The Role of Anti-Interferon-α Autoantibodies in Severe COVID-19: Implications for Vaccination Prioritization
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
| World Health Organization case definition of COVID-19 disease severity | |
| Asymptomatic/Presymptomatic | Test Positive for SARS-CoV-2 with a Virologic Test but Have No Symptoms Consistent with COVID-19 |
| Mild | Individuals showing any signs/symptoms of COVID-19 (e.g., fever, cough, sore throat, malaise, headache, myalgia, nausea, vomiting, diarrhea, loss of taste/smell) but who do not have shortness of breath or clinical signs of pneumonia or abnormal chest imaging |
| Moderate | Individuals showing evidence of lower respiratory tract disease during clinical assessment or imaging and who have a SpO2 ≥ 94% on room air |
| Severe | Individuals who have a SpO2 of <94% on room air or a P/F ratio of <300 mmHg, a respiratory rate of >30 breaths/min, or lung infiltrates occupying >50% of lung fields |
| Critical | Individuals with respiratory failure, septic shock, and/or multiple organ dysfunction |
2.2. Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Anti-IFN Alpha (Anti-IFN-α) Autoantibodies and Neutralization Assay
2.3. Functional Evaluation of Anti-IFN-α Autoantibodies
2.4. Statistical Method
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAb | Autoantibody |
| ACE2 | Angiotensin-converting enzyme 2 |
| AEFI | Adverse events following immunization |
| ANOVA | Analysis of variance |
| APS | Autoimmune polyendocrine syndrome |
| ARDS | Acute respiratory distress syndrome |
| BMI | Body mass index |
| CBC | Complete blood count |
| CCI | Charlson Comorbidity Index |
| CRP | C-reactive protein |
| COVID-19 | Coronavirus disease 2019 |
| CXR | Chest X-ray |
| ELISA | Enzyme-linked immunosorbent assay |
| IC | Inhibitory concentration |
| ICU | Intensive care unit |
| IFN | Interferon |
| IFNAR | Interferon alpha and beta receptor subunit |
| IQR | Interquartile range |
| IRF | Interferon regulatory factor |
| ISGs | Interferon-stimulated genes |
| JAK/STAT | Janus kinase/signal transducers and activators of transcription |
| LDH | Lactate dehydrogenase |
| mRNA | Messenger ribonucleic acid |
| NCID | National Centre for Infectious Diseases |
| OR | Odds ratio |
| PBS | Phosphate buffered saline |
| RT | Reverse transcriptase |
| RT-PCR | Reverse transcriptase–polymerase chain reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SD | Standard deviation |
| STAT1 | Signal Transducer and Activator Transcription 1 |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor α |
| WHO | World Health Organization |
References
- Negishi, H.; Taniguchi, T.; Yanai, H. The Interferon (IFN) Class of Cytokines and the IFN Regulatory Factor (IRF) Transcription Factor Family. Cold Spring Harb. Perspect. Biol. 2018, 10, a028423. [Google Scholar] [CrossRef] [PubMed]
- Members of the COVID Human Genetic Effort; Su, H.C.; Jing, H.; Zhang, Y.; Casanova, J.L. Interfering with Interferons: A Critical Mechanism for Critical COVID-19 Pneumonia. Annu. Rev. Immunol. 2023, 41, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Beziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Galipeau, Y.; Cooper, C.; Langlois, M.A. Autoantibodies in COVID-19: Implications for disease severity and clinical outcomes. Front. Immunol. 2024, 15, 1509289. [Google Scholar] [CrossRef]
- Leong, K.P.; Ng, C.Y.L.; Fan, B.E.; Loh, C.M.; Wong, L.T.; Goh, V.H.H.; Tan, G.L.X.; Chua, C.R.; Tan, J.S.; Lee, S.S.M.; et al. Antiphospholipid and other autoantibodies in COVID-19 patients: A Singapore series. Ann. Acad. Med. Singap. 2022, 51, 586–588. [Google Scholar] [CrossRef]
- Geanes, E.S.; McLennan, R.; LeMaster, C.; Bradley, T. Autoantibodies to ACE2 and immune molecules are associated with COVID-19 disease severity. Commun. Med. 2024, 4, 47. [Google Scholar] [CrossRef]
- Worldometer. Available online: https://www.worldometers.info/coronavirus/country/singapore/ (accessed on 29 June 2025).
- World Health Organization COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/deaths (accessed on 29 June 2025).
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. World Health Organization. 2020. Available online: https://iris.who.int/handle/10665/332196 (accessed on 29 June 2025).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Akbari, A.; Hadizadeh, A.; Amiri, M.; Najafi, N.N.; Shahriari, Z.; Jamialahmadi, T.; Sahebkar, A. Role of autoantibodies targeting interferon type 1 in COVID-19 severity: A systematic review and meta-analysis. J. Transl. Autoimmun. 2023, 7, 100219. [Google Scholar] [CrossRef]
- Troya, J.; Bastard, P.; Planas-Serra, L.; Ryan, P.; Ruiz, M.; de Carranza, M.; Torres, J.; Martínez, A.; Abel, L.; Casanova, J.L.; et al. Neutralizing Autoantibodies to Type I IFNs in >10% of Patients with Severe COVID-19 Pneumonia Hospitalized in Madrid, Spain. J. Clin. Immunol. 2021, 41, 914–922. [Google Scholar] [CrossRef]
- Solanich, X.; Rigo-Bonnin, R.; Gumucio, V.D.; Bastard, P.; Rosain, J.; Philippot, Q.; Perez-Fernandez, X.L.; Fuset-Cabanes, M.P.; Gordillo-Benitez, M.Á.; Suarez-Cuartin, G.; et al. Pre-existing Autoantibodies Neutralizing High Concentrations of Type I Interferons in Almost 10% of COVID-19 Patients Admitted to Intensive Care in Barcelona. J. Clin. Immunol. 2021, 41, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Chen, J.; Zhao, M.; Tang, Y.; Zhao, C.; Jin, Y.; Tian, D.; Liao, Y.; Wang, X.; Wang, W.; et al. Prevalence of Neutralizing Autoantibodies Against Type I Interferon in a Multicenter Cohort of Severe or Critical COVID-19 Cases in Shanghai. J. Clin. Immunol. 2024, 44, 80. [Google Scholar] [CrossRef]
- Arrestier, R.; Bastard, P.; Belmondo, T.; Voiriot, G.; Urbina, T.; Luyt, C.E.; Gervais, A.; Bizien, L.; Segaux, L.; Ben Ahmed, M.; et al. Auto-antibodies against type I IFNs in > 10% of critically ill COVID-19 patients: A prospective multicentre study. Ann. Intensive Care 2022, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Subbian, S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin. Microbiol. Rev. 2021, 34, e00299-20. [Google Scholar] [CrossRef]
- Frasca, F.; Scordio, M.; Santinelli, L.; Gabriele, L.; Gandini, O.; Criniti, A.; Pierangeli, A.; Angeloni, A.; Mastroianni, C.M.; d’Ettorre, G.; et al. Anti-IFN-alpha/-omega neutralizing antibodies from COVID-19 patients correlate with downregulation of IFN response and laboratory biomarkers of disease severity. Eur. J. Immunol. 2022, 52, 1120–1128. [Google Scholar] [CrossRef]
- Bastard, P.; Gervais, A.; Taniguchi, M.; Saare, L.; Sarekannu, K.; Le Voyer, T.; Philippot, Q.; Rosain, J.; Bizien, L.; Asano, T.; et al. Higher COVID-19 pneumonia risk associated with anti-IFN-alpha than with anti-IFN-omega auto-Abs in children. J. Exp. Med. 2024, 221, e20231353. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Q.; Li, H.; Jiang, H.; Xu, J.; Bergquist, R.; Qin, Z. Autoantibodies against type I interferons in COVID-19 infection: A systematic review and meta-analysis. Int. J. Infect. Dis. 2023, 130, 147–152. [Google Scholar] [CrossRef]
- Akbil, B.; Meyer, T.; Stubbemann, P.; Thibeault, C.; Staudacher, O.; Niemeyer, D.; Jansen, J.; Muhlemann, B.; Doehn, J.; Tabeling, C.; et al. Early and Rapid Identification of COVID-19 Patients with Neutralizing Type I Interferon Auto-antibodies. J. Clin. Immunol. 2022, 42, 1111–1129. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Zovi, A.; Ferrara, F.; Langella, R.; Cavallaro, F.; Vitiello, A. Sex affects immune response capacity against COVID-19 infection. Rev. Med. Virol. 2023, 33, e2450. [Google Scholar] [CrossRef]
- Browne, S.K.; Zaman, R.; Sampaio, E.P.; Jutivorakool, K.; Rosen, L.B.; Ding, L.; Pancholi, M.J.; Yang, L.M.; Priel, D.L.; Uzel, G.; et al. Anti-CD20 (rituximab) therapy for anti-IFN-γ autoantibody-associated nontuberculous mycobacterial infection. Blood 2012, 119, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Vazquez, S.E.; Liu, J.; Laurie, M.T.; Wang, C.Y.; Gervais, A.; Le Voyer, T.; Bizien, L.; Zamecnik, C.; Philippot, Q.; et al. Vaccine breakthrough hypoxemic COVID-19 pneumonia in patients with auto-Abs neutralizing type I IFNs. Sci. Immunol. 2023, 8, eabp8966. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.S.B.; Hansen, L.; Grytaas, M.A.; Oftedal, B.E.; Breivik, L.; Zhou, F.; Hufthammer, K.O.; Sjøgren, T.; Olofsson, J.S.; Trieu, M.C.; et al. Vaccination prevents severe COVID-19 outcome in patients with neutralizing type 1 interferon autoantibodies. iScience 2023, 26, 107084. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Michailidis, E.; Hoffmann, H.H.; Chbihi, M.; Le Voyer, T.; Rosain, J.; Philippot, Q.; Seeleuthner, Y.; Gervais, A.; Materna, M.; et al. Auto-antibodies to type I IFNs can underlie adverse reactions to yellow fever live attenuated vaccine. J. Exp. Med. 2021, 218, e20202486. [Google Scholar] [CrossRef]
- Yalcinkaya, A.; Cavalli, M.; Cederholm, A.; Aranda-Guillén, M.; Behere, A.; Mildner, H.; Lakshmikanth, T.; Gonzalez, L.; Mugabo, C.H.; Johnsson, A.; et al. No link between type I interferon autoantibody positivity and adverse reactions to COVID-19 vaccines. NPJ Vaccines 2024, 9, 42. [Google Scholar] [CrossRef]

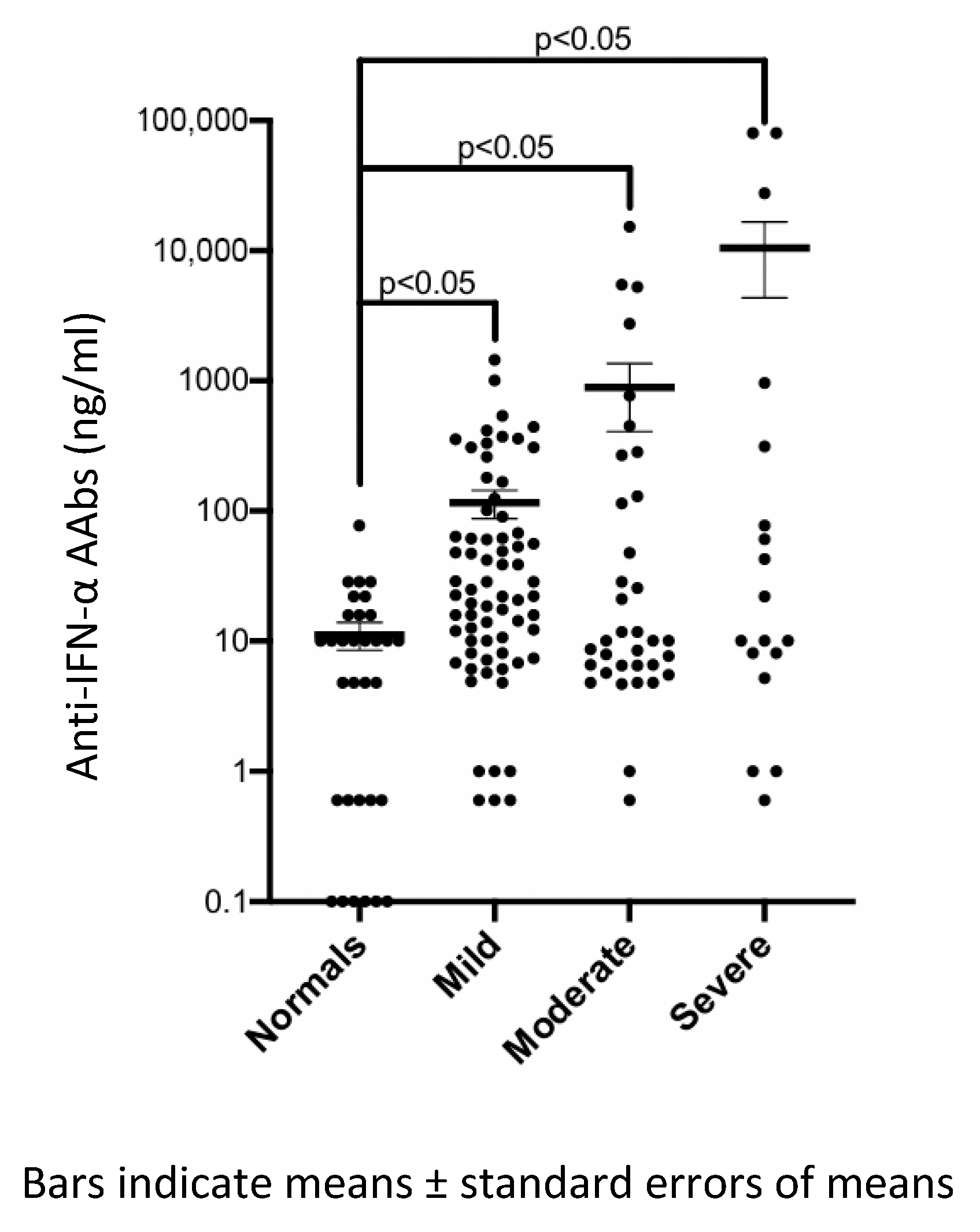
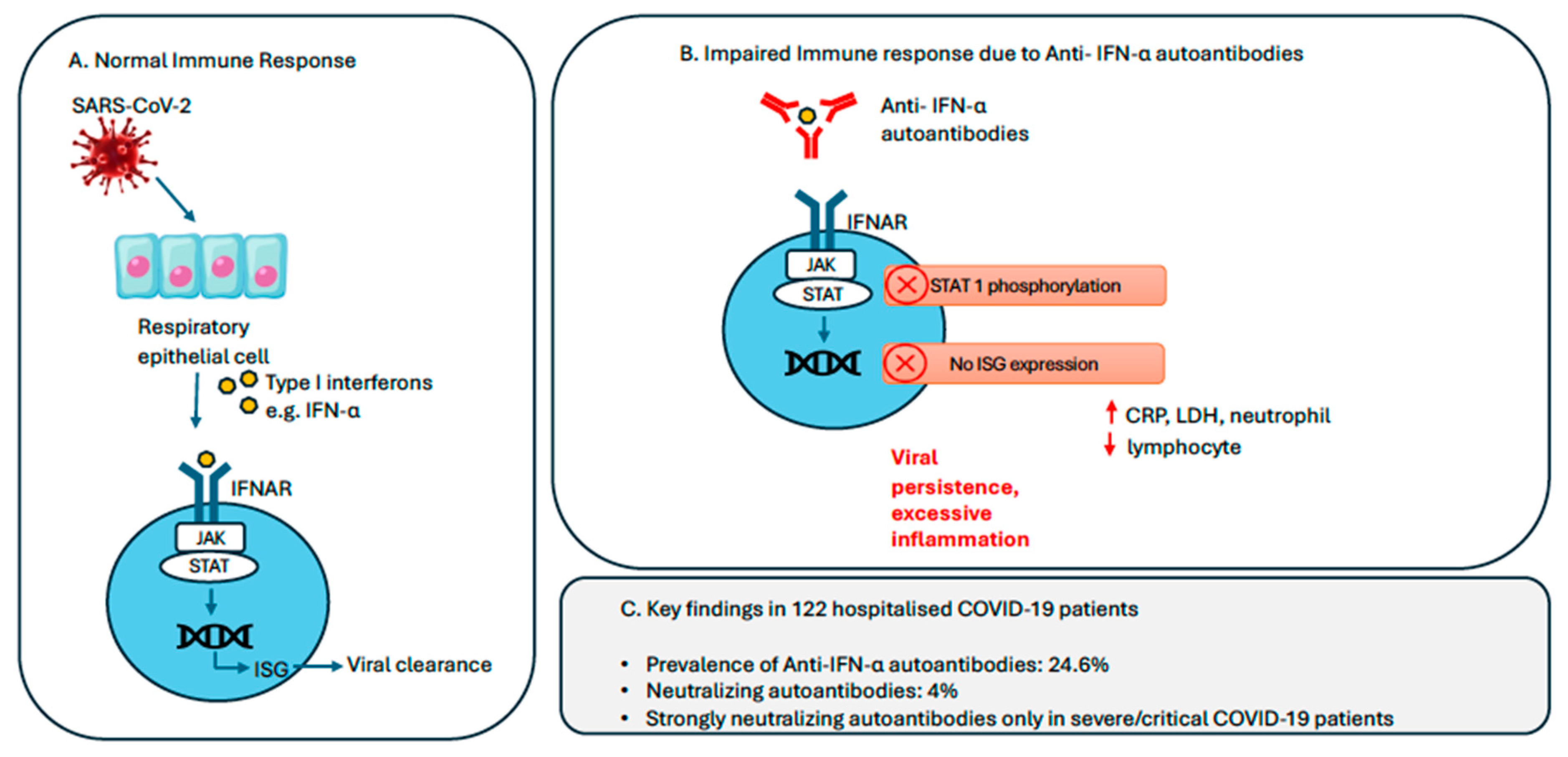
| Clinical Characteristics | Patients with Asymptomatic/Mild COVID-19 (N = 69) | Patients with Moderate COVID-19 (N = 35) | Patients with Severe/Critical COVID-19 (N = 18) | p Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 40 ± 13.6 | 50 ± 13.8 | 54.83 ± 16.53 | <0.001 |
| Male, number (%) | 49 (71%) | 24 (68.6%) | 14 * (77.78%) | 0.779 |
| Charlson Comorbidity Index (mean ± SD) | 0.59 ± 0.92 | 1.34 ± 1.60 | 1.89 ± 1.66 | <0.001 |
| BMI > 25, number (%) | 7 (10.1%) | 6 (17.1%) | 6 (33.33%) | 0.052 |
| Abnormal CXR (opacities/consolidation, no. (%) | 0 (0%) | 35 (100%) | 18 (100%) | <0.001 |
| CRP, mg/L (mean ± SD) | 7.67 ± 14.76 | 35.72 ± 46.33 | 115.23 ± 94.88 | <0.001 |
| LDH, U/L (mean ± SD) | 372.6 ± 78.7 | 457.9 ± 167.3 | 812.06 ± 655.28 | <0.001 |
| Lymphocyte count, 109/L (mean ± SD) | 1.60 ± 0.53 | 1.43 ± 0.70 | 1.36 ± 0.67 | 0.433 |
| Neutrophil count, 109/L (mean ± SD) | 3.71 ± 1.84 | 3.74 ± 1.87 | 6.86 ± 3.65 | <0.001 |
| Platelet count, 109/L (mean ± SD) | 227.3 ± 73.8 | 219.2 ± 80.4 | 315.22 ± 149.71 | <0.001 |
| Anti-IFN-α AAbs, ng/mL (mean ± SD) | 115.7 ± 235.3 | 886.6 ± 2827 | 10,511.3 ± 26,097 | 0.418 |
| Anti-IFN-α AAbs and Clinical Correlations | Pearson Correlation Coefficient (r) | p Value (p) |
| Anti-IFN-α AAbs and CRP | 0.80 | <0.0001 |
| Anti-IFN-α AAbs and LDH | 0.80 | 0.001 |
| Anti-IFN-α AAbs and lymphocyte count | −0.59 | 0.0006 |
| Anti-IFN-α AAbs and neutrophil count | 0.52 | 0.003 |
| Anti-IFN-α AAbs and platelet | 0.060 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, X.R.; Liu, S.; Howe, H.S.; Leong, K.P.; Elangovan, E.; Huang, C.-H.; Kong, K.O.; Thong, B.Y.H.; Vasoo, S.; Leung, B.P.L. The Role of Anti-Interferon-α Autoantibodies in Severe COVID-19: Implications for Vaccination Prioritization. Vaccines 2025, 13, 742. https://doi.org/10.3390/vaccines13070742
Lim XR, Liu S, Howe HS, Leong KP, Elangovan E, Huang C-H, Kong KO, Thong BYH, Vasoo S, Leung BPL. The Role of Anti-Interferon-α Autoantibodies in Severe COVID-19: Implications for Vaccination Prioritization. Vaccines. 2025; 13(7):742. https://doi.org/10.3390/vaccines13070742
Chicago/Turabian StyleLim, Xin Rong, Shiyu Liu, Hwee Siew Howe, Khai Pang Leong, Elampirai Elangovan, Chiung-Hui Huang, Kok Ooi Kong, Bernard Yu Hor Thong, Shawn Vasoo, and Bernard Pui Lam Leung. 2025. "The Role of Anti-Interferon-α Autoantibodies in Severe COVID-19: Implications for Vaccination Prioritization" Vaccines 13, no. 7: 742. https://doi.org/10.3390/vaccines13070742
APA StyleLim, X. R., Liu, S., Howe, H. S., Leong, K. P., Elangovan, E., Huang, C.-H., Kong, K. O., Thong, B. Y. H., Vasoo, S., & Leung, B. P. L. (2025). The Role of Anti-Interferon-α Autoantibodies in Severe COVID-19: Implications for Vaccination Prioritization. Vaccines, 13(7), 742. https://doi.org/10.3390/vaccines13070742





