Abstract
Background/Objectives: The COVID-19 pandemic significantly threatened cancer patients and oncologic care. The rollout of vaccines emerged as a critical milestone, despite the initial lack of evidence regarding their safety and efficacy in this population. This systematic review and meta-analysis evaluate the current evidence on COVID-19 vaccination in patients undergoing radiotherapy (RT). Methods: PubMed, Livivo, Scopus, and Cochrane Library were systematically reviewed for relevant publications on COVID-19 vaccination in the context of radiation oncology, published by 19 April 2024. The treatment effects were calculated as the proportion of seroconverted individuals. Results: A total of 22 studies published between 2021 and 2024 were included, covering various aspects of vaccination, including safety, tolerability, qualitative and quantitative humoral responses, cellular responses, vaccination efficacy, and booster vaccinations. Notably, patients undergoing RT exhibited a high willingness to receive vaccination. Vaccination was overall well tolerated and safe, with a low incidence of side effects, which were primarily mild. The primary meta-analysis showed a seroconversion proportion of 91% [95% CI: 84–96%] overall, with a somewhat higher proportion of 93% in patients receiving RT alone, compared to 90% in patients receiving either RT or RT combined with chemotherapy. Furthermore, immunization during RT led to a sustained increase in antibody titers, with a notable long-term persistence of IgG. Conclusions: COVID-19 vaccines demonstrate excellent safety, immunogenicity, and efficacy in patients receiving RT, who also exhibit a high willingness to be vaccinated. The outcomes observed are comparable to those in healthy controls and superior to those seen in patients receiving other cancer treatments, such as chemotherapy. The vaccination of radiation oncology patients in future pandemics or epidemics is strongly advocated even during active treatment.
1. Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has profoundly affected radiation oncology as well as oncologic healthcare in general. Within the first year of the pandemic, reports indicated a notable decrease in both cancer treatments and new cancer diagnoses [1]. Concurrently, the number of radiotherapy (RT) sessions declined markedly [2]. Cancer patients were found to have an increased susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections [3] and were at higher risk of adverse outcomes [4].
The development of vaccines marked a crucial milestone in managing the pandemic and showed excellent efficacy and tolerability compared to placebos in healthy subjects [5]. In cancer patients, the use of COVID-19 vaccines was initially approached cautiously due to the lack of clinical evidence. However, subsequent research demonstrated their high efficacy and safety in the majority of patients with cancer [6,7]. Data on SARS-CoV-2 vaccination in patients undergoing RT were initially even more limited. An early review of the literature on RT and COVID-19 found that only 1.4% of the included publications addressed both RT and vaccination [8]. Since then, several institutions actively monitoring vaccine administration in the context of radiation oncology have published their results, contributing valuable evidence that could inform strategies for future pandemics.
This systematic review primarily focuses on current evidence regarding immunogenicity parameters in radiation oncology patients, specifically humoral immune response, cellular immune response, response persistency, and vaccination-related side effects. Additionally, a meta-analysis was conducted to pool findings on seroconversion (SC). A secondary aim was to systematically review the circumstances of vaccination, including willingness, hesitancy, and other factors influencing patient decision making.
2. Materials and Methods
Study protocol. A systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines to minimize potential bias [9,10]. All included studies were evaluated using the Newcastle–Ottawa Risk of Bias assessment scale [11]. On 19 April 2024, the databases PubMed, LIVIVO, Scopus, and Cochrane Library were searched. The following search string was employed: >”radiotherapy” OR “radiation oncology” AND “SARS-coV-2” OR “COVID-19” AND “vaccin*” OR “immunisation” OR “immunization”<. The initial search yielded 1195 articles, from which 346 duplicates were removed, leaving 849 articles for screening. Following a multi-stage screening process—comprising title, abstract, and full text—22 studies were included (Figure 1). The inclusion criteria were as follows:
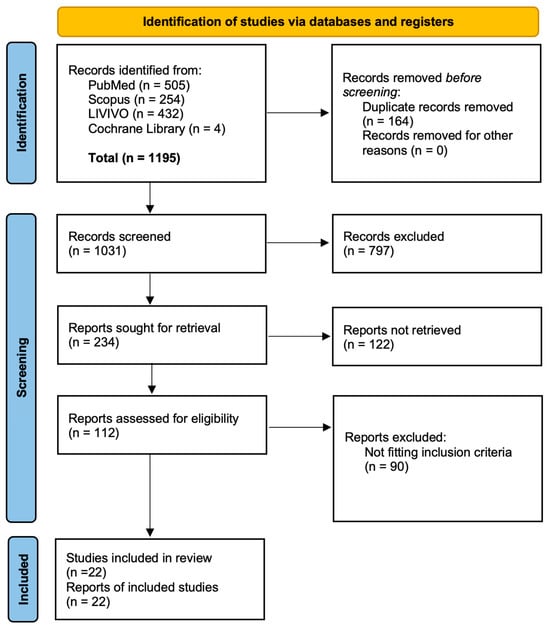
Figure 1.
Flow chart of the study conception in detail.
- (1)
- The study investigated an oncologic population of individuals aged 18 years or older.
- (2)
- Individuals received COVID-19 vaccination and underwent RT within the preceding 12 months.
- (3)
- Vaccine administration preceded RT, except in studies considering the circumstances of vaccination, e.g., willingness, hesitancy, or decision-influencing factors.
- (4)
- Immunological information, including side effects and/or data about the circumstances of vaccination, was reported.
- (5)
- The study design corresponded to at least level II evidence, in line with the Oxford Centre for Evidence-Based Medicine [12].
Only original papers were considered, and literature reviews were excluded. The PICO search strategy was used to guide the literature review (Appendix A Table A1) [13]. The review process was conducted using CADIMA [14]. Of the 22 studies, 9 studies provided detailed SC data of COVID-19 vaccines in radiation oncology patients and were thus suitable for a meta-analysis. The study designs were classified as cross-sectional if patients were recruited after vaccination only, and as longitudinal if patients were recruited prior to vaccination and therefore recruitment and follow-up were at least two distinct time points.
Statistical analysis. Confidence intervals for proportions were calculated using the method of Clopper–Pearson [15]. The primary meta-analytic model for pooling SC proportions was a generalized linear mixed-effects model—additionally stratified by the additional use of chemotherapy as a cancer treatment, the time of RT, study type, and the exclusion of patients with previous SARS-CoV-2 infection. The inverse-variance method was used as a sensitivity analysis. Heterogeneity among studies was assessed using the following metrics: between-study variance Tau2, percent variation across studies due to heterogeneity I2, and a Chi-squared test of the null hypothesis of no between-study heterogeneity. The results from each meta-analysis are graphically presented using forest plots. Publication bias was assessed visually with a funnel plot. All statistical analyses were based on the software R (version 4.3.1) [16], just as in the R packages tidyverse and meta [17,18].
Ethics. No ethical approval had to be obtained for this study.
3. Results
For the systematic review of current evidence, relevant data were extracted from 22 studies [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] published between 2021 and 2024, all of which explored the intersection between vaccination and radiation oncology (Table 1). The majority of studies primarily analyzed general cancer patient samples, which included radiation oncology subgroups, while six studies exclusively investigated radiation oncology patients. Ten studies were conducted exclusively within cohorts of patients with solid tumors. Seven studies explicitly reported to have included a certain proportion of hematologic malignancies, varying from 4.3 to 33.0%. The studies evaluated various vaccines: BNT162b2 was administered in 15 studies, mRNA-1273 in 12, ChAdOx1 nCoV-19 in five, Ad26.COV2.S in six, and Sinovac in seven.

Table 1.
Studies included into the systematic review. The level of evidence according to the Oxford Centre for Evidence-Based Medicine classification.
Circumstances of vaccination. Four studies reported data on vaccination circumstances [19,22,24,27,37]. Suzuki et al. observed that RT and/or chemotherapy patients adjusted their vaccination behavior according to their treatment schedules, opting to vaccinate or delay depending on planned therapies [37]. In a cross-sectional survey by Hong et al., among 82 patients receiving RT, 65 were vaccinated, while 17 declined [24]. Liu et al. similarly reported for a cross-sectional survey that more than half of RT patients (95 of 177) received vaccination. They presented only slightly lower odds for vaccination hesitancy compared to patients receiving other treatment modalities (unadjusted Odds Ratio (OR) = 0.932 [95% CI: 0.646–1.344], adjusted OR = 0.827 [95%CI: 0.510–1.340]) [27]. Geinitz et al. reported in their cross-sectional study a high willingness to vaccinate of 90.3% among RT candidates, with 15.5% receiving vaccination during antineoplastic therapy. Vaccination was declined by 9.7%, where the most common reasons for hesitancy included the intention to wait until treatment completion, indecision, distrust of the available vaccines, concerns about interactions with comorbidities, and prior infection [22].
Safety and tolerability. Six studies evaluated the tolerability of COVID-19 vaccines in RT patients [27,28,30,33,37,39]. Scoccianti et al. (2021) compared the side effects of mRNA-1273 in RT patients and healthy controls, finding similar patterns of early or late side effects attributable to the first or second dose [33]. Commonly reported side effects included fatigue, headache, pain at injection site, fever, chills, and redness at injection site, with higher incidence observed within 7 days after the second dose. However, RT patients experienced fewer side effects after both doses [33]. Thöne et al. reported excellent tolerability in patients during RT, with no severe side effects, and observed a positive association between the occurrence of side effects and humoral response kinetics [39]. Prayongrat et al. observed only mild side effects, particularly after the second dose of messenger ribonucleic acid (mRNA) vaccines, and a favorable tolerability in general [30]. Kian et al. found that three of nine RT patients experienced side effects, two of whom underwent RT alone and one combined with chemotherapy [28]. Liu et al. reported lower odds for side effects in RT patients compared to other treatment modalities (unadjusted OR = 0.478 [95% CI: 0.247–0.926]) [27]. Suzuki et al. highlighted patient anxiety related to the specific side effect of lymph node swelling, as they related it to potential cancer recurrence, especially in those with a history of lymphatic metastases. In this study, 37% of the 709 patients reported a swelling at the injection site [37]. Apart from original research articles, a unique RT-related side effect, the radiation recall phenomenon, has been described in some case reports as a consequence of COVID-19 vaccination [41,42,43,44].
Qualitative humoral vaccination response. Ten studies assessed the humoral vaccination response of COVID-19 vaccines in cancer patients undergoing or having completed RT, out of which Joudi et al. [25] is, however, based on a subset of patients of Ariamanesh et al. [19] and therefore not included in the meta-analysis. Five of the nine studies included comparisons to healthy controls (Table 2, Figure 2).

Table 2.
Studies included in the meta-analysis of quantitative humoral vaccination response. The following vaccines were administered: 1 BNT162b2, 2 mRNA-1273, 3 ChAdOx1 nCov-19, 4 Ad26.COV2.S, 5 Sinovac.
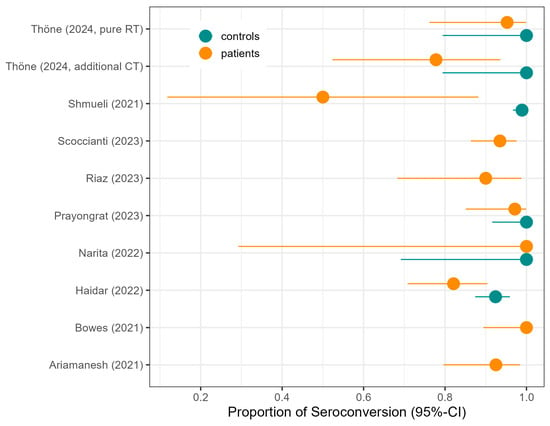
Figure 2.
Proportion of seroconversion in patients as reported in the original studies of the meta-analysis, with seroconversion in controls where reported, along with 95% confidence intervals (CIs).
Provencio et al. analyzed the association of seronegativity and cancer treatment modality. In detail, six months after vaccine administration, the adjusted odds for a negative serologic response were 1.46-fold [95% CI: 0.74–2.70] for RT patients compared to patients not receiving RT, and were also increased for patients receiving chemotherapy (odds ratio = 2.29 [95% CI: 1.12–4.85]) [31]. Scoccianti et al. (2023) observed that a younger age and breast irradiation were associated with a higher SC proportion [34]. Thöne et al. demonstrated a slower development of SARS-CoV-2-specific antibodies in RT patients, particularly in those receiving both RT and chemotherapy as compared to healthy controls [39]. Prayongrat et al. found an SC of 85% after a single adenoviral vaccination in RT patients, increasing to 89.5% after a second adenoviral vaccination, and 100% after a second immunization with an mRNA vaccine, while healthy controls achieved 100% [30].
Comparing pure RT to RT combined with chemoradiotherapy revealed higher SC proportions in RT-only patients. Ariamanesh et al. found that patients with RT only had higher frequencies of both binding (92.5% vs. 70.1%) and neutralizing antibodies (92.5% vs. 76.3%) compared with patients receiving chemotherapy with or without RT [19]. Joudi et al., which is a subset of the data in Ariamanesh et al., also described a higher SC in RT-only patients of 95%, compared to chemotherapy ± RT with an SC proportion of 66.7% [25]. These findings are in line with Thöne et al., who found that 95.3% of patients under pure RT seroconverted: 100% in healthy controls and 77.8% in radio-chemotherapy patients [39].
The meta-analytic model based on all SC studies estimated a pooled SC proportion of 91% [95% CI: 84–96%] in RT patients, derived from a generalized linear mixed-effects model (Figure 3). Variability in the estimates was considerable (I2 = 45%, Chi2 test p-value = 0.06). Using inverse-variance weighting as a sensitivity analysis, the pooled SC was estimated to be 89% [95% CI: 81–94%] (Appendix A Figure A1). The funnel plot shows the potential of publication bias, as smaller studies with low proportions of SC may have not been reported (Appendix A Figure A2). Stratifying the meta-analysis by whether chemotherapy was given to the patients additionally to RT, we found somewhat higher SC proportions among RT-only patients with a much lower variability between the studies (I2 = 0%, Chi2 test p-value = 0.98). For those RT patients who additionally received chemotherapy, the proportion was somewhat lower at 90% [95%CI: 67–97%], with considerable heterogeneity (I2 = 47%, Chi2 test p-value = 0.11) (Figure 4). A further stratification indicated somewhat higher seroconversion proportions when the start of RT preceded vaccination by less than three months (ongoing RT: 87%, prior RT < 3 months prior: 99%, prior RT < 1 year: 89%) (Appendix A Figure A3). Stratifying by study design shows the tendency that cross-sectional studies report a lower seroconversion proportion (83%) compared to longitudinal studies (92%), however limited by the inclusion of only two cross-sectional studies (Appendix A Figure A4). A potentially more important design aspect is that most studies excluded patients with a history of COVID-19 or positive nucleocapsid antibodies prior to vaccination. As expected, these studies reported a lower seroconversion proportion (89%) than studies not mentioning this exclusion criteria (95%) (Appendix A Figure A5).
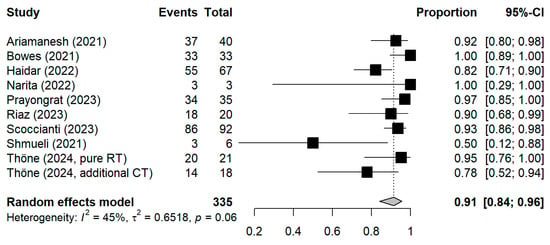
Figure 3.
Forest plot of all studies included in the meta-analysis with the pooled result. Each square represents the point estimate with 95% CI for an individual study; The pooled estimate is shown as a diamond. CI = Confidence interval.
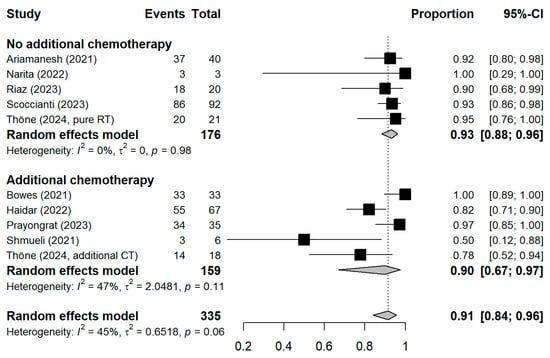
Figure 4.
Stratified forest plot with the respective pooled result, discriminating between studies comprising patients receiving only radiotherapy and patients additionally receiving chemotherapy. CI = Confidence interval.
Quantitative humoral vaccination response. Six studies evaluated the quantitative humoral response [20,30,34,38,39,40]. Scoccianti et al. (2023) reported a median Immunoglobulin G (IgG) titer of 300 BAU/mL (range 7–1633) after a median of 147 days (range 144–154) after the second dose [34]. Uslu et al. found lower IgG levels in RT patients (mean: 1044.75 AU/mL, min–max: 2.00–40,000) compared to non-RT individuals (mean: 2278.00, min-max 353.20–40,000) [40]. Prayongrat et al. noted reduced anti-RBD total IgG levels in RT patients compared to those of healthy controls [30]. Thöne et al. observed similar mean titer values between pure RT patients and healthy controls at four (2001.34 IU/mL vs. 1640.06 IU/mL) and five (3165.71 IU/mL vs. 2994.13 IU/mL) weeks post-vaccination, although RT patients exhibited a slower initial increase [39]. Bowes et al. reported a geometric mean neutralizing antibody titer for RT patients 12 weeks after complete RT of 2.42 log10 U/mL [95% Cl: 2.13–2.72] for patients with thoracic malignancies who did not receive RT of 2.62 log10 U/mL [95% CI: 2.46–2.77], and 2.80 log10 U/mL [95% CI: 2.63–2.97] for healthy controls [20]. Antibody titers were inversely associated with immunosuppressive conditions, co-medication, chemotherapy, comorbidities, a palliative treatment intention [20], just as with age [34]. The concentration levels of anti-RBD total Ig were similar between patients receiving high-dose RT (>50 Gy) compared to low-dose (≤50 Gy) (e.g., three months after second dose, geometric mean of low dose: 115.4 [95% CI: 4.9–2734] vs. high dose: 142 [95% CI: 37.7–536.3]), according to Prayongrat et al. [30].
Cellular response. Two studies reported data on the cellular vaccination response [34,39]. In five sero-nonresponders, Scoccianti et al. (2023) found two positive and one borderline T-cell response out of five [34]. Thöne et al. assessed the T-cell immune response in four sero-nonresponders, all of which were negative [39].
Vaccination efficacy. In a registry-based case-control study, Lee et al. found that individuals with a recent cancer diagnosis, systematic cancer treatment, or RT within the past 12 months exhibited lower vaccine effectiveness against breakthrough and symptomatic SARS-CoV-2 infections [45]. In contrast, Seegers et al. reported significantly fewer breakthrough infections in cancer patients who underwent RT as compared to those who received other treatment modalities, such as chemotherapy [35]. Long-term measurements by Thöne et al., as well as measurements between the 2nd and 3rd dose by Scoccianti et al., found no nucleocapsid positivity, indicating an absence of viral exposure in these patients [34,39]. Bowes et al. concluded that RT does not interfere with the humoral vaccination response [20], while Prayongrat et al. considered effects of RT to be negligible [30].
Booster vaccinations (third dose). Six studies have reported findings of booster vaccinations [20,21,29,34,37,39]. Bowes et al. suggested the administration of an additional booster dose to vaccine non-responders in oncological patients [20]. In Thöne et al. booster vaccinations caused excellent results in terms of SC and good tolerability in all four individuals involved [39]. Similarly, Scoccianti et al. (2023) administered a third dose to 81 vaccinated individuals and observed a markedly enhanced humoral response of initially seronegatives, poor-, regular-, and ultra-responders [34]. However, Chen et al. investigated Sinovac booster vaccinations in lung cancer patients and found lower titers caused by booster vaccinations in RT patients than in other treatments [21].
4. Discussion
Summary. This review comprehensively evaluated the safety, immunogenicity, and efficacy of COVID-19 vaccination in oncology patients undergoing radiotherapy (RT). RT patients showed a high willingness to receive COVID-19 vaccinations, with acceptance rates between 79.3% and 90.3%, exceeding those of cancer patients undergoing other treatments. Vaccines were well tolerated, with only mild side effects. Quantitative humoral responses varied considerably across studies, with some patients showing reduced antibody titers, particularly those with immunosuppressive conditions or those undergoing chemotherapy, although the radiation dose itself had no impact. Findings on vaccine efficacy in RT patients were also heterogenous. While cancer patients, irrespective of treatment status, remain vulnerable to breakthrough infections, those undergoing RT demonstrated a significantly lower incidence of breakthrough infections compared to patients treated with chemotherapy or other treatments. Booster vaccinations are strongly recommended to optimize immunogenicity and reduce seronegativity, particularly in sero-nonresponders. The meta-analysis found a high pooled proportion of seroconversion of 91% in RT patients [95% CI: 84–96%], with higher and more consistent responses in those receiving RT alone. While early publications raised concerns that immunization during RT might impair immune response [7,46], later studies published during the pandemic supported vaccination for RT patients [8]. The results of this review and meta-analysis support the later studies in their conclusion.
Comparison to Literature. The high vaccination acceptance among RT patients found in this review, ranging from 79.3% to 90.3%, contrasts with a meta-analysis showing only a 59% acceptance among general cancer patients [47] and a Korean multicenter study reporting only 62% [48]. Identified major factors influencing hesitancy were treatment plans, timing, side effects, and uncertainties about efficacy and safety, which is consistent with prior findings from a systematic review and meta-analysis [47]. COVID-19 vaccines, however, demonstrated excellent tolerability in RT patients, with six studies reporting only mild to moderate side effects, comparable to the general population [49,50], and decreased reactogenicity has also been observed in cancer patients [51].
The meta-analysis found a high SC proportion of 91% among cancer patients who received RT, where patients receiving only RT had an even higher SC of 93%, compared to 90% with an additional treatment of chemotherapy. Other meta-analyses of cancer patient populations reported proportions of 73% [52], 90% in solid cancer and 63% hematologic cancer patients [26]. A self-conducted reanalysis of a meta-analysis by Yin et al. [53] estimated a pooled SC proportion of 49.5% (95% CI: 30.2–69.0%) for cancer patients after the first dose (vs. 90.6 (73.7; 97.1) in healthy controls) and 86.6% (95% CI: 81.8–90.3%) after the second (vs. 99.5% (98.4–99.9) in healthy controls). These findings suggest that although healthy controls have the highest proportions of SC, RT does not strongly compromise SC, especially not in comparison to other cancer treatments.
One study in this review showed that SC levels in RT patients remained stable over months [39], consistent with evidence that common Western vaccines (mRNA-1273, BNT162b2, Ad26.COV2.S, NVX-CoV2373) sustain humoral and cellular responses for over 6 months [54]. The findings on quantitative humoral response were heterogeneous, where some reported lower titers in RT patients [30,40], while others found not significantly different [38] or comparable titers to healthy controls [39]. In nine seronegative subjects pooled from two studies, a cumulative cellular nonresponse of 78% was found. Other meta-analyses of unselected cancer patients reported humoral response proportions of 78% at only 60% cellular response [52] or 79% humoral at only 61% cellular [55].
Reduced vaccine response in RT patients and the observed heterogeneity of findings highlight the importance of modifying factors such as prior or concurrent chemotherapy, immunosuppressive co-medications, poor overall health, and hematologic malignancies, as recognized both in the included studies [19,20,22,25,39] and previous meta-analyses [56,57,58]. Variability in SC and antibody titers may also be attributed to differences in vaccine platforms. For instance, systematically acting mRNA vaccines have demonstrated better performance compared to conventional vaccine platforms, both in RT patients [30] and in cancer patients more broadly [59]. Although RT is predominantly localized and induces primarily focal effects, systemic immunologic effects on lymphocyte subpopulations, blood cell count, and cytokine levels have been described [60,61].
Regarding booster or third vaccinations, an excellent tolerability as well as effectiveness/efficacy in overcoming seronegativity and increasing low antibody titers were observed. These findings are consistent with a meta-analysis of sero-nonresponding cancer patients, who achieved an SC proportion of 80% (solid cancer) and 44% (hematologic cancer) following booster doses [62]. Additional systematic reviews further supported the effectiveness of booster vaccinations in sero-nonconverting cancer patients, especially in those with solid tumors [63,64,65].
Strengths and Limitations. Following PRISMA guidelines, data from 22 studies were extracted and analyzed, and we used 9 to perform a meta-analysis on the proportion of seroconverted individuals, covering a broad range of vaccine platforms and cancer types. This review provides a comprehensive and systematic synthesis of the current evidence on COVID-19 vaccination in radiation oncology patients, encompassing vaccine safety, qualitative and quantitative humoral and cellular immune responses, and booster vaccination efficacy. The inclusion of real-world vaccination circumstances such as patient willingness and hesitancy strengthens the clinical relevance of the findings.
However, the interpretability of the findings is limited by the heterogeneity of the included studies, particularly regarding the intervals between RT and vaccination, as well as between vaccination and antibody measurements. Although these variations appear to have a minimal impact on SC, they may nevertheless corroborate the results. The potential for publication bias, suggested by asymmetry in the funnel plot, also limits the generalizability of the meta-analysis. Moreover, a separate analysis of vaccine platforms and types, or a consideration of patient characteristics such as co-morbidities and co-medications, was not possible.
5. Conclusions
This review demonstrated a high willingness among RT patients to receive COVID-19 vaccination and indicated that RT does not substantially impair vaccination response. Seroconversion was high, particularly in comparison to that in patients receiving other treatment modalities, and no evidence of increased adverse effects associated with vaccination in RT patients was observed. However, vaccine efficacy may be reduced in the presence of concomitant chemotherapy, immunosuppressive medication, or poor overall health status in cancer patients. As RT patients are particularly vulnerable to COVID-19 and require stringent protective measures, the vaccination of RT patients, even during active (pure) radiation treatment is strongly advocated, ideally under careful medical supervision. Especially patients receiving concomitant chemotherapy or those with other immunosuppressive conditions should undergo close monitoring of antibody levels following vaccination. Furthermore, the administration of booster doses in cancer patients receiving RT is strongly recommended, particularly in sero-nonresponders and in those with low antibody titers or absent cellular immune responses.
Author Contributions
Conceptualization, P.T., M.E. and H.G.; methodology, P.T., M.S.G., A.A. and H.G.; software, P.T. and A.A.; validation, M.E., M.S.G., L.T. and H.G.; data extraction, P.T. and A.A.; formal analysis, P.T. and A.A.; investigation, P.T., A.A. and H.G.; resources, P.T.; data curation, A.A.; writing—original draft preparation, P.T. and A.A.; writing—review and editing, P.T., M.E., M.S.G., G.G., C.K., D.N., E.W., H.W., L.T., W.L., M.C., E.B., B.D., A.A. and H.G.; visualization, P.T., M.E., M.S.G., G.G., C.K., D.N., E.W., H.W., L.T., W.L., M.C., E.B., B.D., A.A. and H.G.; supervision, A.A. and H.G.; project administration, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Supported by Johannes Kepler University Open Access Publishing Fund.
Conflicts of Interest
All authors declare to have nothing to disclose.
Abbreviations
The following abbreviations are used in this manuscript:
| AU | Arbitrary Units |
| BAU | Binding Antibody Units |
| CI | Confidence Interval |
| COVID-19 | Coronavirus Disease 2019 |
| IgG | Immunoglobulin G |
| mRNA | Messenger Ribonucleic Acid |
| OR | Odds Ratio |
| RT | Radiotherapy |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SC | Seroconversion |
Appendix A

Table A1.
Application of the PICO search strategy to guide the literature review.
Table A1.
Application of the PICO search strategy to guide the literature review.
| PICO-Strategy. | |
|---|---|
| Patients | Cancer patients undergoing radiotherapy, aged 18 years or older |
| Intervention | COVID-19 vaccination administered before or concurrently to radiotherapy |
| Comparison | If applicable: healthy controls or other treatment modalities |
| Outcome | Immunogenicity and circumstances of vaccination |
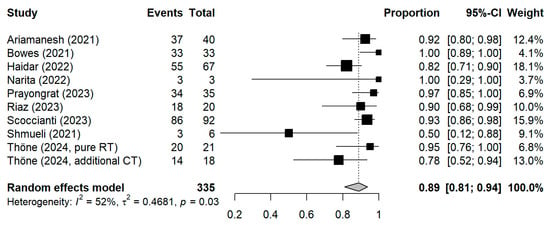
Figure A1.
Forest plot of all studies included in the meta-analysis with the pooled result based on inverse-variance weights as a sensitivity analysis. CI = Confidence interval.
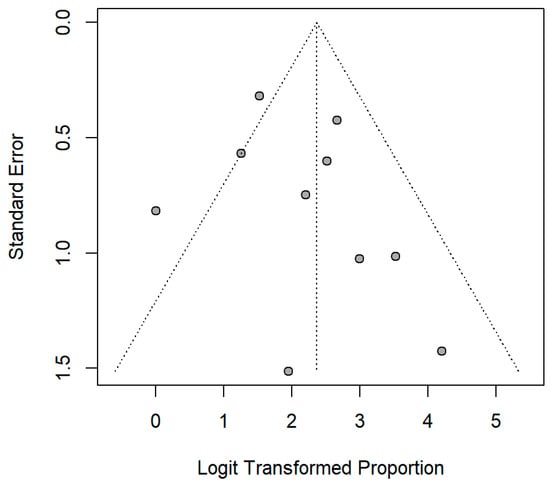
Figure A2.
Funnel plot of the included studies.
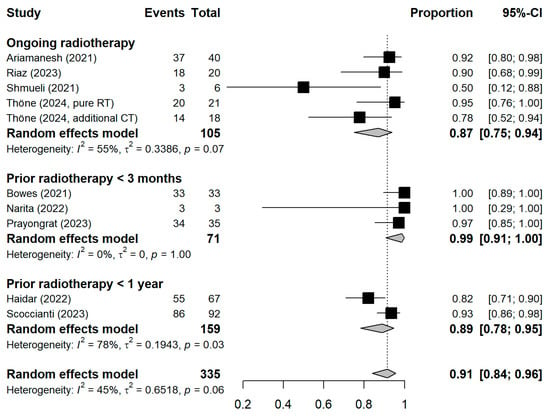
Figure A3.
Stratified forest plot with the respective pooled result, discriminating between studies based on the temporal proximity of radiotherapy to vaccination. CI = Confidence interval.
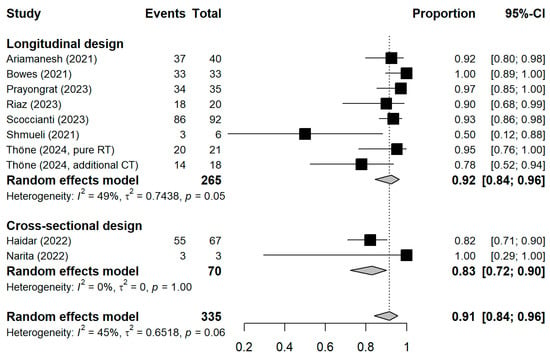
Figure A4.
Stratified forest plots with the respective pooled result, discriminating studies based on the study design. CI = Confidence interval.
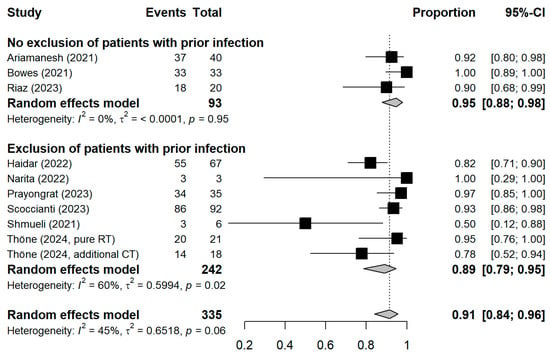
Figure A5.
Stratified forest plot with the respective pooled result, discriminating between studies excluding or not excluding patients with a history of COVID-19 or positive nucleocapsid antibodies prior to vaccination. CI = Confidence interval.
References
- Nogueira, L.M.; Schafer, E.J.; Fan, Q.; Wagle, N.S.; Zhao, J.; Shi, K.S.; Han, X.; Jemal, A.; Yabroff, K.R. Assessment of Changes in Cancer Treatment During the First Year of the COVID-19 Pandemic in the US. JAMA Oncol. 2024, 10, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.; Jones, C.M.; Girdler, R.; Roe, C.; Sharpe, M.; Lawton, S.; Miller, L.; Lewis, P.; Evans, M.; Sebag-Montefiore, D.; et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: A population-based study. Lancet Oncol. 2021, 22, 309–320. [Google Scholar] [CrossRef]
- Lemos, A.E.G.; Silva, G.R.; Gimba, E.R.P.; Matos, A.D.R. Susceptibility of lung cancer patients to COVID-19: A review of the pandemic data from multiple nationalities. Thorac. Cancer 2021, 12, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Di Felice, G.; Visci, G.; Teglia, F.; Angelini, M.; Boffetta, P. Effect of cancer on outcome of COVID-19 patients: A systematic review and meta-analysis of studies of unvaccinated patients. eLife 2022, 11, e74634. [Google Scholar] [CrossRef]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef] [PubMed]
- Javadinia, S.A.; Alizadeh, K.; Mojadadi, M.-S.; Nikbakht, F.; Dashti, F.; Joudi, M.; Harati, H.; Welsh, J.S.; Farahmand, S.A.; Attarian, F. COVID-19 Vaccination in Patients With Malignancy; A Systematic Review and Meta-Analysis of the Efficacy and Safety. Front. Endocrinol. 2022, 13, 860238. [Google Scholar] [CrossRef]
- Hwang, J.K.; Zhang, T.; Wang, A.Z.; Li, Z. COVID-19 vaccines for patients with cancer: Benefits likely outweigh risks. J. Hematol. Oncol. 2021, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Venuti, V.; D’Aviero, A.; Cusumano, D.; Pergolizzi, S.; Daidone, A.; Boldrini, L. COVID-19 and radiotherapy: A systematic review after 2 years of pandemic. Clin. Transl. Imaging 2022, 10, 611–630. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 24 June 2025).
- OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2, Oxford Centre for Evidence-Based Medicine; OCEBM Levels of Evidence Working Group: Oxford, UK, 2011. [Google Scholar]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Kohl, C.; McIntosh, E.J.; Unger, S.; Haddaway, N.R.; Kecke, S.; Schiemann, J.; Wilhelm, R. Online tools supporting the conduct and reporting of systematic reviews and systematic maps: A case study on CADIMA and review of existing tools. Environ. Evid. 2018, 7, 8. [Google Scholar] [CrossRef]
- Clopper, C.J.; Pearson, E.S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 1934, 26, 404–413. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Ariamanesh, M.; Porouhan, P.; PeyroShabany, B.; Fazilat-Panah, D.; Dehghani, M.; Nabavifard, M.; Hatami, F.; Fereidouni, M.; Welsh, J.S.; Javadinia, S.A. Immunogenicity and Safety of the Inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) in Patients with Malignancy. Cancer Investig. 2022, 40, 26–34. [Google Scholar] [CrossRef]
- Bowes, C.L.; Naranbhai, V.; St Denis, K.J.; Lam, E.C.; Bertaux, B.; Keane, F.K.; Khandekar, M.J.; Balazs, A.B.; Iafrate, J.A.; Gainor, J.F.; et al. Heterogeneous immunogenicity of SARS-CoV-2 vaccines in cancer patients receiving radiotherapy. Radiother. Oncol. 2022, 166, 88–91. [Google Scholar] [CrossRef]
- Chen, C.; Dai, L.; Zheng, C.; Li, H.; Li, X.; Yang, M.; Gao, R.; Yao, J.; Zhang, Z.; Shi, Y.; et al. Antibody response to SARS-CoV-2 WT and Omicron BA.4/5 of inactivated -19 vaccine in patients with lung cancer after second and booster immunization. J. Hematol. Oncol. 2023, 16, 47. [Google Scholar] [CrossRef]
- Geinitz, H.; Silberberger, E.; Spiegl, K.; Feichtinger, J.; Wagner, H.; Hermann, P.; Bräutigam, E.; Track, C.; Weis, E.M.; Venhoda, C.; et al. SARS-CoV-2 vaccination willingness and humoral vaccination response in radiation oncology patients. Vaccine 2024, 42, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Haidar, G.; Agha, M.; Bilderback, A.; Lukanski, A.; Linstrum, K.; Troyan, R.; Rothenberger, S.; McMahon, D.K.; Crandall, M.D.; Sobolewksi, M.D.; et al. Prospective Evaluation of Coronavirus Disease 2019 (COVID-19) Vaccine Responses Across a Broad Spectrum of Immunocompromising Conditions: The COVID-19 Vaccination in the Immunocompromised Study (COVICS). Clin. Infect. Dis. 2022, 75, e630–e644. [Google Scholar] [CrossRef]
- Hong, J.; Xu, X.; Yang, J.; Zheng, J.; Dai, S.; Zhou, J.; Zhang, Q.; Ruan, Y.; Ling, C. Knowledge about, attitude and acceptance towards, and predictors of intention to receive the COVID-19 vaccine among cancer patients in Eastern China: A cross-sectional survey. J. Integr. Med. 2022, 20, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Joudi, M.; Moradi Binabaj, M.; Porouhan, P.; PeyroShabany, B.; Tabasi, M.; Fazilat-Panah, D.; Khajeh, M.; Mehrabian, A.; Dehghani, M.; Welsh, J.S.; et al. A Cohort Study on the Immunogenicity and Safety of the Inactivated SARS-CoV-2 Vaccine (BBIBP-CorV) in Patients With Breast Cancer; Does Trastuzumab Interfere With the Outcome? Front. Endocrinol. 2022, 13, 798975. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.R.Y.B.; Wong, S.Y.; Chai, L.Y.A.; Lee, S.C.; Lee, M.X.; Muthiah, M.D.; Tay, S.H.; Teo, C.B.; Tan, B.K.J.; Chan, Y.H.; et al. Efficacy of COVID-19 vaccines in immunocompromised patients: Systematic review and meta-analysis. BMJ 2022, 376, e068632. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Yang, R.; Chen, R.; Huang, Y.; Zhao, X.; Xie, M.; Li, Q.; Wang, Q.; Chen, J. COVID-19 Vaccination Status and Hesitancy among Breast Cancer Patients after Two Years of Pandemic: A Cross-Sectional Survey. Vaccines 2022, 10, 1530. [Google Scholar] [CrossRef]
- Kian, W.; Zemel, M.; Kestenbaum, E.H.; Rouvinov, K.; Alguayn, W.; Levitas, D.; Ievko, A.; Michlin, R.; Abod, M.A.; Massalha, I.; et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in oncologic patients undergoing numerous cancer treatment options: A retrospective single-center study. Medicine 2022, 101, e28561. [Google Scholar] [CrossRef] [PubMed]
- Narita, D.; Ebina-Shibuya, R.; Miyauchi, E.; Tsukita, Y.; Saito, R.; Murakami, K.; Kimura, N.; Sugiura, H. Antibody responses to second doses of COVID-19 vaccination in lung cancer patients undergoing treatment. Respir. Investig. 2023, 61, 247–253. [Google Scholar] [CrossRef]
- Prayongrat, A.; Noppaving, P.; Chobarporn, T.; Sudhinaraset, N.; Teeyapun, N.; Pakvisal, N.; Jantarabenjakul, W.; Sophonphan, J.; Lertbutsayanukul, C.; Poovorawan, Y. Safety and Immunogenicity of Homologous and Heterologous Adenoviral-Vectored and mRNA COVID-19 Vaccine Regimens in Radiotherapy Patients. Vaccines 2023, 11, 1135. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Estival, A.; Franco, F.; López-Vivanco, G.; Saigí, M.; Arasanz, H.; Diz, P.; Carcereny, E.; García, J.; Aguado, C.; et al. Immunogenicity of COVID-19 vaccines in lung cancer patients. Lung Cancer 2023, 184, 107323. [Google Scholar] [CrossRef]
- Riaz, A.; Alam, A.; Saleem, N. Administration of Sinopharm COVID-19 Vaccine in Cancer Patients. Pak. J. Pharm. Sci. 2023, 36, 67–70. [Google Scholar] [CrossRef]
- Scoccianti, S.; Delli Paoli, C.; Grilli Leonulli, B.; Paoletti, L.; Alpi, P.; Caini, S.; Barca, R.; Fondelli, S.; Russo, S.; Perna, M.; et al. Acute tolerance of Moderna mRNA-1273 vaccine against COVID-19 in patients with cancer treated with radiotherapy. Lancet Oncol. 2021, 22, 1212–1214. [Google Scholar] [CrossRef]
- Scoccianti, S.; Delli Paoli, C.; Infantino, M.; Paoletti, L.; Caini, S.; Meacci, F.; Russo, S.; Esposito, M.; Fondelli, S.; Grilli Leonulli, B.; et al. Immunogenicity after two and three doses of mRNA vaccine in patients with cancer treated with exclusive radiotherapy. Int. Immunopharmacol. 2023, 122, 110460. [Google Scholar] [CrossRef]
- Seegers, V.; Rousseau, G.; Zhou, K.; Blanc-Lapierre, A.; Bigot, F.; Mahammedi, H.; Lambert, A.; Moreau-Bachelard, C.; Campone, M.; Conroy, T.; et al. COVID-19 Infection despite Previous Vaccination in Cancer Patients and Healthcare Workers: Results from a French Prospective Multicenter Cohort (PAPESCO-19). Cancers 2023, 15, 4777. [Google Scholar] [CrossRef]
- Shmueli, E.S.; Itay, A.; Margalit, O.; Berger, R.; Halperin, S.; Jurkowicz, M.; Levin, E.G.; Levy, I.; Olmer, L.; Regev-Yochay, G.; et al. Efficacy and safety of BNT162b2 vaccination in patients with solid cancer receiving anticancer therapy—A single centre prospective study. Eur. J. Cancer 2021, 157, 124–131. [Google Scholar] [CrossRef]
- Suzuki, H.; Akiyama, T.; Ueda, N.; Matsumura, S.; Mori, M.; Namiki, M.; Yamada, N.; Tsutsumi, C.; Tozaki, S.; Iwamoto, H.; et al. COVID-19 Vaccination in Patients with Cancer. Cancers 2022, 14, 2556. [Google Scholar] [CrossRef]
- Thakkar, A.; Gonzalez-Lugo, J.D.; Goradia, N.; Gali, R.; Shapiro, L.C.; Pradhan, K.; Rahman, S.; Kim, S.Y.; Ko, B.; Sica, R.A.; et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021, 39, 1081–1090.E2. [Google Scholar] [CrossRef]
- Thöne, P.; Egger, M.; Geroldinger-Simic, M.; Kindermann, H.; Kocik, L.; Karasek, N.; Fischerlehner, B.; Spiegl, K.; Gruber, G.; Aschacher, B.; et al. Immunogenicity Parameters of Cancer Patients Receiving the mRNA Vaccine BNT162b2 While Obtaining Radiotherapy: A Longitudinal Cohort Evaluation. Vaccines 2024, 12, 275. [Google Scholar] [CrossRef]
- Uslu, G.H.; Rakici, S.Y.; Cicek, A.C.; Yazici, Z.A. COVID-19 vaccine immunity in oncology patients. Bratisl. Med. J. 2023, 124, 187–192. [Google Scholar] [CrossRef]
- Soyfer, V.; Gutfeld, O.; Shamai, S.; Schlocker, A.; Merimsky, O. COVID-19 Vaccine-Induced Radiation Recall Phenomenon. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 957–961. [Google Scholar] [CrossRef]
- Marples, R.; Douglas, C.; Xavier, J.; Collins, A.-J. Breast Radiation Recall Phenomenon After Astra-Zeneca COVID-19 Vaccine: A Case Series. Cureus 2022, 14, e21499. [Google Scholar] [CrossRef] [PubMed]
- Tatekawa, S.; Hoshino, S.; Takemoto, N.; Oda, M.; Akino, Y.; Iwahori, K.; Hirata, T.; Hayashi, K.; Tamari, K.; Seo, Y.; et al. COVID-19 vaccine-induced Recurrence of the Radiation Recall Phenomenon in the Laryngeal Mucosa Due to a VEGF Inhibitor. Adv. Radiat. Oncol. 2022, 7, 101048. [Google Scholar] [CrossRef] [PubMed]
- Steber, C.R.; Ponnatapura, J.; Hughes, R.T.; Farris, M.K. Rapid Development of Clinically Symptomatic Radiation Recall Pneumonitis Immediately Following COVID-19 Vaccination. Cureus 2021, 13, e14303. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.W.; Ionescu, M.C.; Starkey, T.; Little, M.; Tilby, M.; Tripathy, A.R.; Mckenzie, H.S.; Al-Hajji, Y.; Appanna, N.; Barnard, M.; et al. COVID-19: Third dose booster vaccine effectiveness against breakthrough coronavirus infection, hospitalisations and death in patients with cancer: A population-based study. Eur. J. Cancer 2022, 175, 1–10. [Google Scholar] [CrossRef]
- Mariniello, D.F.; Aronne, L.; Vitale, M.; Schiattarella, A.; Pagliaro, R.; Komici, K. Current challenges and perspectives in lung cancer care during COVID-19 waves. Curr. Opin. Pulm. Med. 2023, 29, 239–247. [Google Scholar] [CrossRef]
- Prabani, K.I.P.; Weerasekara, I.; Damayanthi, H.D.W.T. COVID-19 vaccine acceptance and hesitancy among patients with cancer: A systematic review and meta-analysis. Public Health 2022, 212, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.Y.; Kim, S.I.; Park, E.Y.; Park, S.-Y.; Koh, S.-J.; Cha, Y.; Yoo, H.J.; Joung, J.Y.; Yoon, H.M.; Eom, B.W.; et al. Cancer Patients’ Willingness to Take COVID-19 Vaccination: A Nationwide Multicenter Survey in Korea. Cancers 2021, 13, 3883. [Google Scholar] [CrossRef]
- Thomas, S.J.; Perez, J.L.; Lockhart, S.P.; Hariharan, S.; Kitchin, N.; Bailey, R.; Liau, K.; Lagkadinou, E.; Türeci, Ö.; Şahin, U.; et al. Efficacy and safety of the BNT162b2 mRNA COVID-19 vaccine in participants with a history of cancer: Subgroup analysis of a global phase 3 randomized clinical trial. Vaccine 2022, 40, 1483–1492. [Google Scholar] [CrossRef]
- Shear, S.L.; Shams, K.; Weisberg, J.; Hamidi, N.; Scott, S.C. COVID-19 Vaccination Safety Profiles in Patients With Solid Tumour Cancers: A Systematic Review. Clin. Oncol. 2023, 35, e421–e433. [Google Scholar] [CrossRef]
- So, A.C.P.; McGrath, H.; Ting, J.; Srikandarajah, K.; Germanou, S.; Moss, C.; Russell, B.; Monroy-Iglesias, M.; Dolly, S.; Irshad, S.; et al. COVID-19 Vaccine Safety in Cancer Patients: A Single Centre Experience. Cancers 2021, 13, 3573. [Google Scholar] [CrossRef]
- Becerril-Gaitan, A.; Vaca-Cartagena, B.F.; Ferrigno, A.S.; Mesa-Chavez, F.; Barrientos-Gutiérrez, T.; Tagliamento, M.; Lambertini, M.; Villarreal-Garza, C. Immunogenicity and risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection after Coronavirus Disease 2019 (COVID-19) vaccination in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2022, 160, 243–260. [Google Scholar] [CrossRef]
- Yin, J.; Chen, Y.; Li, Y.; Zhang, X.; Wang, C. Seroconversion rate after COVID-19 vaccination in patients with solid cancer: A systematic review and meta-analysis. Hum. Vaccines Immunother. 2022, 18, 2119763. [Google Scholar] [CrossRef]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451.E17. [Google Scholar] [CrossRef] [PubMed]
- Martins-Branco, D.; Nader-Marta, G.; Tecic Vuger, A.; Debien, V.; Ameye, L.; Brandão, M.; Punie, K.; Loizidou, A.; Willard-Gallo, K.; Spilleboudt, C.; et al. Immune response to anti-SARS-CoV-2 prime-vaccination in patients with cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2023, 149, 3075–3080. [Google Scholar] [CrossRef]
- Gong, I.Y.; Vijenthira, A.; Betschel, S.D.; Hicks, L.K.; Cheung, M.C. COVID-19 vaccine response in patients with hematologic malignancy: A systematic review and meta-analysis. Am. J. Hematol. 2022, 97, E132–E135. [Google Scholar] [CrossRef]
- Tang, K.; Wei, Z.; Wu, X. Impaired serological response to COVID-19 vaccination following anticancer therapy: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 4860–4868. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, D.; Li, Z.; Zhang, K. Predictors of poor serologic response to COVID-19 vaccine in patients with cancer: A systematic review and meta-analysis. Eur. J. Cancer 2022, 172, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rachman, A.; Iriani, A.; Sukrisman, L.; Rajabto, W.; Mulansari, N.A.; Lubis, A.M.; Cahyanur, R.; Prasetyawati, F.; Priantono, D.; Rumondor, B.B.; et al. A comparative study of the COVID-19 vaccine efficacy among cancer patients: mRNA versus non-mRNA. PLoS ONE 2023, 18, e0281907. [Google Scholar] [CrossRef] [PubMed]
- Geinitz, H.; Zimmermann, F.B.; Stoll, P.; Thamm, R.; Kaffenberger, W.; Ansorg, K.; Keller, M.; Busch, R.; Van Beuningen, D.; Molls, M. Fatigue, serum cytokine levels, and blood cell counts during radiotherapy of patients with breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 691–698. [Google Scholar] [CrossRef]
- Sage, E.K.; Schmid, T.E.; Sedelmayr, M.; Gehrmann, M.; Geinitz, H.; Duma, M.N.; Combs, S.E.; Multhoff, G. Comparative analysis of the effects of radiotherapy versus radiotherapy after adjuvant chemotherapy on the composition of lymphocyte subpopulations in breast cancer patients. Radiother. Oncol. 2016, 118, 176–180. [Google Scholar] [CrossRef]
- Mai, A.S.; Lee, A.R.Y.B.; Tay, R.Y.K.; Shapiro, L.; Thakkar, A.; Halmos, B.; Grinshpun, A.; Herishanu, Y.; Benjamini, O.; Tadmor, T.; et al. Booster doses of COVID-19 vaccines for patients with haematological and solid cancer: A systematic review and individual patient data meta-analysis. Eur. J. Cancer 2022, 172, 65–75. [Google Scholar] [CrossRef]
- Petrelli, F.; Luciani, A.; Borgonovo, K.; Ghilardi, M.; Parati, M.C.; Petrò, D.; Lonati, V.; Pesenti, A.; Cabiddu, M. Third dose of SARS-CoV-2 vaccine: A systematic review of 30 published studies. J. Med. Virol. 2022, 94, 2837–2844. [Google Scholar] [CrossRef]
- Al Hajji, Y.; Taylor, H.; Starkey, T.; Lee, L.Y.W.; Tilby, M. Antibody response to a third booster dose of SARS-CoV-2 vaccination in adults with haematological and solid cancer: A systematic review. Br. J. Cancer 2022, 127, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Antonarelli, G.; Scotté, F.; Spano, J.P.; Barrière, J.; Michot, J.M.; André, F.; Curigliano, G. Seroconversion rate after vaccination against COVID-19 in patients with cancer—A systematic review. Ann. Oncol. 2022, 33, 158–168. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).