterol. Hepatol. 2022, 7, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, S.; Dütschler, J.; König, M.; Graf, N.; Oikonomou, V.; Krieger, C.; Truniger, S.; Franke, A.; Eckhold, A.; Forsch, K.; et al. Systemic and T Cell-associated Responses to SARS-CoV-2 Immunisation in Gut Inflammation (STAR SIGN Study): Effects of Biologics on Vaccination Efficacy of the Third Dose of mRNA Vaccines against SARS-CoV-2. Aliment. Pharmacol. Ther. 2023, 57, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Alexander, J.L.; Lin, K.W.; Ahmad, T.; Pollock, K.M.; Powell, N.; Le, K.; Zhou, X.; Ibraheim, H.; Anandabaskaran, S.; et al. Infliximab and Tofacitinib Attenuate Neutralizing Antibody Responses Against SARS-CoV-2 Ancestral and Omicron Variants in Inflammatory Bowel Disease Patients After 3 Doses of COVID-19 Vaccine. Gastroenterology 2023, 164, 300–303.e3. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, S.; Brand, S. Editorial: Updated COVID-19 Boosters—Tailoring Protection for Patients With IBD. Authors’ Reply. Aliment. Pharmacol. Ther. 2025, 61, 382–383. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Kennedy, N.A.; Saifuddin, A.; Sandoval, D.M.; Reynolds, C.J.; Seoane, R.C.; Kottoor, S.H.; Pieper, F.P.; Lin, K.-M.; Butler, D.K.; et al. Antibody Decay, T Cell Immunity and Breakthrough Infections Following Two SARS-CoV-2 Vaccine Doses in Inflammatory Bowel Disease Patients Treated with Infliximab and Vedolizumab. Nat. Commun. 2022, 13, 1379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Le, K.; Zhou, X.; Alexander, J.L.; Lin, S.; Bewshea, C.; Chanchlani, N.; Nice, R.; McDonald, T.J.; Lamb, C.A.; et al. Neutralising Antibody Potency against SARS-CoV-2 Wild-Type and Omicron BA.1 and BA.4/5 Variants in Patients with Inflammatory Bowel Disease Treated with Infliximab and Vedolizumab after Three Doses of COVID-19 Vaccine (CLARITY IBD): An Analysis of a Prospective Multicentre Cohort Study. Lancet Gastroenterol. Hepatol. 2023, 8, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, S.; Dütschler, J.; Junker, D.; König, M.; Graf, N.; Krieger, C.; Truniger, S.; Oikonomou, V.; Leinenkugel, G.; Koller, S.; et al. XBB.1.5-Adapted COVID-19 mRNA Vaccines but Not Infections With Previous Omicron Variants Boost Neutralisation Against the SARS-CoV-2 JN.1 Variant in Patients With Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2025, 61, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Jian, F.; Wang, J.; Yisimayi, A.; Song, W.; Xu, Y.; Chen, X.; Niu, X.; Yang, S.; Yu, Y.; Wang, P.; et al. Evolving Antibody Response to SARS-CoV-2 Antigenic Shift from XBB to JN.1. Nature 2025, 637, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, S.; Dütschler, J.; König, M.; Dulovic, A.; Graf, N.; Junker, D.; Oikonomou, V.; Krieger, C.; Truniger, S.; Franke, A.; et al. STAR SIGN Study: Evaluation of COVID-19 Vaccine Efficacy against the SARS-CoV-2 Variants BQ.1.1 and XBB.1.5 in Patients with Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2023, 58, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Wagstaffe, H.R.; Thwaites, R.S.; Reynaldi, A.; Sidhu, J.K.; McKendry, R.; Ascough, S.; Papargyris, L.; Collins, A.M.; Xu, J.; Lemm, N.-M.; et al. Mucosal and Systemic Immune Correlates of Viral Control after SARS-CoV-2 Infection Challenge in Seronegative Adults. Sci. Immunol. 2024, 9, eadj9285. [Google Scholar] [CrossRef] [PubMed]
- Pisanic, N.; Antar, A.A.R.; Hetrich, M.K.; Demko, Z.O.; Zhang, X.; Spicer, K.; Kruczynski, K.L.; Detrick, B.; Clarke, W.; Knoll, M.D.; et al. Early, Robust Mucosal Secretory Immunoglobulin A but Not Immunoglobulin G Response to Severe Acute Respiratory Syndrome Coronavirus 2 Spike in Oral Fluid Is Associated With Faster Viral Clearance and Coronavirus Disease 2019 Symptom Resolution. J. Infect. Dis. 2025, 231, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Rowe, M.; McMahan, K.; Hachmann, N.P.; Miller, J.; Jacob-Dolan, C.; Liu, J.; Verrette, B.; Gotthardt, K.A.; Ty, D.M.; et al. SARS-CoV-2 XBB.1.5 mRNA Booster Vaccination Elicits Limited Mucosal Immunity. Sci. Transl. Med. 2024, 16, eadp8920. [Google Scholar] [CrossRef] [PubMed]
- Declercq, J.; Gerlo, S.; Van Nevel, S.; De Ruyck, N.; Holtappels, G.; Delesie, L.; Tobback, E.; Lammens, I.; Gerebtsov, N.; Sedeyn, K.; et al. Repeated COVID-19 mRNA-Based Vaccination Contributes to SARS-CoV-2 Neutralizing Antibody Responses in the Mucosa. Sci. Transl. Med. 2024, 16, eadn2364. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, S.; Dütschler, J.; Junker, D.; König, M.; Leinenkugel, G.; Graf, N.; Krieger, C.; Truniger, S.; Franke, A.; Koller, S.; et al. Systemic and Mucosal Immunogenicity of Monovalent XBB.1.5-Adapted COVID-19 mRNA Vaccines in Patients with Inflammatory Bowel Disease. Vaccines 2024, 12, 774. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Strengert, M.; Junker, D.; Kaiser, P.D.; Kerrinnes, T.; Traenkle, B.; Dinter, H.; Häring, J.; Ghozzi, S.; Zeck, A.; et al. Exploring beyond Clinical Routine SARS-CoV-2 Serology Using MultiCoV-Ab to Evaluate Endemic Coronavirus Cross-Reactivity. Nat. Commun. 2021, 12, 1152. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Ayoub, H.H.; Coyle, P.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al Thani, A.A.; Al-Kanaani, Z.; Al-Kuwari, E.; Jeremijenko, A.; et al. Differential Protection against SARS-CoV-2 Reinfection Pre- and Post-Omicron. Nature 2025, 639, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Marking, U.; Svensson, J.; Greilert-Norin, N.; Bacchus, P.; Nilsson, P.; Hober, S.; Gordon, M.; Blom, K.; Klingström, J.; et al. Anti-Spike Mucosal IgA Protection against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 387, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Marking, U.; Bladh, O.; Havervall, S.; Greilert-Norin, N.; Gordon, M.; Alm, J.J.; Blom, K.; Åberg, M.; Klingström, J.; Thålin, C. Mucosal IgA Protects against BQ.1 and BQ.1.1 Infection. Lancet Infect. Dis. 2023, 23, e272–e273. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Marcotte, H.; Hammarström, L.; Pan-Hammarström, Q. Mucosal IgA against SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 387, e55. [Google Scholar] [CrossRef] [PubMed]
- Woelfel, S.; Junker, D.; Bergamin, I.; Meyer-Herbon, P.; Stillhard, R.; Graf, N.; Leinenkugel, G.; Dütschler, J.; König, M.; Kammerlander, L.; et al. STAR LIGHT Study: XBB.1.5 COVID-19 mRNA Vaccines Boost Systemic but Not Mucosal Immunity Against the SARS-CoV-2 JN.1 Variant in Patients with Chronic Liver Disease. Vaccines 2024, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A.; Pezzullo, A.M.; Cristiano, A.; Boccia, S. Global Estimates of Lives and Life-Years Saved by COVID-19 Vaccination during 2020-2024. medRxiv 2024. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D.; et al. COVID-19-Neutralizing Antibodies Predict Disease Severity and Survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mellis, I.A.; Wu, M.; Bowen, A.; Gherasim, C.; Valdez, R.; Shah, J.G.; Purpura, L.J.; Yin, M.T.; Gordon, A.; et al. KP.2-Based Monovalent mRNA Vaccines Robustly Boost Antibody Responses to SARS-CoV-2. Lancet Infect. Dis. 2025, 25, e133–e134. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Pinedo, K.; Stabile, M.A.; Hamlin, R.E.; Pienkos, S.M.; Ratnasiri, K.; Stanford COVID-19 Biobank; Yang, S.; Blomkalns, A.L.; Nadeau, K.C.; et al. Prior Vaccination Prevents Overactivation of Innate Immune Responses during COVID-19 Breakthrough Infection. Sci. Transl. Med. 2025, 17, eadq1086. [Google Scholar] [CrossRef] [PubMed]
- Mellors, J.; Dhaliwal, R.; Longet, S.; Tipton, T.; OCTAVE Consortium; McInnes, I.; Siebert, S.; Kearns, P.; Rea, D.; Cook, G.; et al. Complement-Mediated Enhancement of SARS-CoV-2 Antibody Neutralisation Potency in Vaccinated Individuals. Nat. Commun. 2025, 16, 2666. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.C.; Hentenaar, I.T.; Morrison-Porter, A.; Solano, D.; Haddad, N.S.; Castrillon, C.; Runnstrom, M.C.; Lamothe, P.A.; Andrews, J.; Roberts, D.; et al. SARS-CoV-2-Specific Plasma Cells Are Not Durably Established in the Bone Marrow Long-Lived Compartment after mRNA Vaccination. Nat. Med. 2025, 31, 235–244. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Wegmann, F.; Aid, M.; Sciacca, M.; Liu, J.; Hachmann, N.P.; Miller, J.; Jacob-Dolan, C.; Powers, O.; Hope, D.; et al. Mucosal Boosting Enhances Vaccine Protection against SARS-CoV-2 in Macaques. Nature 2024, 626, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Gagne, M.; Flynn, B.J.; Andrew, S.F.; Marquez, J.; Flebbe, D.R.; Mychalowych, A.; Lamb, E.; Davis-Gardner, M.E.; Burnett, M.R.; Serebryannyy, L.A.; et al. Mucosal Adenovirus Vaccine Boosting Elicits IgA and Durably Prevents XBB.1.16 Infection in Nonhuman Primates. Nat. Immunol. 2024, 25, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- Darling, T.L.; Harastani, H.H.; Joshi, A.; Bricker, T.L.; Soudani, N.; Seehra, K.; Hassan, A.O.; Diamond, M.S.; Boon, A.C.M. Mucosal Immunization with ChAd-SARS-CoV-2-S Prevents Sequential Transmission of SARS-CoV-2 to Unvaccinated Hamsters. Sci. Adv. 2024, 10, eadp1290. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zeng, C.; Cox, T.M.; Li, C.; Son, Y.M.; Cheon, I.S.; Wu, Y.; Behl, S.; Taylor, J.J.; Chakaraborty, R.; et al. Respiratory Mucosal Immunity against SARS-CoV-2 after mRNA Vaccination. Sci. Immunol. 2022, 7, eadd4853. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woelfel, S.; Dütschler, J.; Junker, D.; König, M.; Leinenkugel, G.; Krieger, C.; Truniger, S.; Franke, A.; Koller, S.; Metzger-Peter, K.; et al. XBB.1.5 COVID-19 mRNA Vaccines Induce Inadequate Mucosal Immunity in Patients with Inflammatory Bowel Disease. Vaccines 2025, 13, 759. https://doi.org/10.3390/vaccines13070759
Woelfel S, Dütschler J, Junker D, König M, Leinenkugel G, Krieger C, Truniger S, Franke A, Koller S, Metzger-Peter K, et al. XBB.1.5 COVID-19 mRNA Vaccines Induce Inadequate Mucosal Immunity in Patients with Inflammatory Bowel Disease. Vaccines. 2025; 13(7):759. https://doi.org/10.3390/vaccines13070759
Chicago/Turabian StyleWoelfel, Simon, Joel Dütschler, Daniel Junker, Marius König, Georg Leinenkugel, Claudia Krieger, Samuel Truniger, Annett Franke, Seraina Koller, Katline Metzger-Peter, and et al. 2025. "XBB.1.5 COVID-19 mRNA Vaccines Induce Inadequate Mucosal Immunity in Patients with Inflammatory Bowel Disease" Vaccines 13, no. 7: 759. https://doi.org/10.3390/vaccines13070759
APA StyleWoelfel, S., Dütschler, J., Junker, D., König, M., Leinenkugel, G., Krieger, C., Truniger, S., Franke, A., Koller, S., Metzger-Peter, K., Frei, N., STAR SIGN Study Investigators, Albrich, W. C., Friedrich, M., Niess, J. H., Schneiderhan-Marra, N., Dulovic, A., Korte, W., Bürgi, J. J., & Brand, S. (2025). XBB.1.5 COVID-19 mRNA Vaccines Induce Inadequate Mucosal Immunity in Patients with Inflammatory Bowel Disease. Vaccines, 13(7), 759. https://doi.org/10.3390/vaccines13070759






