Immunogenicity, Efficacy and Twelve-Month Storage Stability Studies of a Lyophilized Rabies mRNA Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines, Antibodies, Vaccines, Viruses, and Animals
2.2. mRNA Preparation and LNP Formulation
2.3. Cryo-EM
2.4. ELISA
2.5. Western Blot Analysis
2.6. Flow Cytometry Assay for Detection of the Expression of RABV-G
2.7. Flow Cytometry Assay for Intracellular Cytokine Staining (ICS)
2.8. ELISpot
2.9. Virus Neutralization Titer Assay
2.10. Vaccine Potency Test
2.11. Post-Exposure Prophylaxis (PEP) Study in Beagle Dogs
2.12. Statistical Analysis
3. Results
3.1. Construction and Characterization of ABO1005
3.2. ABO1005 Induced Robust Humoral Responses in Mice
3.3. Low Dose of ABO1005 Protected Mice Against Lethal RABV Challenge
3.4. ABO1005 Induced Specific T Cell Activation and Germinal Center B Cell Reaction
3.5. ABO1005 Elicited Long-Lasting Neutralizing Antibodies and Immunogenicity Was Not Affected by Pre-Administration of Hyperimmune Serum
3.6. Lyophilized ABO1005 Protected Beagle Dogs in a Post-Exposure Prophylaxis Model
3.7. ABO1005 Has a Potency That Meets the Standards for Human Vaccine Release
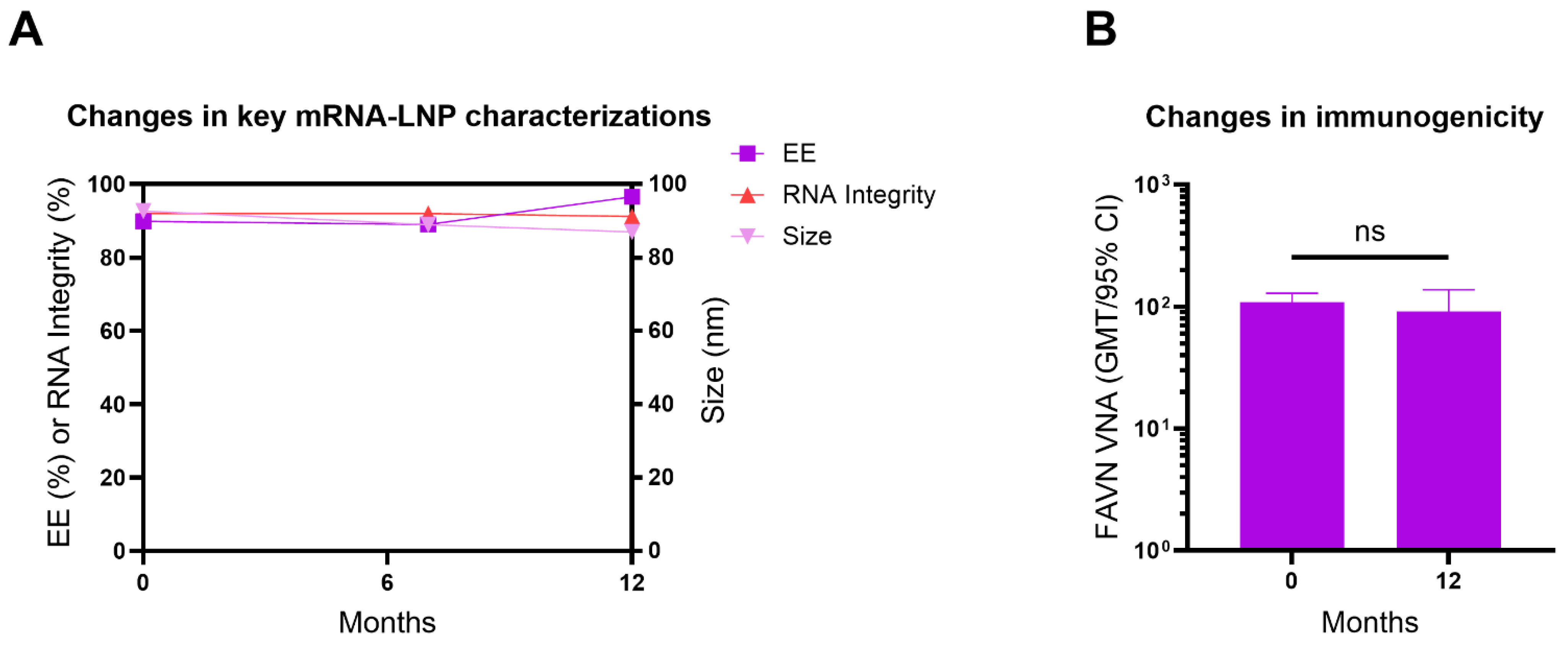
3.8. Lyophilized ABO1005 Vaccine Maintained Long-Term Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, A.D.; Yale, G.; Corfmat, J.; Appupillai, M.; Gigante, C.M.; Lopes, M.; Betodkar, U.; Costa, N.C.; Fernandes, K.A.; Mathapati, P.; et al. Elimination of human rabies in Goa, India through an integrated One Health approach. Nat. Commun. 2022, 13, 2788. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.M.; Fedosyuk, S.; English, S.; Augusto, G.; Berg, A.; Thorley, L.; Haselon, A.S.; Segireddy, R.R.; Bowden, T.A.; Douglas, A.D. Structure of trimeric pre-fusion rabies virus glycoprotein in complex with two protective antibodies. Cell Host Microbe 2022, 30, 1219–1230.e1217. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.L.; Wang, H.; Zhao, W.; Zhang, S.F.; Miao, F.M.; Cao, Y.; Chen, C.; Li, Y.F.; Gao, J.; Lv, R.Y.; et al. Efficacy of ormutivimab, a novel recombinant human anti-rabies monoclonal antibody, in post-exposure prophylaxis animal models. Travel Med. Infect. Dis. 2022, 46, 102267. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Lin, S.; Ye, F.; Yang, J.; Qi, J.; Chen, Z.; Lin, X.; Wang, J.; Yue, D.; Cheng, Y.; et al. Structural Analysis of Rabies Virus Glycoprotein Reveals pH-Dependent Conformational Changes and Interactions with a Neutralizing Antibody. Cell Host Microbe 2020, 27, 441–453.e447. [Google Scholar] [CrossRef]
- Morgeaux, S.; Tordo, N.; Gontier, C.; Perrin, P. Beta-propiolactone treatment impairs the biological activity of residual DNA from BHK-21 cells infected with rabies virus. Vaccine 1993, 11, 82–90. [Google Scholar] [CrossRef]
- Frazatti-Gallina, N.M.; Mourao-Fuches, R.M.; Paoli, R.L.; Silva, M.L.; Miyaki, C.; Valentini, E.J.; Raw, I.; Higashi, H.G. Vero-cell rabies vaccine produced using serum-free medium. Vaccine 2004, 23, 511–517. [Google Scholar] [CrossRef]
- Xu, C.; Lau, C.L.; Clark, J.; Rafferty, A.C.; Mills, D.J.; Ramsey, L.; Gilbert, B.; Doi, S.A.R.; Furuya-Kanamori, L. Immunogenicity after pre- and post-exposure rabies vaccination: A systematic review and dose-response meta-analysis. Vaccine 2021, 39, 1044–1050. [Google Scholar] [CrossRef]
- Li, J.; Liu, Q.; Liu, J.; Wu, X.; Lei, Y.; Li, S.; Zhao, D.; Li, Z.; Luo, L.; Peng, S.; et al. An mRNA-based rabies vaccine induces strong protective immune responses in mice and dogs. Virol. J. 2022, 19, 184. [Google Scholar] [CrossRef]
- Warrell, M.J. Developments in human rabies prophylaxis. Rev. Sci. Tech. 2018, 37, 629–647. [Google Scholar] [CrossRef]
- Fisher, C.R.; Schnell, M.J. New developments in rabies vaccination. Rev. Sci. Tech. 2018, 37, 657–672. [Google Scholar] [CrossRef]
- Aldrich, C.; Leroux-Roels, I.; Huang, K.B.; Bica, M.A.; Loeliger, E.; Schoenborn-Kellenberger, O.; Walz, L.; Leroux-Roels, G.; von Sonnenburg, F.; Oostvogels, L. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: A phase 1 trial. Vaccine 2021, 39, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yang, T.; Zhu, C.; Feng, M.; Zhang, L.; Zhang, Z.; Wang, X.; Yu, R.; Pan, X.; Zhao, C.; et al. A single vaccination of nucleoside-modified Rabies mRNA vaccine induces prolonged highly protective immune responses in mice. Front. Immunol. 2022, 13, 1099991. [Google Scholar] [CrossRef]
- Callaway, H.M.; Zyla, D.; Larrous, F.; de Melo, G.D.; Hastie, K.M.; Avalos, R.D.; Agarwal, A.; Corti, D.; Bourhy, H.; Saphire, E.O. Structure of the rabies virus glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Sci. Adv. 2022, 8, eabp9151. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, C.; Zhang, S.; Zhang, X.; Liu, Y.; Wang, Y.; Zhang, F.; Wu, X.; Hu, R. Glycoprotein from street rabies virus BD06 induces early and robust immune responses when expressed from a non-replicative adenovirus recombinant. Arch. Virol. 2015, 160, 2315–2323. [Google Scholar] [CrossRef]
- Qin, Q.; Yan, H.; Gao, W.; Cao, R.; Liu, G.; Zhang, X.; Wang, N.; Zuo, W.; Yuan, L.; Gao, P.; et al. Engineered mRNAs With Stable Structures Minimize Double-stranded RNA Formation and Increase Protein Expression. J. Mol. Biol. 2024, 436, 168822. [Google Scholar] [CrossRef]
- Zhang, N.N.; Li, X.F.; Deng, Y.Q.; Zhao, H.; Huang, Y.J.; Yang, G.; Huang, W.J.; Gao, P.; Zhou, C.; Zhang, R.R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Deng, Y.Q.; Zhang, N.N.; Zhang, Y.F.; Zhong, X.; Xu, S.; Qiu, H.Y.; Wang, T.C.; Zhao, H.; Zhou, C.; Zu, S.L.; et al. Lipid nanoparticle-encapsulated mRNA antibody provides long-term protection against SARS-CoV-2 in mice and hamsters. Cell Res. 2022, 32, 375–382. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, P.; Shan, Y.; Thirumeni, N.; Li, M.; Lv, Y.; Li, J.; Ren, W.; Huang, L.; Wei, J.; et al. Rabies virus glycoprotein serology ELISA for measurement of neutralizing antibodies in sera of vaccinated human subjects. Vaccine 2019, 37, 6060–6067. [Google Scholar] [CrossRef]
- Li, D.; Wang, X.; Li, G.; Zhou, J.; Bian, L.; Zhao, X.; Xing, L.; Zeng, J.; Cui, J.; Cui, L.; et al. Optimizing rabies mRNA vaccine efficacy via RABV-G structural domain screening and heterologous prime-boost immunization. NPJ Vaccines 2025, 10, 43. [Google Scholar] [CrossRef]
- Li, M.; Fang, E.; Wang, Y.; Shi, L.; Li, J.; Peng, Q.; Li, X.; Zhao, D.; Liu, X.; Liu, X.; et al. An mRNA vaccine against rabies provides strong and durable protection in mice. Front. Immunol. 2023, 14, 1288879. [Google Scholar] [CrossRef]
- Cliquet, F.; Wasniewski, M. The Fluorescent Antibody Virus Neutralization Test. In Current Laboratory Techniques in Rabies Diagnosis, Research and Prevention; Academic Press: Cambridge, MA, USA, 2015; Volume 2, pp. 217–231. [Google Scholar]
- Yager, M.L.; Moore, S.M. The Rapid Fluorescent Focus Inhibition Test. In Current Laboratory Techniques in Rabies Diagnosis, Research and Prevention; Academic Press: Cambridge, MA, USA, 2015; Volume 2, pp. 199–215. [Google Scholar]
- China Press of Traditional Chinese Medicine. Pharmacopoeia of the People’s Republic of China, 2020 ed.; China Press of Traditional Chinese Medicine: Beijing, China, 2020; Volume 3, pp. 578–579. [Google Scholar]
- Liu, Y.; Chen, Q.; Zhang, F.; Zhang, S.; Li, N.; Lian, H.; Wang, Y.; Zhang, J.; Hu, R. Evaluation of rabies biologics against Irkut virus isolated in China. J. Clin. Microbiol. 2013, 51, 3499–3504. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.N.; Isloor, S.; Veeresh, B.H.; Rathnamma, D.; Sharada, R.; Das, L.J.; Satyanarayana, M.L.; Hegde, N.R.; Rahman, S.A. Application and Comparative Evaluation of Fluorescent Antibody, Immunohistochemistry and Reverse Transcription Polymerase Chain Reaction Tests for the Detection of Rabies Virus Antigen or Nucleic Acid in Brain Samples of Animals Suspected of Rabies in India. Vet. Sci. 2018, 5, 24. [Google Scholar] [CrossRef]
- Askri, H.; Akrouti, I.; Rourou, S.; Kallel, H. Production, purification, and characterization of recombinant rabies virus glycoprotein expressed in PichiaPink yeast. Biotechnol. Rep. 2022, 35, e00736. [Google Scholar] [CrossRef]
- Zhang, J.N.; Meng, Y.J.; Bai, Y.H.; Li, Y.F.; Yang, L.Q.; Shi, N.M.; Han, H.X.; Gao, J.; Zhu, L.J.; Li, S.P.; et al. Rabies Virus Neutralizing Activity, Safety, and Immunogenicity of Recombinant Human Rabies Antibody Compared with Human Rabies Immunoglobulin in Healthy Adults. Biomed. Env. Sci. 2022, 35, 782–791. [Google Scholar] [CrossRef]
- Overduin, L.A.; van Dongen, J.J.M.; Visser, L.G. The Cellular Immune Response to Rabies Vaccination: A Systematic Review. Vaccines 2019, 7, 110. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Consultation on Rabies: Third Report; World Health Organization: Geneva, Switzerland, 2018; 183p. [Google Scholar]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczko, D.; Kariko, K.; Schreiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol. Ther. 2022, 30, 1941–1951. [Google Scholar] [CrossRef]
- Li, J.; Yu, P.; Liu, Q.; Xu, L.; Chen, Y.; Li, Y.; Zhang, F.; Zhu, W.; Peng, Y. Safety and efficacy assessment of an mRNA rabies vaccine in dogs, rodents, and cynomolgus macaques. NPJ Vaccines 2024, 9, 130. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Li, L.; Hu, R.; Lin, H.; Liu, H.; Liu, F.; Shao, H.; Liu, Y. Ineffectiveness of rabies vaccination alone for post-exposure protection against rabies infection in animal models. Antivir. Res. 2016, 135, 56–61. [Google Scholar] [CrossRef]
- Quiambao, B.P.; Dy-Tioco, H.Z.; Dizon, R.M.; Crisostomo, M.E.; Teuwen, D.E. Rabies post-exposure prophylaxis with purified equine rabies immunoglobulin: One-year follow-up of patients with laboratory-confirmed category III rabies exposure in the Philippines. Vaccine 2009, 27, 7162–7166. [Google Scholar] [CrossRef]
- H, S.R.; Khobragade, A.; Satapathy, D.; Gupta, M.; Kumar, S.; Bhomia, V.; V, R.; Desai, M.; Agrawal, A.D. Safety and Immunogenicity of a novel three-dose recombinant nanoparticle rabies G protein vaccine administered as simulated post exposure immunization: A randomized, comparator controlled, multicenter, phase III clinical study. Hum. Vaccin. Immunother. 2021, 17, 4239–4245. [Google Scholar] [CrossRef]
- Schnee, M.; Vogel, A.B.; Voss, D.; Petsch, B.; Baumhof, P.; Kramps, T.; Stitz, L. An mRNA Vaccine Encoding Rabies Virus Glycoprotein Induces Protection against Lethal Infection in Mice and Correlates of Protection in Adult and Newborn Pigs. PLoS Negl. Trop. Dis. 2016, 10, e0004746. [Google Scholar] [CrossRef] [PubMed]
- Kalimuddin, S.; Wijaya, L.; Chan, Y.F.Z.; Wong, A.W.L.; Oh, H.M.L.; Wang, L.F.; Kassim, J.A.; Zhao, J.; Shi, Z.; Low, J.G. A phase II randomized study to determine the safety and immunogenicity of the novel PIKA rabies vaccine containing the PIKA adjuvant using an accelerated regimen. Vaccine 2017, 35, 7127–7132. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Yang, J.; Wang, Z.; Shen, R.; Zhang, C.; Wu, Y.; Zhou, M.; Chen, H.; Fu, Z.F.; Sun, H.; et al. A single immunization with core-shell structured lipopolyplex mRNA vaccine against rabies induces potent humoral immunity in mice and dogs. Emerg. Microbes Infect. 2023, 12, 2270081. [Google Scholar] [CrossRef]
- Lebrun, A.; Garcia, S.; Li, J.; Kean, R.B.; Hooper, D.C. Protection Against CNS-Targeted Rabies Virus Infection is Dependent upon Type-1 Immune Mechanisms Induced by Live-Attenuated Rabies Vaccines. Trop. Med. Infect. Dis. 2017, 2, 22. [Google Scholar] [CrossRef]
- Dangi, T.; Sanchez, S.; Lew, M.H.; Awakoaiye, B.; Visvabharathy, L.; Richner, J.M.; Koralnik, I.J.; Penaloza-MacMaster, P. Pre-existing immunity modulates responses to mRNA boosters. Cell. Rep. 2023, 42, 112167. [Google Scholar] [CrossRef]
- CDC. RSV Vaccine Guidance for Older Adults. Available online: https://www.cdc.gov/rsv/hcp/vaccine-clinical-guidance/older-adults.html (accessed on 8 January 2025).
- Kasper, J.C.; Winter, G.; Friess, W. Recent advances and further challenges in lyophilization. Eur. J. Pharm. Biopharm. 2013, 85, 162–169. [Google Scholar] [CrossRef]
- Franze, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef]
- Pillay, J.; Gaudet, L.; Wingert, A.; Bialy, L.; Mackie, A.S.; Paterson, D.I.; Hartling, L. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: Living evidence syntheses and review. BMJ 2022, 378, e069445. [Google Scholar] [CrossRef]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschope, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis following COVID-19 vaccine: Incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2022, 24, 2000–2018. [Google Scholar] [CrossRef]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef]
- Parry, R.; Gifford, R.J.; Lytras, S.; Ray, S.C.; Coin, L.J.M. No evidence of SARS-CoV-2 reverse transcription and integration as the origin of chimeric transcripts in patient tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2109066118. [Google Scholar] [CrossRef] [PubMed]






| Group | Dilution | Survival | Accumulated | Result | ||||
|---|---|---|---|---|---|---|---|---|
| Death | Live | Death | Live | Death Rate | Log ED50 | Potency | ||
| PBS | \ | 16 | 0 | \ | \ | 100% | \ | \ |
| 9th Std 1 | ×125 | 3 | 13 | 3 | 23 | 11.5% | 2.75 | 11.4 IU/mL |
| ×625 | 8 | 8 | 11 | 10 | 52.3% | |||
| ×3125 | 14 | 2 | 25 | 2 | 92.6% | |||
| ABO1005 | ×125 | 0 | 16 | 0 | 26 | 0% | 2.94 | 8.85 IU/dose (0.5 mL) |
| ×625 | 6 | 10 | 6 | 10 | 37.5% | |||
| ×3125 | 16 | 0 | 22 | 0 | 100% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Ling, D.; Ji, K.; Tang, L.; Zhang, X.; Lu, X.; Leng, X.; Tan, C.; Wu, H.; Pang, W.; et al. Immunogenicity, Efficacy and Twelve-Month Storage Stability Studies of a Lyophilized Rabies mRNA Vaccine. Vaccines 2025, 13, 743. https://doi.org/10.3390/vaccines13070743
Chen C, Ling D, Ji K, Tang L, Zhang X, Lu X, Leng X, Tan C, Wu H, Pang W, et al. Immunogenicity, Efficacy and Twelve-Month Storage Stability Studies of a Lyophilized Rabies mRNA Vaccine. Vaccines. 2025; 13(7):743. https://doi.org/10.3390/vaccines13070743
Chicago/Turabian StyleChen, Chen, Dandan Ling, Kai Ji, Liang Tang, Xiaojing Zhang, Xishan Lu, Xuemei Leng, Changyao Tan, Hongchao Wu, Wenqiang Pang, and et al. 2025. "Immunogenicity, Efficacy and Twelve-Month Storage Stability Studies of a Lyophilized Rabies mRNA Vaccine" Vaccines 13, no. 7: 743. https://doi.org/10.3390/vaccines13070743
APA StyleChen, C., Ling, D., Ji, K., Tang, L., Zhang, X., Lu, X., Leng, X., Tan, C., Wu, H., Pang, W., He, Q., Zhang, J., Gao, P., Wang, X., Wang, L., & Ying, B. (2025). Immunogenicity, Efficacy and Twelve-Month Storage Stability Studies of a Lyophilized Rabies mRNA Vaccine. Vaccines, 13(7), 743. https://doi.org/10.3390/vaccines13070743






