Immune Responses Induced by Recombinant Membrane Proteins of Mycoplasma agalactiae in Goats
Abstract
1. Introduction
2. Materials and Methods
2.1. Expression and Purification of P40 and MAG_1560 Recombinant Proteins
2.2. Mycoplasma Agalactiae Inactivation
2.3. Membrane Protein Extraction
2.4. Animal Immunization and Experimental Groups
2.5. Determination of Specific IgG Production Levels by Indirect ELISA
2.6. Avidity Test for IgG Antibodies
2.7. Colony Immunoblotting Analysis
2.8. Analysis of Gene Expression of Inflammatory Markers Triggered by In Vitro Stimulation of Peripheral Blood Mononuclear Cells (PBMCs)
2.9. RNA Extraction, cDNA Synthesis, and Gene Expression
2.10. Statistical Analysis
3. Results
3.1. Purified P40 and MAG_1560
3.2. The Recombinant Vaccine Induced a High Rate of Specific IgG Antibody Production
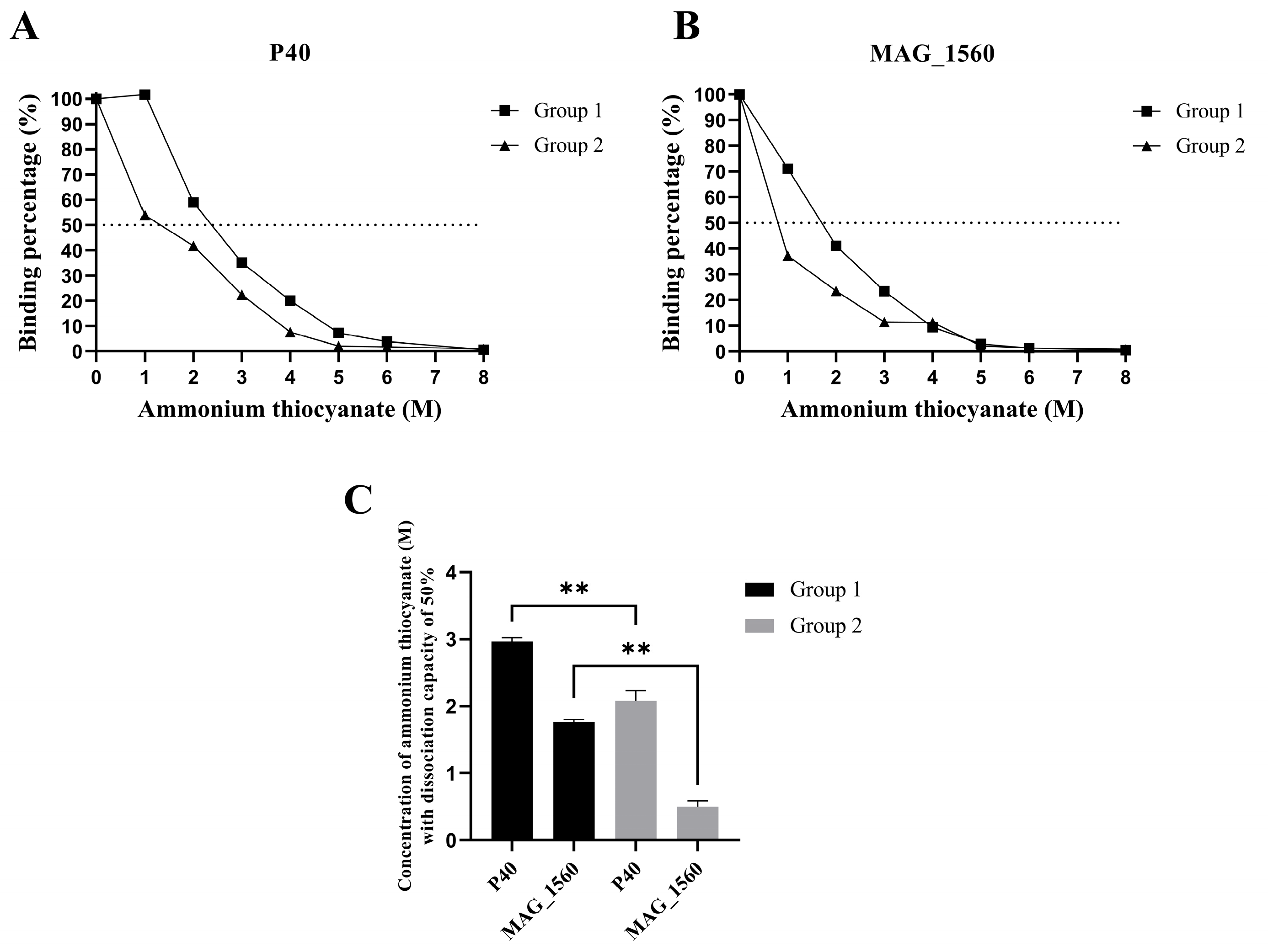
3.3. Colony Recognition by Specific Antibodies Over Time Post-Immunization
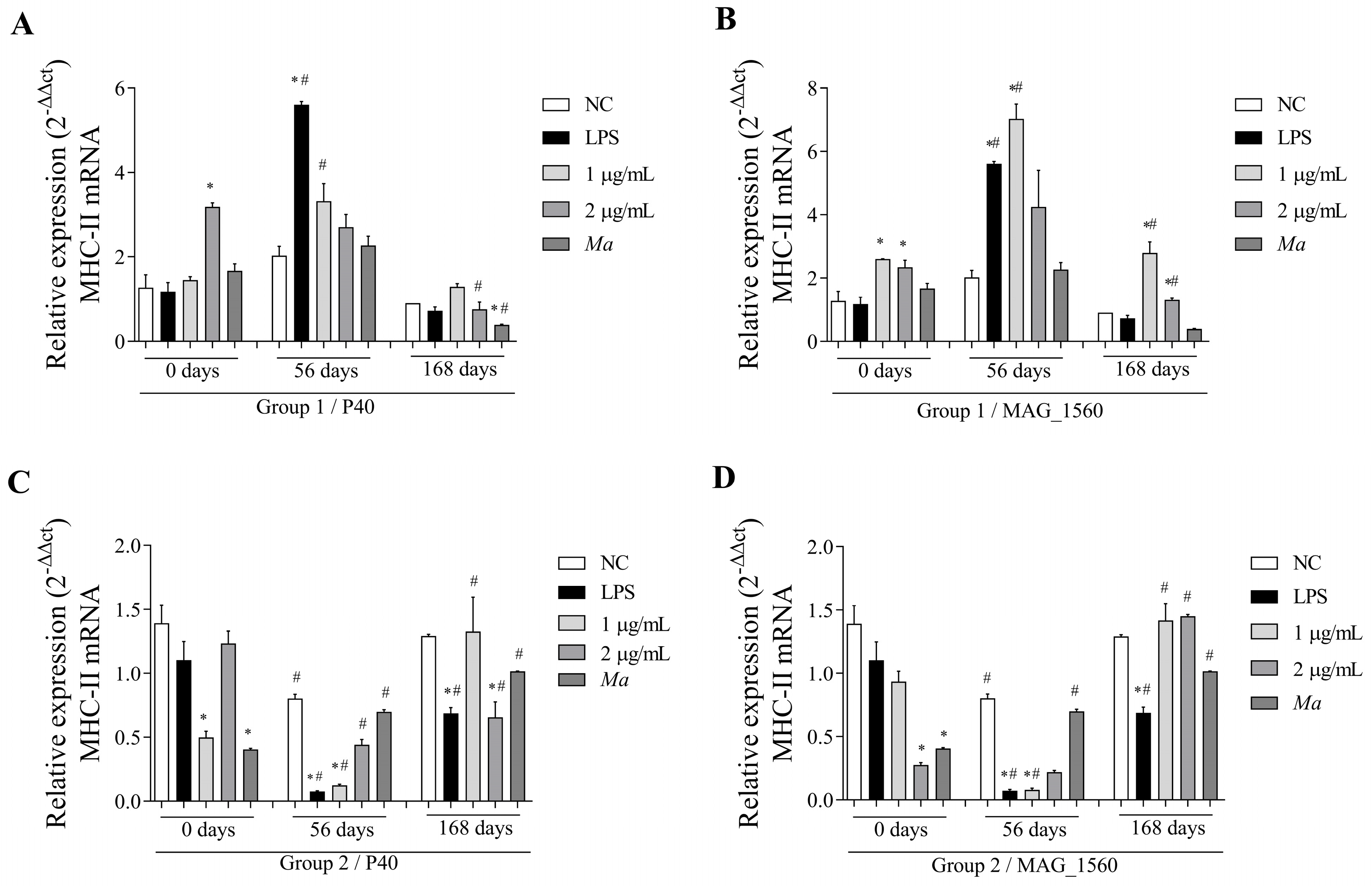
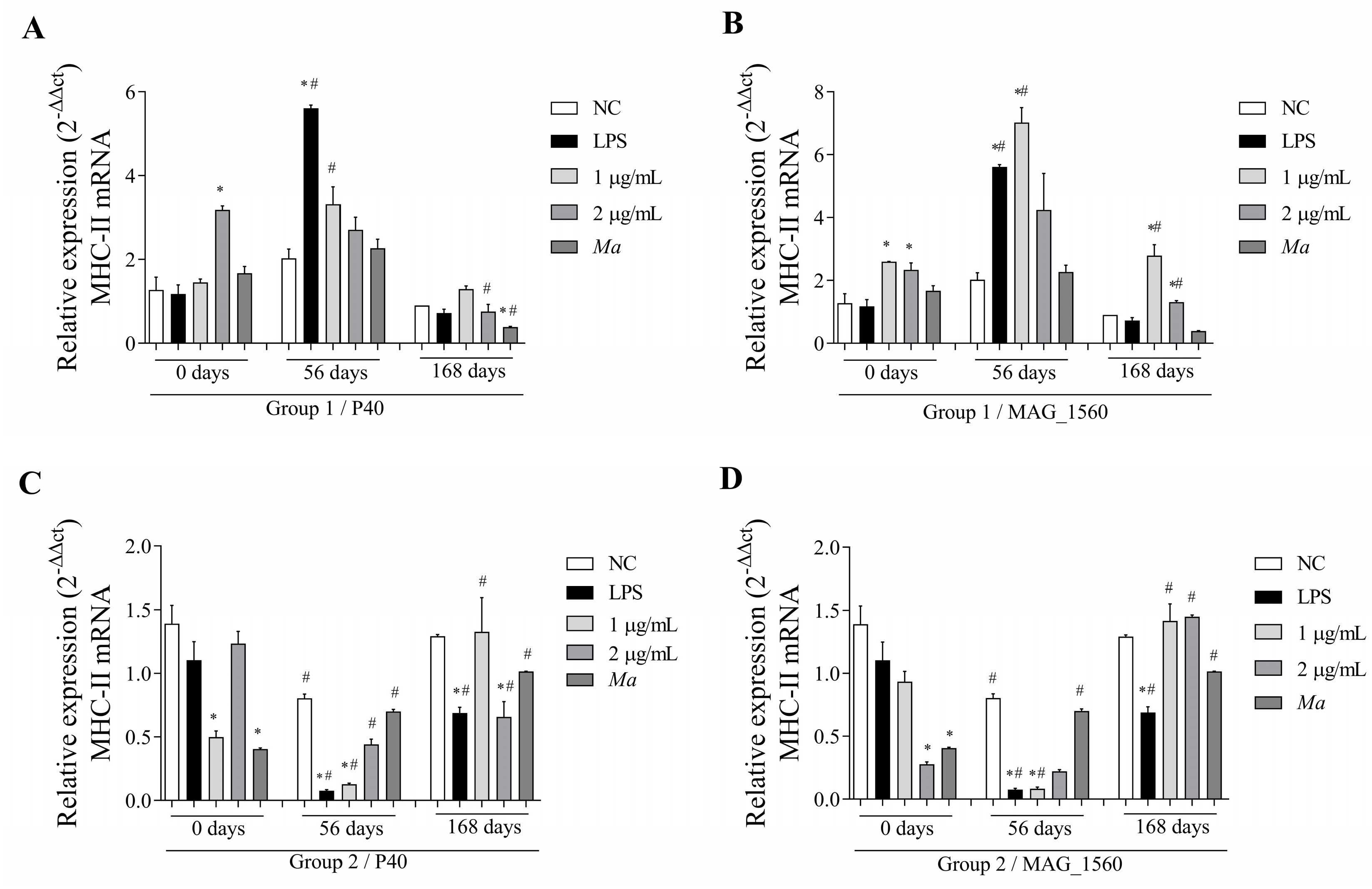
3.4. The P40 and MAG_1560 Proteins Can Alter the Gene Expression of Cytokines and MHC-II in Different Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, M.C. Agalaxie Contagieuse Des Brebis et Des Chèvres. Rev. Sci. Tech. L’oie 1987, 6, 681–711. [Google Scholar] [CrossRef]
- Abdollahi, M.; Lotfollahzadeh, S.; Salehi, T.Z.; Moosakhani, F.; Raoofi, A. Assessment of the Duration of Maternal-Derived Antibodies Specific to the Mycoplasma agalactiae Vaccine in Goat Kids. Vet. Med. Sci. 2022, 8, 2119–2125. [Google Scholar] [CrossRef]
- Gómez-Martín, Á.; Amores, J.; Paterna, A.; De la Fe, C. Contagious Agalactia Due to Mycoplasma Spp. in Small Dairy Ruminants: Epidemiology and Prospects for Diagnosis and Control. Vet. J. 2013, 198, 48–56. [Google Scholar] [PubMed]
- Bergonier, D.; Berthelot, X.; Poumarat, F. Contagious Agalactia of Small Ruminants: Current Knowledge Concerning Epidemiology, Diagnosis and Control. Rev. Sci. Tech. L’oie 1997, 16, 848–873. [Google Scholar] [CrossRef]
- Madanat, A.; Zendulková, D.; Pospíšil, Z. Contagious Agalactia of Sheep and Goats. A Review. Acta Vet. Brno 2001, 70, 403–412. [Google Scholar] [CrossRef]
- Nicholas, R.A.J.; Ayling, R.D.; Loria, G.R. Ovine Mycoplasmal Infections. Small Rumin. Res. 2008, 76, 92–98. [Google Scholar] [CrossRef]
- Campos, A.C.; Azevedo, E.O.; Alcântara, M.D.B.; Silva, R.B.S.; Cordeiro, A.A.; Mamede, A.G.; Melo, M.A.; Nascimento, E.R.; Castro, R.S. Efficiency of Inactive Vaccines against Contagious Agalactia in Brazil. Arq. Bras. Med. Vet. Zootec. 2013, 65, 1394–1402. [Google Scholar] [CrossRef]
- De Azevedo, E.O.; De Alcântara, M.D.B.; Do Nascimento, E.R.; Tabosa, I.M.; Barreto, M.L.; De Almeida, J.F.; Araújo, M.D.O.; Rodrigues, A.R.O.; Riet-Correa, F.; De Castro, R.S. Contagious Agalactia by Mycoplasma agalactiae in Small Ruminants in Brazil: First Report. Braz. J. Microbiol. 2006, 37, 576–581. [Google Scholar] [CrossRef]
- Peixoto, R.M.; Andrioli, A.; Pinheiro, R.R.; Alves, F.S.F.; Dos Santos, V.W.S.; De Souza, M.M.; De Azevedo, D.A.A.; Damasceno, E.M.; Teixeira, M.F.d.S. Mycoplasma agalactiae in Dairy Goat Flocks Bred in State of Ceará in Association with Caprine Arthritis Encephalitis Virus. Acta Sci. Vet. 2018, 46, 7. [Google Scholar] [CrossRef]
- Alcântara, M.D.B.; Campos, A.C.; Melo, M.A.; Pereira Filho, J.M.; Nascimento, E.R.; Farias, A.A.; Sousa, D.R.M.; Azevedo, E.O. Immune Response in Goats Vaccinated against Contagious Agalactia. Pesqui. Vet. Bras. 2013, 33, 561–564. [Google Scholar] [CrossRef]
- Chopra-Dewasthaly, R.; Baumgartner, M.; Gamper, E.; Innerebner, C.; Zimmermann, M.; Schilcher, F.; Tichy, A.; Winter, P.; Jechlinger, W.; Rosengarten, R.; et al. Role of Vpma Phase Variation in Mycoplasma agalactiae Pathogenesis. FEMS Immunol. Med. Microbiol. 2012, 66, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Fleury, B.; Bergonier, D.; Berthelot, X.; Peterhans, E.; Frey, J.; Vilei, E.M. Characterization of P40, a Cytadhesin of Mycoplasma agalactiae. Infect. Immun. 2002, 70, 5612–5621. [Google Scholar] [CrossRef] [PubMed]
- Rosati, S.; Robino, P.; Fadda, M.; Pozzi, S.; Mannelli, A.; Pittau, M. Expression and Antigenic Characterization of Recombinant Mycoplasma Agalactiae P48 Major Surface Protein. Vet. Microbiol. 2000, 71, 201–210. [Google Scholar] [CrossRef]
- Barbosa, M.S.; dos Santos Alves, R.P.; de Souza Rezende, I.; Pereira, S.S.; Campos, G.B.; Freitas, L.M.; Chopra-Dewasthaly, R.; de Souza Ferreira, L.C.; de Sá Guimarães, A.M.; Marques, L.M.; et al. Novel Antigenic Proteins of Mycoplasma agalactiae as Potential Vaccine and Serodiagnostic Candidates. Vet. Microbiol. 2020, 251, 108866. [Google Scholar] [CrossRef]
- Marchioro, S.B.; Fisch, A.; Gomes, C.K.; Jorge, S.; Galli, V.; Haesebrouck, F.; Maes, D.; Dellagostin, O.; Conceição, F.R. Local and Systemic Immune Responses Induced by a Recombinant Chimeric Protein Containing Mycoplasma hyopneumoniae Antigens Fused to the B Subunit of Escherichia Coli Heat-Labile Enterotoxin LTB. Vet. Microbiol. 2014, 173, 166–171. [Google Scholar] [CrossRef]
- Nkando, I.; Perez-Casal, J.; Mwirigi, M.; Prysliak, T.; Townsend, H.; Berberov, E.; Kuria, J.; Mugambi, J.; Soi, R.; Liljander, A.; et al. Recombinant Mycoplasma mycoides Proteins Elicit Protective Immune Responses against Contagious Bovine Pleuropneumonia. Vet. Immunol. Immunopathol. 2016, 171, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.; Chen, S.; Zhang, M.; Cheng, Z.; Zhang, W.; Liu, D.; Shan, Y.; Du, G.; Li, W.; et al. Evaluation of Immune Effect to Recombinant Potential Protective Antigens of Mycoplasma ovipneumoniae in Mice. Microb. Pathog. 2025, 204, 107555. [Google Scholar] [CrossRef]
- Avramidis, N.; Victoratos, P.; Yiangou, M.; Hadjipetrou-Kourounakis, L. Adjuvant Regulation of Cytokine Profile and Antibody Isotype of Immune Responses to Mycoplasma agalactiae in Mice. Vet. Microbiol. 2002, 88, 325–338. [Google Scholar] [CrossRef]
- Rawadi, G.; Roman-Roman, S. Mycoplasma Membrane Lipoproteins Induce Proinflammatory Cytokines by a Mechanism Distinct from That of Lipopolysaccharide. Infect. Immun. 1996, 64, 637–643. [Google Scholar] [CrossRef]
- Chávez González, Y.R.; Ros Bascuñana, C.; Bölske, G.; Mattsson, J.G.; Fernández Molina, C.; Johansson, K.E. In Vitro Amplification of the 16S RRNA Genes from Mycoplasma bovis and Mycoplasma agalactiae by PCR. Vet. Microbiol. 1995, 47, 183–190. [Google Scholar]
- Reina, R.; Juganaru, M.M.; Profiti, M.; Cascio, P.; Cerruti, F.; Bertolotti, L.; De Meneghi, D.; Amorena, B.; Rosati, S. Immunological Parameters in Goats Experimentally Infected with SRLV Genotype E, Strain Roccaverano. Vet. Immunol. Immunopathol. 2011, 139, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.B.; Purdie, A.C.; Whittington, R.J.; Begg, D.J. Cellular and Humoral Immune Responses in Sheep Vaccinated with Candidate Antigens MAP2698c and MAP3567 from Mycobacterium avium Subspecies Paratuberculosis. Front. Cell. Infect. Microbiol. 2014, 4, 93. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Li, Y.; Wang, Q.; Shao, J.; Chen, Y.; Xin, J. Mycoplasma Bovis-Derived Lipid-Associated Membrane Proteins Activate IL-1β Production through the NF-ΚB Pathway via Toll-like Receptor 2 and MyD88. Dev. Comp. Immunol. 2016, 55, 111–118. [Google Scholar] [CrossRef]
- Amills, M.; Francino, O.; Sánchez, A. Nested PCR Allows the Characterization of TaqI and PstI RFLPs in the Second Exon of the Caprine MHC Class II DRB Gene. Vet. Immunol. Immunopathol. 1995, 48, 313–321. [Google Scholar] [CrossRef]
- Angulo, M.; Reyes-Becerril, M.; Cepeda-Palacios, R.; Tovar-Ramírez, D.; Esteban, M.Á.; Angulo, C. Probiotic Effects of Marine Debaryomyces Hansenii CBS 8339 on Innate Immune and Antioxidant Parameters in Newborn Goats. Appl. Microbiol. Biotechnol. 2019, 103, 2339–2352. [Google Scholar] [CrossRef] [PubMed]
- Walia, V.; Kumar, R.; Mitra, A. Lipopolysaccharide and Concanavalin A Differentially Induce the Expression of Immune Response Genes in Caprine Monocyte Derived Macrophages. Anim. Biotechnol. 2015, 26, 298–303. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, S.V.; Saxena, V.K.; Singh, M.K.; Singh, A.V.; Sohal, J.S. Expression Profiles of Different Cytokine Genes in Peripheral Blood Mononuclear Cells of Goats Infected Experimentally with Native Strain of Mycobacterium Avium Subsp. Paratuberculosis. Anim. Biotechnol. 2013, 24, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.; Muñoz, M.C.; Molina, J.M.; Rodríguez, F.; Perez, D.; Lopez, A.; Ferrer, O.; Hermosilla, C.; Taubert, A.; Ruiz, A. Protective Immune Responses during Prepatency in Goat Kids Experimentally Infected with Eimeria Ninakohlyakimovae. Vet. Parasitol. 2017, 242, 1–9. [Google Scholar] [CrossRef]
- Loria, G.R.; Puleio, R.; Filioussis, G.; Rosales, R.S.; Nicholas, R.A.J. Contagious Agalactia: Costs and Control Revisited. Rev. Sci. Tech. 2019, 38, 695–702. [Google Scholar] [CrossRef]
- Lacasta, D.; Ferrer, L.M.; Ramos, J.J.; González, J.M.; Ortín, A.; Fthenakis, G.C. Vaccination Schedules in Small Ruminant Farms. Vet. Microbiol. 2015, 181, 34–46. [Google Scholar] [CrossRef]
- Tola, S.; Manunta, D.; Cocco, M.; Turrini, F.; Rocchigiani, A.M.; Idini, G.; Angioi, A.; Leori, G. Characterization of Membrane Surface Proteins of Mycoplasma Agalactiae during Natural Infection. FEMS Microbiol. Lett. 1997, 154, 355–362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buonavoglia, D.; Greco, G.; Corrente, M.; Fiorella, M.; Abramo, M.D.; Latronico, F.; Fasanella, A.; Decaro, N. Long-Term Immunogenicity and Protection against Mycoplasma Agalactiae Induced by an Oil Adjuvant Vaccine in Sheep. Res. Vet. Sci. 2010, 88, 16–19. [Google Scholar] [CrossRef]
- Bobbala, S.; Hook, S. Is There an Optimal Formulation and Delivery Strategy for Subunit Vaccines? Pharm. Res. 2016, 33, 2078–2097. [Google Scholar] [CrossRef]
- Budroni, S.; Buricchi, F.; Cavallone, A.; Bourguignon, P.; Caubet, M.; Dewar, V.; D’Oro, U.; Finco, O.; Garçon, N.; El Idrissi, M.; et al. Antibody Avidity, Persistence, and Response to Antigen Recall: Comparison of Vaccine Adjuvants. NPJ Vaccines 2021, 6, 78. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, F.Z.; Skwarczynski, M.; Toth, I. Vaccine Design, 2nd ed.; Thomas, S., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2022; Volume 2412, ISBN 978-1-0716-1891-2. [Google Scholar]
- Huang, Z.; Gong, H.; Sun, Q.; Yang, J.; Yan, X.; Xu, F. Research Progress on Emulsion Vaccine Adjuvants. Heliyon 2024, 10, e24662. [Google Scholar]
- Jorge, S.; de Oliveira, N.R.; Marchioro, S.B.; Fisch, A.; Gomes, C.K.; Hartleben, C.P.; Conceição, F.R.; Dellagostin, O.A. The Mycoplasma Hyopneumoniae Recombinant Heat Shock Protein P42 Induces an Immune Response in Pigs under Field Conditions. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 229–236. [Google Scholar] [CrossRef]
- Adamu, J.Y.; Wawegama, N.K.; Browning, G.F.; Markham, P.F. Membrane Proteins of Mycoplasma Bovis and Their Role in Pathogenesis. Res. Vet. Sci. 2013, 95, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Gondaira, S.; Okamoto, M.; Nebu, T.; Koiwa, M.; Ohtsuka, H.; Murai, K.; Matsuda, K.; Fujiki, J.; Iwano, H.; et al. Effect of Mycoplasma Bovis on Expression of Inflammatory Cytokines and Matrix Metalloproteinases MRNA in Bovine Synovial Cells. Vet. Immunol. Immunopathol. 2019, 216, 109920. [Google Scholar] [CrossRef] [PubMed]
- Gelgie, A.E.; Gelalcha, B.D.; Freeman, T.; Ault-Seay, T.B.; Beever, J.; Kerro Dego, O. Whole Transcriptome Analysis of Mycoplasma bovis-Host Interactions under in vitro and in vivo Conditions. Vet. Microbiol. 2025, 303, 110426. [Google Scholar] [CrossRef]
- Fields, J.K.; Günther, S.; Sundberg, E.J. Structural Basis of IL-1 Family Cytokine Signaling. Front. Immunol. 2019, 10, 1412. [Google Scholar]
- Jain, A.; Song, R.; Wakeland, E.K.; Pasare, C. T Cell-Intrinsic IL-1R Signaling Licenses Effector Cytokine Production by Memory CD4 T Cells. Nat. Commun. 2018, 9, 3185. [Google Scholar] [CrossRef] [PubMed]
- Künzli, M.; Masopust, D. CD4+ T Cell Memory. Nat. Immunol. 2023, 24, 903–914. [Google Scholar]
- Yu, Y.; Chen, Y.; Wang, Y.; Li, Y.; Zhang, L.; Xin, J. TLR2/MyD88/NF-ΚB Signaling Pathway Regulates IL-1β Production in DF-1 Cells Exposed to Mycoplasma gallisepticum LAMPs. Microb. Pathog. 2018, 117, 225–231. [Google Scholar] [CrossRef]
- Campbell, D.J. MyD88 and IL-1: Loosening Treg Cells’ Firm Grip. Trends Immunol. 2014, 35, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.; Qiao, J.; Weng, Y.; Zhang, H.; Liao, Q.; Qiu, J.; Chen, C.; Allain, J.-P.; Li, C. Evaluation of Humoral and Cellular Immune Responses to BP26 and OMP31 Epitopes in the Attenuated Brucella melitensis Vaccinated Sheep. Vaccine 2014, 32, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Kakh, M.K.; Doroudchi, M.; Talepoor, A.G. Induction of Regulatory T Cells After Virus Infection and Vaccination. Immunology 2025. [Google Scholar] [CrossRef]
- La Manna, M.P.; Agnone, A.; Villari, S.; Puleio, R.; Vitale, M.; Nicholas, R.; Sireci, G.; Dieli, F.; Loria, G.R. Expansion of Intracellular IFN-γ Positive Lymphocytes during Mycoplasma Agalactiae Infection in Sheep. Res. Vet. Sci. 2011, 91, e64–e67. [Google Scholar] [CrossRef]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-γ: An Overview of Signals, Mechanisms and Functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-Γin Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar]
- Castro, F.; Cardoso, A.P.; Gonçalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Mohr, E.; Cunningham, A.F.; Toellner, K.-M.; Bobat, S.; Coughlan, R.E.; Bird, R.A.; MacLennan, I.C.M.; Serre, K. IFN- γ Produced by CD8 T Cells Induces T-Bet-Dependent and -Independent Class Switching in B Cells in Responses to Alum-Precipitated Protein Vaccine. Proc. Natl. Acad. Sci. USA 2010, 107, 17292–17297. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the Regulation of Innate Resistance and Adaptive Immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Chessa, B.; Pittau, M.; Puricelli, M.; Zobba, R.; Coradduzza, E.; Dall’Ara, P.; Rosati, S.; Poli, G.; Alberti, A. Genetic Immunization with the Immunodominant Antigen P48 of Mycoplasma agalactiae Stimulates a Mixed Adaptive Immune Response in BALBc Mice. Res. Vet. Sci. 2009, 86, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Elsner, R.A.; Smita, S.; Shlomchik, M.J. IL-12 Induces a B Cell-Intrinsic IL-12/IFNγ Feed-Forward Loop Promoting Extrafollicular B Cell Responses. Nat. Immunol. 2024, 25, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, W.; Jiang, H.; Zhao, Q.; Hu, Y.; Tang, X.; Liu, X.; Dai, H.; Rui, H.; Liu, B. IL-12 Family Cytokines and Autoimmune Diseases: A Potential Therapeutic Target? J. Transl. Autoimmun. 2025, 10, 100263. [Google Scholar]
- Amills, M.; Ramiya, V.; Norimine, J.; Lewin, H.A. The Major Hystocompatibility Complex of Ruminants. Rev. Sci. Tech. L’oie 1998, 17, 108–120. [Google Scholar] [CrossRef]
- Hoffman, W.; Lakkis, F.G.; Chalasani, G. B Cells, Antibodies, and More. Clin. J. Am. Soc. Nephrol. 2016, 11, 137–154. [Google Scholar] [CrossRef]
- Saraav, I.; Wang, Q.; Brown, K.M.; Sibley, L.D. Secretory Microneme Proteins Induce T-Cell Recall Responses in Mice Chronically Infected with Toxoplasma gondii. mSphere 2019, 4, 10–1128. [Google Scholar] [CrossRef]
- Rodriguez, F.; Sarradell, J.; Poveda, J.B.; Ball, H.J.; Fernández, A. Immunohistochemical Characterization of Lung Lesions Induced Experimentally by Mycoplasma agalactiae AndMycoplasma bovis in Goats. J. Comp. Pathol. 2000, 123, 285–293. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio, B.A.; Barbosa, M.S.; de Oliveira, M.G.; Santos Júnior, M.N.; Guimarães, B.C.d.B.; Andres, E.S.S.; da Silva, Á.M.B.; Gomes, C.P.; Bittencourt, R.d.S.; Correia, T.M.L.; et al. Immune Responses Induced by Recombinant Membrane Proteins of Mycoplasma agalactiae in Goats. Vaccines 2025, 13, 746. https://doi.org/10.3390/vaccines13070746
Sampaio BA, Barbosa MS, de Oliveira MG, Santos Júnior MN, Guimarães BCdB, Andres ESS, da Silva ÁMB, Gomes CP, Bittencourt RdS, Correia TML, et al. Immune Responses Induced by Recombinant Membrane Proteins of Mycoplasma agalactiae in Goats. Vaccines. 2025; 13(7):746. https://doi.org/10.3390/vaccines13070746
Chicago/Turabian StyleSampaio, Beatriz Almeida, Maysa Santos Barbosa, Matheus Gonçalves de Oliveira, Manoel Neres Santos Júnior, Bruna Carolina de Brito Guimarães, Emilly Stefane Souza Andres, Ágatha Morgana Bertoti da Silva, Camila Pacheco Gomes, Rafaela de Souza Bittencourt, Thiago Macêdo Lopes Correia, and et al. 2025. "Immune Responses Induced by Recombinant Membrane Proteins of Mycoplasma agalactiae in Goats" Vaccines 13, no. 7: 746. https://doi.org/10.3390/vaccines13070746
APA StyleSampaio, B. A., Barbosa, M. S., de Oliveira, M. G., Santos Júnior, M. N., Guimarães, B. C. d. B., Andres, E. S. S., da Silva, Á. M. B., Gomes, C. P., Bittencourt, R. d. S., Correia, T. M. L., da Silva, L. S. C., da Cruz, J. F., Chopra-Dewasthaly, R., Campos, G. B., Timenetsky, J., Bastos, B. L., & Marques, L. M. (2025). Immune Responses Induced by Recombinant Membrane Proteins of Mycoplasma agalactiae in Goats. Vaccines, 13(7), 746. https://doi.org/10.3390/vaccines13070746





