Ferritin-Based HA DNA Vaccine Outperforms Conventional Designs in Inducing Protective Immunity Against Seasonal Influenza
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. Animals
2.3. Plasmid Design
2.4. Plasmid Preparation
2.5. Protein Expression and Purification
2.6. PNGase F Treatment
2.7. Polyacrylamide Gel Electrophoresis (PAGE)
2.8. Western Blot Analysis
2.9. Dynamic Light Scattering (DLS)
2.10. Transmission Electron Microscopy (TEM)
2.11. Immunization and Challenge Experiments
2.12. Enzyme-Linked Immunosorbent Assay (ELISA)
2.13. Hemagglutination Inhibition (HI) Assay
2.14. Enzyme-Linked Immunospot (ELISPOT) Assay
2.15. Quantitative Real-Time PCR (qPCR)
2.16. Histopathological Analysis
2.17. Statistical Analysis
3. Results
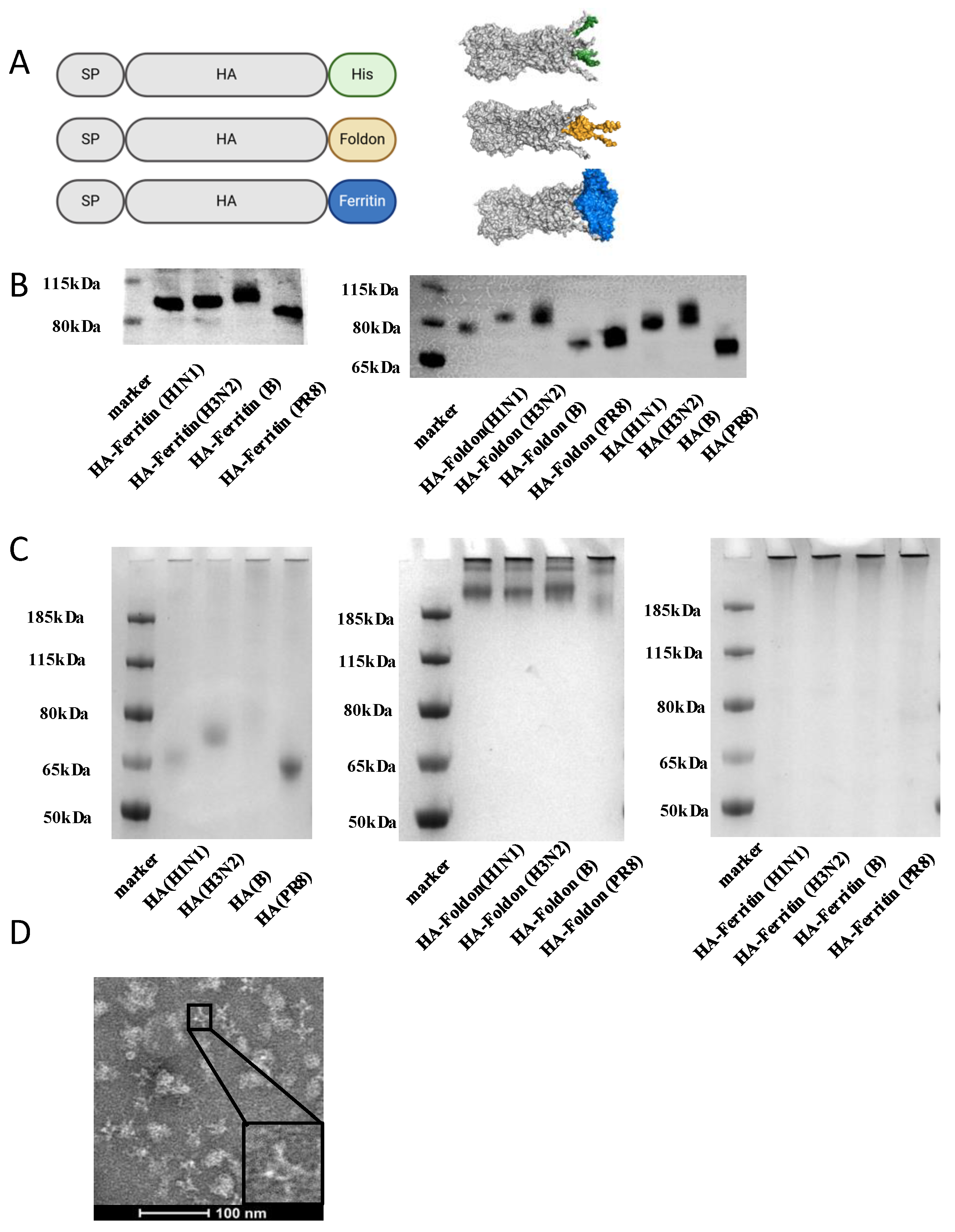
3.1. Design and Characterization of Influenza HA Constructs
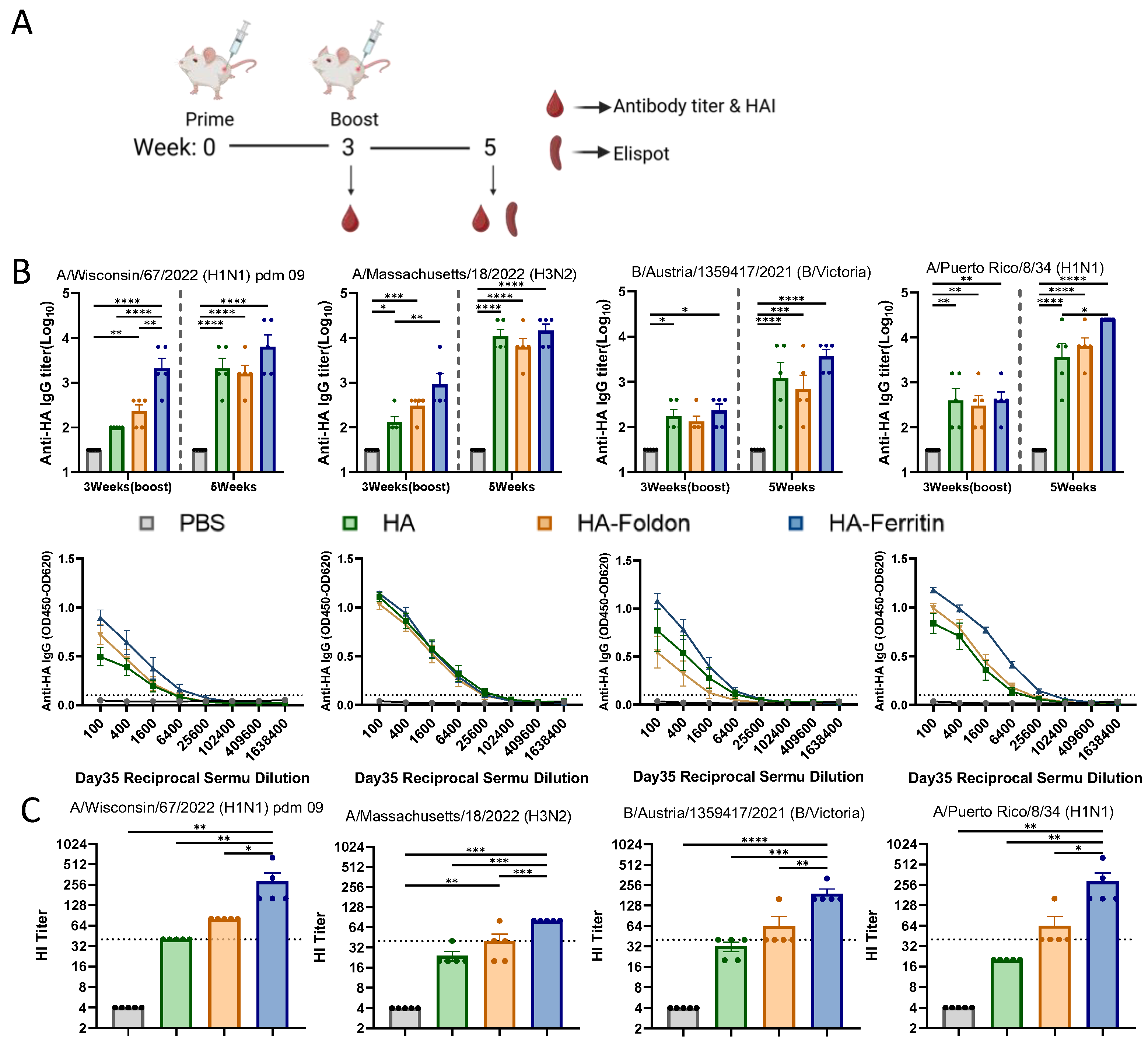
3.2. Humoral Immune Responses Following Vaccination
3.3. T Cell Immune Responses Following Vaccination
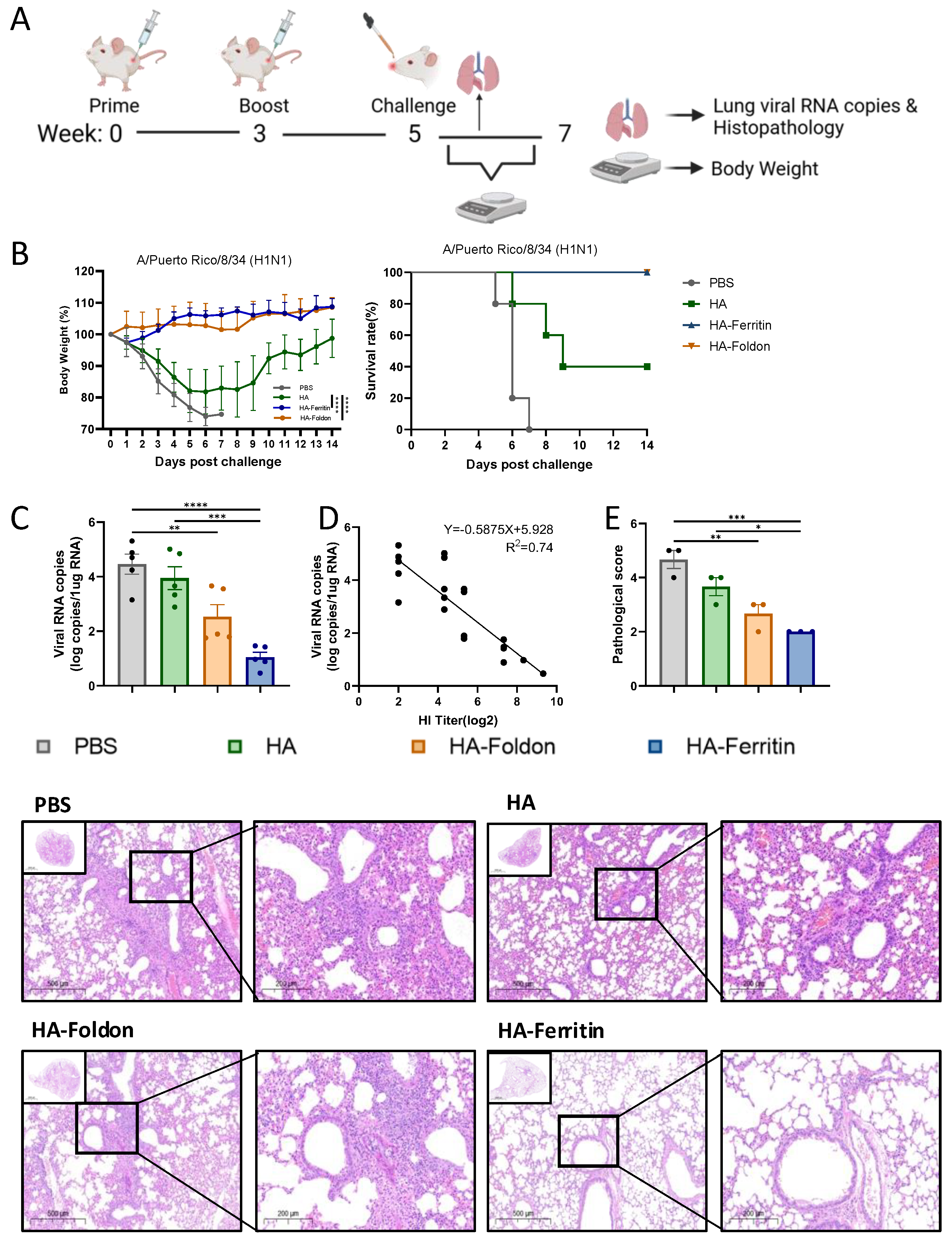
3.4. Protective Efficacy of Vaccination Against Homologous Viral Challenge
3.5. Immunogenicity of Trivalent Seasonal Influenza Vaccines
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of Global Seasonal Influenza-Associated Respiratory Mortality: A Modelling Study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ren, W.; Wang, Q.; Li, J. Advances in Nucleic Acid Universal Influenza Vaccines. Vaccines 2024, 12, 664. [Google Scholar] [CrossRef]
- Fatima, M.; Hong, K.-J. Innovations, Challenges, and Future Prospects for Combination Vaccines Against Human Infections. Vaccines 2025, 13, 335. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, H.; An, Y.; Chen, Z. Intranasal Immunization with DNA Vaccine HA-CCL19/Polyethylenimine/Chitosan Composite Provides Immune Protection Against H7N9 Infection. Vaccines 2024, 13, 10. [Google Scholar] [CrossRef]
- Litvinova, V.R.; Rudometov, A.P.; Rudometova, N.B.; Kisakov, D.N.; Borgoyakova, M.B.; Kisakova, L.A.; Starostina, E.V.; Fando, A.A.; Yakovlev, V.A.; Tigeeva, E.V.; et al. DNA Vaccine Encoding a Modified Hemagglutinin Trimer of Avian Influenza A Virus H5N8 Protects Mice from Viral Challenge. Vaccines 2024, 12, 538. [Google Scholar] [CrossRef]
- Kuiken, T.; Holmes, E.C.; McCauley, J.; Rimmelzwaan, G.F.; Williams, C.S.; Grenfell, B.T. Host Species Barriers to Influenza Virus Infections. Science 2006, 312, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Skehel, J.J.; Wiley, D.C. Receptor Binding and Membrane Fusion in Virus Entry: The Influenza Hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef]
- McMillan, C.L.D.; Cheung, S.T.M.; Modhiran, N.; Barnes, J.; Amarilla, A.A.; Bielefeldt-Ohmann, H.; Lee, L.Y.Y.; Guilfoyle, K.; van Amerongen, G.; Stittelaar, K.; et al. Development of Molecular Clamp Stabilized Hemagglutinin Vaccines for Influenza A Viruses. NPJ Vaccines 2021, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Vishwakarma, P.; Saxena, S.; Kumar, V.; Khatri, R.; Kumar, A.; Singh, M.; Mishra, S.; Asthana, S.; Ahmed, S.; et al. Intradermal Immunization of Soluble Influenza HA Derived from a Lethal Virus Induces High Magnitude and Breadth of Antibody Responses and Provides Complete Protection In Vivo. Vaccines 2023, 11, 780. [Google Scholar] [CrossRef]
- Hendin, H.E.; Lavoie, P.-O.; Gravett, J.M.; Pillet, S.; Saxena, P.; Landry, N.; D’Aoust, M.-A.; Ward, B.J. Elimination of Receptor Binding by Influenza Hemagglutinin Improves Vaccine-Induced Immunity. NPJ Vaccines 2022, 7, 42. [Google Scholar] [CrossRef]
- Kumar, S.; Moral-Sánchez, I.D.; Singh, S.; Newby, M.L.; Allen, J.D.; Bijl, T.P.L.; Vaghani, Y.; Jing, L.; Lodha, R.; Ortlund, E.A.; et al. The Design and Immunogenicity of an HIV-1 Clade C Pediatric Envelope Glycoprotein Stabilized by Multiple Platforms. Vaccines 2025, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, Y.; Zhang, S.; Sun, J.; Sun, W.; Li, J.; Liu, Y.; Li, M.; Cheng, L.; Jiang, Y.; et al. RBD Trimer mRNA Vaccine Elicits Broad and Protective Immune Responses against SARS-CoV-2 Variants. iScience 2022, 25, 104043. [Google Scholar] [CrossRef] [PubMed]
- Tjärnhage, E.; Brown, D.; Bogen, B.; Andersen, T.K.; Grødeland, G. Trimeric, APC-Targeted Subunit Vaccines Protect Mice against Seasonal and Pandemic Influenza. J. Virol. 2023, 97, e01694-22. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, M.J.G.; Cox, F.; Koornneef, A.; Yu, X.; van Overveld, D.; Le, L.; van den Hoogen, W.; Vaneman, J.; Thoma, A.; Voorzaat, R.; et al. A Foldon-Free Prefusion F Trimer Vaccine for Respiratory Syncytial Virus to Reduce off-Target Immune Responses. Nat. Microbiol. 2024, 9, 3254–3267. [Google Scholar] [CrossRef]
- Milder, F.J.; Jongeneelen, M.; Ritschel, T.; Bouchier, P.; Bisschop, I.J.M.; De Man, M.; Veldman, D.; Le, L.; Kaufmann, B.; Bakkers, M.J.G.; et al. Universal Stabilization of the Influenza Hemagglutinin by Structure-Based Redesign of the pH Switch Regions. Proc. Natl. Acad. Sci. USA 2022, 119, e2115379119. [Google Scholar] [CrossRef]
- Qiao, Y.; Jin, S.; Nie, J.; Chang, Y.; Wang, B.; Guan, S.; Li, Q.; Shi, Y.; Kong, W.; Shan, Y. Hemagglutinin-Based DNA Vaccines Containing Trimeric Self-Assembling Nanoparticles Confer Protection against Influenza. J. Leukoc. Biol. 2022, 112, 547–556. [Google Scholar] [CrossRef]
- Salinas, N.D.; Ma, R.; McAleese, H.; Ouahes, T.; Long, C.A.; Miura, K.; Lambert, L.E.; Tolia, N.H. A Self-Assembling Pfs230D1-Ferritin Nanoparticle Vaccine Has Potent and Durable Malaria Transmission-Reducing Activity. Vaccines 2024, 12, 546. [Google Scholar] [CrossRef]
- Wang, X.; Qin, Z.; Zhang, M.; Shang, B.; Li, Z.; Zhao, M.; Tang, Q.; Tang, Q.; Luo, J. Immunogenicity and Protection of Recombinant Self-Assembling Ferritin-Hemagglutinin Nanoparticle Influenza Vaccine in Mice. Clin. Exp. Vaccine Res. 2025, 14, 23–34. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.; Yang, Y.; Ma, G.; Su, Z.; Zhang, S. An Apoferritin-Hemagglutinin Conjugate Vaccine with Encapsulated Nucleoprotein Antigen Peptide from Influenza Virus Confers Enhanced Cross Protection. Bioconjugate Chem. 2020, 31, 1948–1959. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Xue, W.; Zeng, Y.; Liu, L.; Cui, L.; Liu, H.; Zhang, Y.; Chen, L.; Nie, M.; et al. Glycoprotein E-Displaying Nanoparticles Induce Robust Neutralizing Antibodies and T-Cell Response Against Varicella Zoster Virus. Int. J. Mol. Sci. 2024, 25, 9872. [Google Scholar] [CrossRef]
- Widge, A.T.; Hofstetter, A.R.; Houser, K.V.; Awan, S.F.; Chen, G.L.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Holman, L.A.; et al. An Influenza Hemagglutinin Stem Nanoparticle Vaccine Induces Cross-Group 1 Neutralizing Antibodies in Healthy Adults. Sci. Transl. Med. 2023, 15, eade4790. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Joyce, M.G.; Gillespie, R.A.; Gallagher, J.R.; Andrews, S.F.; Yassine, H.M.; Wheatley, A.K.; Fisher, B.E.; Ambrozak, D.R.; Creanga, A.; et al. Mosaic Nanoparticle Display of Diverse Influenza Virus Hemagglutinins Elicits Broad B Cell Responses. Nat. Immunol. 2019, 20, 362–372. [Google Scholar] [CrossRef]
- Swanson, K.A.; Rainho-Tomko, J.N.; Williams, Z.P.; Lanza, L.; Peredelchuk, M.; Kishko, M.; Pavot, V.; Alamares-Sapuay, J.; Adhikarla, H.; Gupta, S.; et al. A Respiratory Syncytial Virus (RSV) F Protein Nanoparticle Vaccine Focuses Antibody Responses to a Conserved Neutralization Domain. Sci. Immunol. 2020, 5, eaba6466. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.O.; Lee, E.; Parks, R.; Martinez, D.R.; Li, D.; Chen, H.; Edwards, R.J.; Gobeil, S.; Barr, M.; Mansouri, K.; et al. Neutralizing Antibody Vaccine for Pandemic and Pre-Emergent Coronaviruses. Nature 2021, 594, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Kanekiyo, M.; Wei, C.-J.; Yassine, H.M.; McTamney, P.M.; Boyington, J.C.; Whittle, J.R.R.; Rao, S.S.; Kong, W.-P.; Wang, L.; Nabel, G.J. Self-Assembling Influenza Nanoparticle Vaccines Elicit Broadly Neutralizing H1N1 Antibodies. Nature 2013, 499, 102–106. [Google Scholar] [CrossRef]
- Tang, P.; Cui, E.; Cheng, J.; Li, B.; Tao, J.; Shi, Y.; Jiao, J.; Du, E.; Wang, J.; Liu, H. A Ferritin Nanoparticle Vaccine Based on the Hemagglutinin Extracellular Domain of Swine Influenza A (H1N1) Virus Elicits Protective Immune Responses in Mice and Pigs. Front. Immunol. 2024, 15, 1361323. [Google Scholar] [CrossRef]
- Yang, R.S.; Traver, M.; Barefoot, N.; Stephens, T.; Alabanza, C.; Manzella-Lapeira, J.; Zou, G.; Wolff, J.; Li, Y.; Resto, M.; et al. Mosaic Quadrivalent Influenza Vaccine Single Nanoparticle Characterization. Sci. Rep. 2024, 14, 4534. [Google Scholar] [CrossRef]
- Chen, J.-R.; Liu, Y.-M.; Tseng, Y.-C.; Ma, C. Better Influenza Vaccines: An Industry Perspective. J. Biomed. Sci. 2020, 27, 33. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Yan, J.; Villarreal, D.O.; Racine, T.; Chu, J.S.; Walters, J.N.; Morrow, M.P.; Khan, A.S.; Sardesai, N.Y.; Kim, J.J.; Kobinger, G.P.; et al. Protective Immunity to H7N9 Influenza Viruses Elicited by Synthetic DNA Vaccine. Vaccine 2014, 32, 2833–2842. [Google Scholar] [CrossRef]
- Honda-Okubo, Y.; Bart Tarbet, E.; Hurst, B.L.; Petrovsky, N. An Advax-CpG Adjuvanted Recombinant H5 Hemagglutinin Vaccine Protects Mice against Lethal Influenza Infection. Vaccine 2023, 41, 5730–5741. [Google Scholar] [CrossRef] [PubMed]
- Kackos, C.M.; DeBeauchamp, J.; Davitt, C.J.H.; Lonzaric, J.; Sealy, R.E.; Hurwitz, J.L.; Samsa, M.M.; Webby, R.J. Seasonal Quadrivalent mRNA Vaccine Prevents and Mitigates Influenza Infection. npj Vaccines 2023, 8, 157. [Google Scholar] [CrossRef]
- Houser, K.V.; Chen, G.L.; Carter, C.; Crank, M.C.; Nguyen, T.A.; Burgos Florez, M.C.; Berkowitz, N.M.; Mendoza, F.; Hendel, C.S.; Gordon, I.J.; et al. Safety and Immunogenicity of a Ferritin Nanoparticle H2 Influenza Vaccine in Healthy Adults: A Phase 1 Trial. Nat. Med. 2022, 28, 383–391. [Google Scholar] [CrossRef]
- Eliasson, D.G.; Omokanye, A.; Schön, K.; Wenzel, U.A.; Bernasconi, V.; Bemark, M.; Kolpe, A.; El Bakkouri, K.; Ysenbaert, T.; Deng, L.; et al. M2e-Tetramer-Specific Memory CD4 T Cells Are Broadly Protective Against Influenza Infection. Mucosal Immunol. 2018, 11, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Santiago, F.; Lambert, K.; Takimoto, T.; Topham, D.J. T Cell-Mediated Protection Against Lethal 2009 Pandemic H1N1 Influenza Virus Infection in a Mouse Model. J. Virol. 2011, 85, 448–455. [Google Scholar] [CrossRef]
- Subbiah, J.; Oh, J.; Kim, K.-H.; Shin, C.-H.; Park, B.R.; Bhatnagar, N.; Seong, B.-L.; Wang, B.-Z.; Kang, S.-M. A Chimeric Thermostable M2e and H3 Stalk-Based Universal Influenza A Virus Vaccine. NPJ Vaccines 2022, 7, 68. [Google Scholar] [CrossRef]
- Co, M.D.T.; Orphin, L.; Cruz, J.; Pazoles, P.; Rothman, A.L.; Ennis, F.A.; Terajima, M. Discordance between Antibody and T Cell Responses in Recipients of Trivalent Inactivated Influenza Vaccine. Vaccine 2008, 26, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.; Patriarca, P.A.; Ensor, K.; Izikson, R.; Goldenthal, K.L.; Cox, M.M. Evaluation of the Safety, Reactogenicity and Immunogenicity of FluBlok® Trivalent Recombinant Baculovirus-Expressed Hemagglutinin Influenza Vaccine Administered Intramuscularly to Healthy Adults 50–64 Years of Age. Vaccine 2011, 29, 2272–2278. [Google Scholar] [CrossRef]
- Couch, R.B.; Patel, S.M.; Wade-Bowers, C.L.; Niño, D. A Randomized Clinical Trial of an Inactivated Avian Influenza A (H7N7) Vaccine. PLoS ONE 2012, 7, e49704. [Google Scholar] [CrossRef]
- Blanchfield, K.; Kamal, R.P.; Tzeng, W.; Music, N.; Wilson, J.R.; Stevens, J.; Lipatov, A.S.; Katz, J.M.; York, I.A. Recombinant Influenza H7 Hemagglutinins Induce Lower Neutralizing Antibody Titers in Mice than Do Seasonal Hemagglutinins. Influenza Respir. Viruses 2014, 8, 628–635. [Google Scholar] [CrossRef]
- Wu, N.C.; Wilson, I.A. Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med. 2020, 10, a038778. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Shi, Y.; Qi, J.; Zhang, W.; Xiao, H.; Gao, G.F. Structural Vaccinology: Structure-Based Design of Influenza A Virus Hemagglutinin Subtype-Specific Subunit Vaccines. Protein Cell 2011, 2, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- du Preez, H.N.; Lin, J.; Maguire, G.E.M.; Aldous, C.; Kruger, H.G. COVID-19 Vaccine Adverse Events: Evaluating the Pathophysiology with an Emphasis on Sulfur Metabolism and Endotheliopathy. Eur. J. Clin. Investig. 2024, 54, e14296. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Goel, K.; Dhanawat, M. Plasmid DNA and mRNA Delivery: Approaches and Challenges. Adv. Immunol. 2025, 165, 63–87. [Google Scholar] [CrossRef]
- Hayashi, H.; Sun, J.; Yanagida, Y.; Otera, T.; Kubota-Koketsu, R.; Shioda, T.; Ono, C.; Matsuura, Y.; Arase, H.; Yoshida, S.; et al. Preclinical Study of a DNA Vaccine Targeting SARS-CoV-2. Curr. Res. Transl. Med. 2022, 70, 103348. [Google Scholar] [CrossRef]
- Tebas, P.; Yang, S.; Boyer, J.D.; Reuschel, E.L.; Patel, A.; Christensen-Quick, A.; Andrade, V.M.; Morrow, M.P.; Kraynyak, K.; Agnes, J.; et al. Safety and Immunogenicity of INO-4800 DNA Vaccine against SARS-CoV-2: A Preliminary Report of an Open-Label, Phase 1 Clinical Trial. eClinicalMedicine 2021, 31, 100689. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Jiang, Y.; Li, Y.; Zhong, Y.; Chen, M.; Jiang, W.; Xiang, R.; Cao, N.; Sun, L.; Wang, X.; et al. Ferritin-Based HA DNA Vaccine Outperforms Conventional Designs in Inducing Protective Immunity Against Seasonal Influenza. Vaccines 2025, 13, 745. https://doi.org/10.3390/vaccines13070745
Lin H, Jiang Y, Li Y, Zhong Y, Chen M, Jiang W, Xiang R, Cao N, Sun L, Wang X, et al. Ferritin-Based HA DNA Vaccine Outperforms Conventional Designs in Inducing Protective Immunity Against Seasonal Influenza. Vaccines. 2025; 13(7):745. https://doi.org/10.3390/vaccines13070745
Chicago/Turabian StyleLin, Hongzhe, Yuxuan Jiang, Yan Li, Yiwei Zhong, Mingyue Chen, Weiyu Jiang, Rong Xiang, Najing Cao, Lei Sun, Xuanyi Wang, and et al. 2025. "Ferritin-Based HA DNA Vaccine Outperforms Conventional Designs in Inducing Protective Immunity Against Seasonal Influenza" Vaccines 13, no. 7: 745. https://doi.org/10.3390/vaccines13070745
APA StyleLin, H., Jiang, Y., Li, Y., Zhong, Y., Chen, M., Jiang, W., Xiang, R., Cao, N., Sun, L., Wang, X., Lu, L., Wang, Q., Han, G., Ma, D., & Wang, B. (2025). Ferritin-Based HA DNA Vaccine Outperforms Conventional Designs in Inducing Protective Immunity Against Seasonal Influenza. Vaccines, 13(7), 745. https://doi.org/10.3390/vaccines13070745






