An In Vitro Approach to Prime or Boost Human Antigen-Specific CD8+ T Cell Responses: Applications to Vaccine Studies
Abstract
1. Introduction
1.1. Need for Human-Based Approaches and Assays in Vaccinology
1.2. Genesis of a Multipurpose CD8+ T Cell Induction Approach
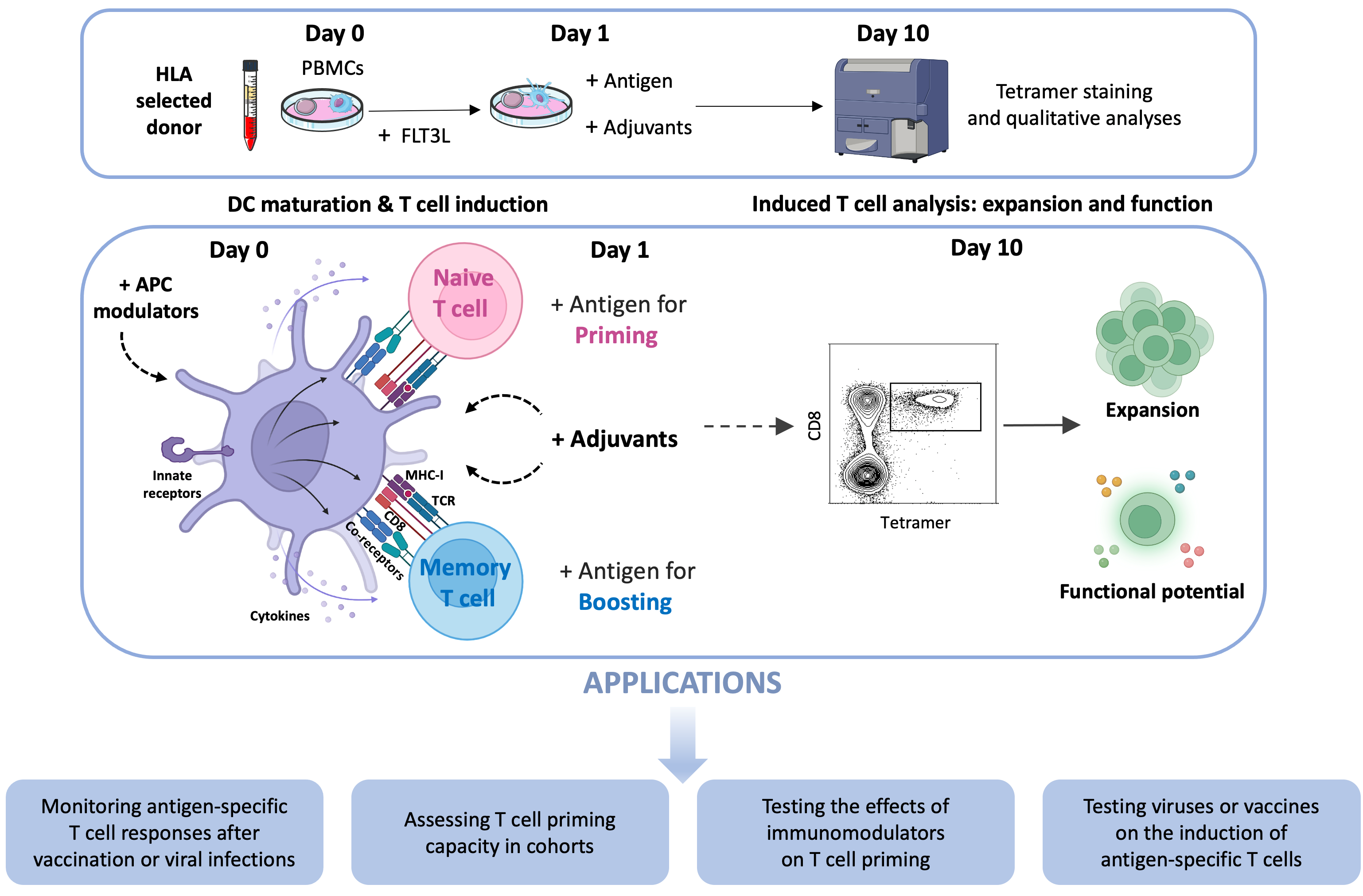
1.3. Description of the Standard Protocol
- Media: AIM-V serum-free medium and R10 medium (RPMI-1640 supplemented with antibiotics (1%), L-glutamine (1%), and FCS (10%)).
- Cells: Fresh or frozen PBMCs from HLA-A2+ donors.
- DC modulation factor: FLT3L: stock at 10 μg/mL. Working concentration is 50 ng/mL.
- Antigens: Peptides for de novo (e.g., ELA Melan-A peptide) or recall (e.g., GIL influenza peptide) responses, reconstituted at 10 mg/mL in DMSO, to be used at a final concentration of 1 μg/mL or 0.01 μg/mL, respectively.
- Stimulating agents: PRR ligands, cytokines…
- •
- Day 0: Mobilizing dendritic cells with FLT3L
- -
- Resuspend PBMCs in AIM-V medium. Count the cells to determine viability and cell number.
- -
- Adjust the AIM-V volume to obtain 10 × 106 cells/mL (adjust concentration according to cell number and frequency of naive T cells specific for any given antigen).
- -
- Take 10 × 106 cells/mL in AIM-V and add FLT3L at final concentration of 50 ng/mL.
- -
- Distribute 250 μL/well (2.5 × 106 cells) into a 48-well plate.
- -
- Incubate at 37 °C/5% CO2 overnight.
- •
- Day 1: Adding antigens and stimuli
- -
- Prepare GIL and ELA peptide antigens in AIM-V medium and add desired stimulating agents.
- -
- Distribute 250 μL/well of antigen and/or stimuli media to the 250 μL already present in the wells from Day 0 by gently rimming across the top of the well not to disturb the adherent cells.
- -
- Incubate at 37 °C/5% CO2.
- ○
- Use AIM-V medium on Day 0 and Day 1. Use R10 for medium replacement on Day 4 and Day 7.
- ○
- Antigens and stimuli are prepared at twice the desired final concentration as their concentration is diluted by half upon addition to the cell suspension.
- •
- Day 2: Add 10% of FCS to each well.
- •
- Day 4 and Day 7: Remove 250 μL of old medium and add 250 μL of fresh R10.
- •
- Day 10–11: Collect cell suspension and wash with PBS to prepare for the staining with antigen-specific tetramers and antibodies.
2. Scope of the Method in Diagnostic Settings
2.1. Monitoring Antigen-Specific T Cell Responses After Vaccination or Viral Infections
2.2. Assessing T Cell Priming Capacity in Cohorts
2.3. Testing the Effects of Immunomodulators on T Cell Priming
2.4. Testing Viruses or Vaccines on the Induction of Antigen-Specific T Cells
3. Limitations and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.W.; Ma, D.; Davoodian, A.; Ayutyanont, N.; Werner, B. COVID-19 vaccination decreased COVID-19 hospital length of stay, in-hospital death, and increased home discharge. Prev. Med. Rep. 2023, 32, 102152. [Google Scholar] [CrossRef]
- Koup, R.A.; Douek, D.C. Vaccine design for CD8 T lymphocyte responses. Cold Spring Harb. Perspect. Med. 2011, 1, a007252. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, M.; Rabaan, A.A.; Fawarah, M.M.A.; Almuthree, S.A.; Alsubki, R.A.; Alfaraj, A.H.; Mashraqi, M.M.; Alshamrani, S.A.; Abduljabbar, W.A.; Alwashmi, A.S.S.; et al. Updated Insights into the T Cell-Mediated Immune Response against SARS-CoV-2: A Step towards Efficient and Reliable Vaccines. Vaccines 2023, 11, 101. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.C. T-cell-inducing vaccines—What’s the future. Immunology 2012, 135, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Panagioti, E.; Klenerman, P.; Lee, L.N.; van der Burg, S.H.; Arens, R. Features of Effective T Cell-Inducing Vaccines against Chronic Viral Infections. Front. Immunol. 2018, 9, 276. [Google Scholar] [CrossRef]
- Liu, J.; Yu, J.; McMahan, K.; Jacob-Dolan, C.; He, X.; Giffin, V.; Wu, C.; Sciacca, M.; Powers, O.; Nampanya, F.; et al. CD8 T cells contribute to vaccine protection against SARS-CoV-2 in macaques. Sci. Immunol. 2022, 7, eabq7647. [Google Scholar] [CrossRef]
- Kalimuddin, S.; Tham, C.Y.L.; Chan, Y.F.Z.; Hang, S.K.; Kunasegaran, K.; Chia, A.; Chan, C.Y.Y.; Ng, D.H.L.; Sim, J.X.Y.; Tan, H.C.; et al. Vaccine-induced T cell responses control Orthoflavivirus challenge infection without neutralizing antibodies in humans. Nat. Microbiol. 2025, 10, 374–387. [Google Scholar] [CrossRef]
- Groscurth, P.; Filgueira, L. Killing Mechanisms of Cytotoxic T Lymphocytes. News Physiol. Sci. 1998, 13, 17–21. [Google Scholar] [CrossRef]
- Sigal, L.J. Activation of CD8 T Lymphocytes during Viral Infections. In Encyclopedia of Immunobiology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 286–290. [Google Scholar]
- Adam, L.; Rosenbaum, P.; Bonduelle, O.; Combadière, B. Strategies for Immunomonitoring after Vaccination and during Infection. Vaccines 2021, 9, 365. [Google Scholar] [CrossRef]
- Clay, T.M.; Hobeika, A.C.; Mosca, P.J.; Lyerly, H.K.; Morse, M.A. Assays for monitoring cellular immune responses to active immunotherapy of cancer. Clin. Cancer Res. 2001, 7, 1127–1135. [Google Scholar]
- Jurk, M.; Heil, F.; Vollmer, J.; Schetter, C.; Krieg, A.M.; Wagner, H.; Lipford, G.; Bauer, S. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 2002, 3, 499. [Google Scholar] [CrossRef] [PubMed]
- Quinn, K.M.; Fox, A.; Harland, K.L.; Russ, B.E.; Li, J.; Nguyen, T.H.O.; Loh, L.; Olshanksy, M.; Naeem, H.; Tsyganov, K.; et al. Age-Related Decline in Primary CD8(+) T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8(+) T Cells. Cell Rep. 2018, 23, 3512–3524. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.D.; Moss, P.A.H.; Goulder, P.J.R.; Barouch, D.H.; McHeyzer-Williams, M.G.; Bell, J.I.; McMichael, A.J.; Davis, M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science 1996, 274, 94–96, Erratum in Science 1998, 280, 1821. [Google Scholar] [CrossRef]
- Lopez-Gomez, A.; Pelaez-Prestel, H.F.; Juarez, I. Approaches to evaluate the specific immune responses to SARS-CoV-2. Vaccine 2023, 41, 6434–6443. [Google Scholar] [CrossRef] [PubMed]
- Malyguine, A.M.; Strobl, S.; Dunham, K.; Shurin, M.R.; Sayers, T.J. ELISPOT Assay for Monitoring Cytotoxic T Lymphocytes (CTL) Activity in Cancer Vaccine Clinical Trials. Cells 2012, 1, 111–126. [Google Scholar] [CrossRef]
- Smith, S.G.; Smits, K.; Joosten, S.A.; van Meijgaarden, K.E.; Satti, I.; Fletcher, H.A.; Caccamo, N.; Dieli, F.; Mascart, F.; McShane, H.; et al. Intracellular Cytokine Staining and Flow Cytometry: Considerations for Application in Clinical Trials of Novel Tuberculosis Vaccines. PLoS ONE 2015, 10, e0138042. [Google Scholar] [CrossRef]
- Bacher, P.; Scheffold, A. Flow-cytometric analysis of rare antigen-specific T cells. Cytometry A 2013, 83, 692–701. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef]
- Wölfl, M.; Greenberg, P.D. Antigen-specific activation and cytokine-facilitated expansion of naive, human CD8+ T cells. Nature protocols 2014, 9, 950–966. [Google Scholar] [CrossRef]
- Martinuzzi, E.; Afonso, G.; Gagnerault, M.C.; Naselli, G.; Mittag, D.; Combadiere, B.; Boitard, C.; Chaput, N.; Zitvogel, L.; Harrison, L.C.; et al. acDCs enhance human antigen-specific T-cell responses. Blood 2011, 118, 2128–2137. [Google Scholar] [CrossRef]
- Pittet, M.J.; Valmori, D.; Dunbar, P.R.; Speiser, D.E.; Lienard, D.; Lejeune, F.; Fleischhauer, K.; Cerundolo, V.; Cerottini, J.C.; Romero, P. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J. Exp. Med. 1999, 190, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Dutoit, V.; Rubio-Godoy, V.; Pittet, M.J.; Zippelius, A.; Dietrich, P.Y.; Legal, F.A.; Guillaume, P.; Romero, P.; Cerottini, J.C.; Houghten, R.A.; et al. Degeneracy of antigen recognition as the molecular basis for the high frequency of naive A2/Melan-a peptide multimer(+) CD8(+) T cells in humans. J. Exp. Med. 2002, 196, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Valmori, D.; Pittet, M.J.; Zippelius, A.; Rimoldi, D.; Levy, F.; Dutoit, V.; Ayyoub, M.; Rubio-Godoy, V.; Michielin, O.; et al. Antigenicity and immunogenicity of Melan-A/MART-1 derived peptides as targets for tumor reactive CTL in human melanoma. Immunol. Rev. 2002, 188, 81–96. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.M.; Larrieu, P.; Sarrabayrouse, G.; Prevost-Blondel, A.; Lengagne, R.; Desfrancois, J.; Labarriere, N.; Jotereau, F. HLA anchor optimization of the melan-A-HLA-A2 epitope within a long peptide is required for efficient cross-priming of human tumor-reactive T cells. J. Immunol. 2012, 188, 2102–2110. [Google Scholar] [CrossRef]
- Rosalia, R.A.; Quakkelaar, E.D.; Redeker, A.; Khan, S.; Camps, M.; Drijfhout, J.W.; Silva, A.L.; Jiskoot, W.; van Hall, T.; van Veelen, P.A.; et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur. J. Immunol. 2013, 43, 2554–2565. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Kleemann, L.; Richardson, J.R.; Rusch, E.; Rammensee, H.G.; Gouttefangeas, C. Simultaneous Identification of Functional Antigen-Specific CD8(+) and CD4(+) Cells after In Vitro Expansion Using Elongated Peptides. Cells 2022, 11, 3451. [Google Scholar] [CrossRef]
- Lissina, A.; Briceno, O.; Afonso, G.; Larsen, M.; Gostick, E.; Price, D.A.; Mallone, R.; Appay, V. Priming of Qualitatively Superior Human Effector CD8+ T Cells Using TLR8 Ligand Combined with FLT3 Ligand. J. Immunol. 2016, 196, 256–263. [Google Scholar] [CrossRef]
- Dallan, B.; Proietto, D.; De Laurentis, M.; Gallerani, E.; Martino, M.; Ghisellini, S.; Zurlo, A.; Volpato, S.; Govoni, B.; Borghesi, M.; et al. Age differentially impacts adaptive immune responses induced by adenoviral versus mRNA vaccines against COVID-19. Nat. Aging 2024, 4, 1121–1136. [Google Scholar] [CrossRef]
- Dorshkind, K.; Montecino-Rodriguez, E.; Signer, R.A. The ageing immune system: Is it ever too old to become young again? Nat. Rev. Immunol. 2009, 9, 57–62. [Google Scholar] [CrossRef]
- Linton, P.J.; Dorshkind, K. Age-related changes in lymphocyte development and function. Nat. Immunol. 2004, 5, 133–139. [Google Scholar] [CrossRef]
- Kiessling, M.; Cole, J.J.; Kubel, S.; Klein, P.; Korn, K.; Henry, A.R.; Laboune, F.; Fourati, S.; Harrer, E.; Harrer, T.; et al. Chronic inflammation degrades CD4 T cell immunity to prior vaccines in treated HIV infection. Nat. Commun. 2024, 15, 10200. [Google Scholar] [CrossRef]
- Hensel, N.; Gu, Z.; Sagar; Wieland, D.; Jechow, K.; Kemming, J.; Llewellyn-Lacey, S.; Gostick, E.; Sogukpinar, O.; Emmerich, F.; et al. Memory-like HCV-specific CD8(+) T cells retain a molecular scar after cure of chronic HCV infection. Nat. Immunol. 2021, 22, 229–239. [Google Scholar] [CrossRef]
- Yin, K.; Peluso, M.J.; Luo, X.; Thomas, R.; Shin, M.G.; Neidleman, J.; Andrew, A.; Young, K.C.; Ma, T.; Hoh, R.; et al. Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 2024, 25, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, A.; Zandi, K.; Cheok, Y.Y.; Saeidi, H.; Wong, W.F.; Lee, C.Y.Q.; Cheong, H.C.; Yong, Y.K.; Larsson, M.; Shankar, E.M. T-Cell Exhaustion in Chronic Infections: Reversing the State of Exhaustion and Reinvigorating Optimal Protective Immune Responses. Front. Immunol. 2018, 9, 2569. [Google Scholar] [CrossRef]
- Piening, A.; Ebert, E.; Gottlieb, C.; Khojandi, N.; Kuehm, L.M.; Hoft, S.G.; Pyles, K.D.; McCommis, K.S.; DiPaolo, R.J.; Ferris, S.T.; et al. Obesity-related T cell dysfunction impairs immunosurveillance and increases cancer risk. Nat. Commun. 2024, 15, 2835. [Google Scholar] [CrossRef]
- Nojima, I.; Eikawa, S.; Tomonobu, N.; Hada, Y.; Kajitani, N.; Teshigawara, S.; Miyamoto, S.; Tone, A.; Uchida, H.A.; Nakatsuka, A.; et al. Dysfunction of CD8 + PD-1 + T cells in type 2 diabetes caused by the impairment of metabolism-immune axis. Sci. Rep. 2020, 10, 14928. [Google Scholar] [CrossRef]
- Hraber, P.; Kuiken, C.; Yusim, K. Evidence for human leukocyte antigen heterozygote advantage against hepatitis C virus infection. Hepatology 2007, 46, 1713–1721. [Google Scholar] [CrossRef]
- Kaslow, R.A.; Carrington, M.; Apple, R.; Park, L.; Munoz, A.; Saah, A.J.; Goedert, J.J.; Winkler, C.; O’Brien, S.J.; Rinaldo, C.; et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 1996, 2, 405–411. [Google Scholar] [CrossRef]
- White, E.; Papagno, L.; Samri, A.; Sugata, K.; Hejblum, B.; Henry, A.R.; Rogan, D.C.; Darko, S.; Recordon-Pinson, P.; Dudoit, Y.; et al. Clonal succession after prolonged antiretroviral therapy rejuvenates CD8(+) T cell responses against HIV-1. Nat. Immunol. 2024, 25, 1555–1564. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Arens, R.; Schoenberger, S.P. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol. Rev. 2010, 235, 190–205. [Google Scholar] [CrossRef]
- Briceno, O.; Lissina, A.; Wanke, K.; Afonso, G.; von Braun, A.; Ragon, K.; Miquel, T.; Gostick, E.; Papagno, L.; Stiasny, K.; et al. Reduced naive CD8(+) T-cell priming efficacy in elderly adults. Aging Cell 2016, 15, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Alanio, C.; Nicoli, F.; Sultanik, P.; Flecken, T.; Perot, B.; Duffy, D.; Bianchi, E.; Lim, A.; Clave, E.; van Buuren, M.M.; et al. Bystander hyperactivation of preimmune CD8 T cells in chronic HCV patients. eLife 2015, 4, e07916. [Google Scholar] [CrossRef]
- Cabral-Piccin, M.P.; Briceno, O.; Papagno, L.; Liouville, B.; White, E.; Perdomo-Celis, F.; Autaa, G.; Volant, S.; Llewellyn-Lacey, S.; Fromentin, R.; et al. CD8 + T-cell priming is quantitatively but not qualitatively impaired in people with HIV-1 on antiretroviral therapy. AIDS 2024, 38, 161–166. [Google Scholar] [CrossRef]
- Alanio, C.; Lemaitre, F.; Law, H.K.; Hasan, M.; Albert, M.L. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood 2010, 115, 3718–3725. [Google Scholar] [CrossRef]
- Kuse, N.; Sun, X.; Akahoshi, T.; Lissina, A.; Yamamoto, T.; Appay, V.; Takiguchi, M. Priming of HIV-1-specific CD8(+) T cells with strong functional properties from naive T cells. EBioMedicine 2019, 42, 109–119. [Google Scholar] [CrossRef]
- Gallerani, E.; Proietto, D.; Dallan, B.; Campagnaro, M.; Pacifico, S.; Albanese, V.; Marzola, E.; Marconi, P.; Caputo, A.; Appay, V.; et al. Impaired Priming of SARS-CoV-2-Specific Naive CD8(+) T Cells in Older Subjects. Front. Immunol. 2021, 12, 693054. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Valiante, N.M. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2003, 2, 727–735. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef]
- Gutjahr, A.; Papagno, L.; Nicoli, F.; Lamoureux, A.; Vernejoul, F.; Lioux, T.; Gostick, E.; Price, D.A.; Tiraby, G.; Perouzel, E.; et al. Cutting Edge: A Dual TLR2 and TLR7 Ligand Induces Highly Potent Humoral and Cell-Mediated Immune Responses. J. Immunol. 2017, 198, 4205–4209. [Google Scholar] [CrossRef]
- Gutjahr, A.; Papagno, L.; Vernejoul, F.; Lioux, T.; Jospin, F.; Chanut, B.; Perouzel, E.; Rochereau, N.; Appay, V.; Verrier, B.; et al. New chimeric TLR7/NOD2 agonist is a potent adjuvant to induce mucosal immune responses. EBioMedicine 2020, 58, 102922. [Google Scholar] [CrossRef]
- Pavot, V.; Climent, N.; Rochereau, N.; Garcia, F.; Genin, C.; Tiraby, G.; Vernejoul, F.; Perouzel, E.; Lioux, T.; Verrier, B.; et al. Directing vaccine immune responses to mucosa by nanosized particulate carriers encapsulating NOD ligands. Biomaterials 2016, 75, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Papagno, L.; Kuse, N.; Lissina, A.; Gostick, E.; Price, D.A.; Appay, V.; Nicoli, F. The TLR9 ligand CpG ODN 2006 is a poor adjuvant for the induction of de novo CD8(+) T-cell responses in vitro. Sci. Rep. 2020, 10, 11620. [Google Scholar] [CrossRef] [PubMed]
- Gutjahr, A.; Papagno, L.; Nicoli, F.; Kanuma, T.; Kuse, N.; Cabral-Piccin, M.P.; Rochereau, N.; Gostick, E.; Lioux, T.; Perouzel, E.; et al. The STING ligand cGAMP potentiates the efficacy of vaccine-induced CD8+ T cells. JCI Insight 2019, 4, e125107. [Google Scholar] [CrossRef]
- Kuse, N.; Akahoshi, T.; Takiguchi, M. STING Ligand-Mediated Priming of Functional CD8(+) T Cells Specific for HIV-1-Protective Epitopes from Naive T Cells. J. Virol. 2021, 95, e0069921. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Botto, S.; Mizuno, N.; Pryke, K.; Gall, B.; Boehm, D.; Sali, T.M.; Jin, H.; Nilsen, A.; Gough, M.; et al. Characterization of a Novel Compound That Stimulates STING-Mediated Innate Immune Activity in an Allele-Specific Manner. Front. Immunol. 2020, 11, 1430. [Google Scholar] [CrossRef]
- Ramanjulu, J.M.; Pesiridis, G.S.; Yang, J.; Concha, N.; Singhaus, R.; Zhang, S.Y.; Tran, J.L.; Moore, P.; Lehmann, S.; Eberl, H.C.; et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature 2018, 564, 439–443. [Google Scholar] [CrossRef]
- Pan, B.S.; Perera, S.A.; Piesvaux, J.A.; Presland, J.P.; Schroeder, G.K.; Cumming, J.N.; Trotter, B.W.; Altman, M.D.; Buevich, A.V.; Cash, B.; et al. An orally available non-nucleotide STING agonist with antitumor activity. Science 2020, 369, eaba6098. [Google Scholar] [CrossRef]
- Lu, D.; Shang, G.; Li, J.; Lu, Y.; Bai, X.C.; Zhang, X. Activation of STING by targeting a pocket in the transmembrane domain. Nature 2022, 604, 557–562. [Google Scholar] [CrossRef]
- Heath, A.W.; Playfair, J.H. Cytokines as immunological adjuvants. Vaccine 1992, 10, 427–434. [Google Scholar] [CrossRef]
- Autaa, G.; Papagno, L.; Nogimori, T.; Boizard-Moracchini, A.; Korenkov, D.; Roy, M.; Suzuki, K.; Masuta, Y.; White, E.; Llewellyn-Lacey, S.; et al. Ageing and inflammation limit the induction of SARS-CoV-2-specific CD8+ T cell responses in severe COVID-19. JCI Insight 2025, 10, e180867. [Google Scholar] [CrossRef] [PubMed]
- Mussap, M.; Puddu, M.; Fanos, V. Metabolic Reprogramming of Immune Cells Following Vaccination: From Metabolites to Personalized Vaccinology. Curr. Med. Chem. 2024, 31, 1046–1068. [Google Scholar] [CrossRef]
- Adamik, J.; Munson, P.V.; Maurer, D.M.; Hartmann, F.J.; Bendall, S.C.; Argüello, R.J.; Butterfield, L.H. Immuno-metabolic dendritic cell vaccine signatures associate with overall survival in vaccinated melanoma patients. Nat. Commun. 2023, 14, 7211. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.; Lochner, M.; Berod, L.; Sparwasser, T. Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 2016, 28, 514–524. [Google Scholar] [CrossRef]
- Nicoli, F.; Papagno, L.; Frere, J.J.; Cabral-Piccin, M.P.; Clave, E.; Gostick, E.; Toubert, A.; Price, D.A.; Caputo, A.; Appay, V. Naïve CD8(+) T-Cells Engage a Versatile Metabolic Program Upon Activation in Humans and Differ Energetically From Memory CD8(+) T-Cells. Front. Immunol. 2018, 9, 2736. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, F.; Cabral-Piccin, M.P.; Papagno, L.; Gallerani, E.; Fusaro, M.; Folcher, V.; Dubois, M.; Clave, E.; Vallet, H.; Frere, J.J.; et al. Altered Basal Lipid Metabolism Underlies the Functional Impairment of Naive CD8(+) T Cells in Elderly Humans. J. Immunol. 2022, 208, 562–570. [Google Scholar] [CrossRef]
- Lahaye, X.; Satoh, T.; Gentili, M.; Cerboni, S.; Conrad, C.; Hurbain, I.; El Marjou, A.; Lacabaratz, C.; Lelievre, J.D.; Manel, N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity 2013, 39, 1132–1142. [Google Scholar] [CrossRef]
- Lahaye, X.; Gentili, M.; Silvin, A.; Conrad, C.; Picard, L.; Jouve, M.; Zueva, E.; Maurin, M.; Nadalin, F.; Knott, G.J.; et al. NONO Detects the Nuclear HIV Capsid to Promote cGAS-Mediated Innate Immune Activation. Cell 2018, 175, 488–501.e22. [Google Scholar] [CrossRef]
- Malim, M.H.; Emerman, M. HIV-1 sequence variation: Drift, shift, and attenuation. Cell 2001, 104, 469–472. [Google Scholar] [CrossRef]
- Li, G.; Piampongsant, S.; Faria, N.R.; Voet, A.; Pineda-Pena, A.C.; Khouri, R.; Lemey, P.; Vandamme, A.M.; Theys, K. An integrated map of HIV genome-wide variation from a population perspective. Retrovirology 2015, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Piccin, M.P.; Papagno, L.; Lahaye, X.; Perdomo-Celis, F.; Volant, S.; White, E.; Monceaux, V.; Llewellyn-Lacey, S.; Fromentin, R.; Price, D.A.; et al. Primary role of type I interferons for the induction of functionally optimal antigen-specific CD8(+) T cells in HIV infection. EBioMedicine 2023, 91, 104557. [Google Scholar] [CrossRef]
- Koutsoumpli, G.; Stasiukonyte, N.; Hoogeboom, B.N.; Daemen, T. An in vitro CD8 T-cell priming assay enables epitope selection for hepatitis C virus vaccines. Vaccine 2024, 42, 126032. [Google Scholar] [CrossRef]
- Liu, Y.; Keib, A.; Neuber, B.; Wang, L.; Riemer, A.B.; Bonsack, M.; Huckelhoven-Krauss, A.; Schmitt, A.; Muller-Tidow, C.; Schmitt, M. Definition and Characterization of SOX11-Derived T Cell Epitopes towards Immunotherapy of Glioma. Int. J. Mol. Sci. 2023, 24, 1943. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Goulding, S.P.; Conn, B.P.; McGann, C.D.; Dietze, J.L.; Kohler, J.; Lenkala, D.; Boudot, A.; Rothenberg, D.A.; Turcott, P.J.; et al. Systematic discovery and validation of T cell targets directed against oncogenic KRAS mutations. Cell Rep. Methods 2021, 1, 100084. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.N.; Tosch, C.; Kotsias, F.; Claudepierre, M.C.; Schmitt, D.; Remy-Ziller, C.; Hoffmann, C.; Ricordel, M.; Nourtier, V.; Farine, I.; et al. Pseudocowpox virus, a novel vector to enhance the therapeutic efficacy of antitumor vaccination. Clin. Transl. Immunol. 2022, 11, e1392. [Google Scholar] [CrossRef]
- Beuneu, H.; Lemaître, F.; Deguine, J.; Moreau, H.D.; Bouvier, I.; Garcia, Z.; Albert, M.L.; Bousso, P. Visualizing the functional diversification of CD8+ T cell responses in lymph nodes. Immunity 2010, 33, 412–423. [Google Scholar] [CrossRef]
- Bar-Ephraim, Y.E.; Kretzschmar, K.; Clevers, H. Organoids in immunological research. Nat. Rev. Immunol. 2020, 20, 279–293. [Google Scholar] [CrossRef]
- Wagar, L.E.; Salahudeen, A.; Constantz, C.M.; Wendel, B.S.; Lyons, M.M.; Mallajosyula, V.; Jatt, L.P.; Adamska, J.Z.; Blum, L.K.; Gupta, N.; et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 2021, 27, 125–135. [Google Scholar] [CrossRef]
- Yin, Q.; Luo, W.; Mallajosyula, V.; Bo, Y.; Guo, J.; Xie, J.; Sun, M.; Verma, R.; Li, C.; Constantz, C.M.; et al. A TLR7-nanoparticle adjuvant promotes a broad immune response against heterologous strains of influenza and SARS-CoV-2. Nat. Mater. 2023, 22, 380–390. [Google Scholar] [CrossRef]
- Santos, A.J.M.; van Unen, V.; Lin, Z.; Chirieleison, S.M.; Ha, N.; Batish, A.; Chan, J.E.; Cedano, J.; Zhang, E.T.; Mu, Q.; et al. A human autoimmune organoid model reveals IL-7 function in coeliac disease. Nature 2024, 632, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ghanizada, M.; Mallajosyula, V.; Sola, E.; Capasso, R.; Kathuria, K.R.; Davis, M.M. Differential roles of human CD4(+) and CD8(+) regulatory T cells in controlling self-reactive immune responses. Nat. Immunol. 2025, 26, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Jeger-Madiot, R.; Planas, D.; Staropoli, I.; Debarnot, H.; Kervevan, J.; Mary, H.; Collina, C.; Fonseca, B.F.; Robinot, R.; Gellenoncourt, S.; et al. Modeling memory B cell responses in a lymphoid organ-chip to evaluate mRNA vaccine boosting. J. Exp. Med. 2024, 221, e20240289. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.O.; Cabral-Piccin, M.P.; Appay, V.; Papagno, L. An In Vitro Approach to Prime or Boost Human Antigen-Specific CD8+ T Cell Responses: Applications to Vaccine Studies. Vaccines 2025, 13, 729. https://doi.org/10.3390/vaccines13070729
Nguyen HO, Cabral-Piccin MP, Appay V, Papagno L. An In Vitro Approach to Prime or Boost Human Antigen-Specific CD8+ T Cell Responses: Applications to Vaccine Studies. Vaccines. 2025; 13(7):729. https://doi.org/10.3390/vaccines13070729
Chicago/Turabian StyleNguyen, Hoang Oanh, Mariela P. Cabral-Piccin, Victor Appay, and Laura Papagno. 2025. "An In Vitro Approach to Prime or Boost Human Antigen-Specific CD8+ T Cell Responses: Applications to Vaccine Studies" Vaccines 13, no. 7: 729. https://doi.org/10.3390/vaccines13070729
APA StyleNguyen, H. O., Cabral-Piccin, M. P., Appay, V., & Papagno, L. (2025). An In Vitro Approach to Prime or Boost Human Antigen-Specific CD8+ T Cell Responses: Applications to Vaccine Studies. Vaccines, 13(7), 729. https://doi.org/10.3390/vaccines13070729





