Replication of Vectored Herpesvirus of Turkey (HVT) in a Continuous, Microcarrier-Independent Suspension Cell Line from Muscovy Duck
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Infection of Adherent Cells
2.3. Infection of Suspension Cultures
2.4. DNA Extraction and Droplet Digital PCR (ddPCR) for Quantification of HVT Genome Copy Numbers
2.5. Focus Quantification
2.6. Software
3. Results
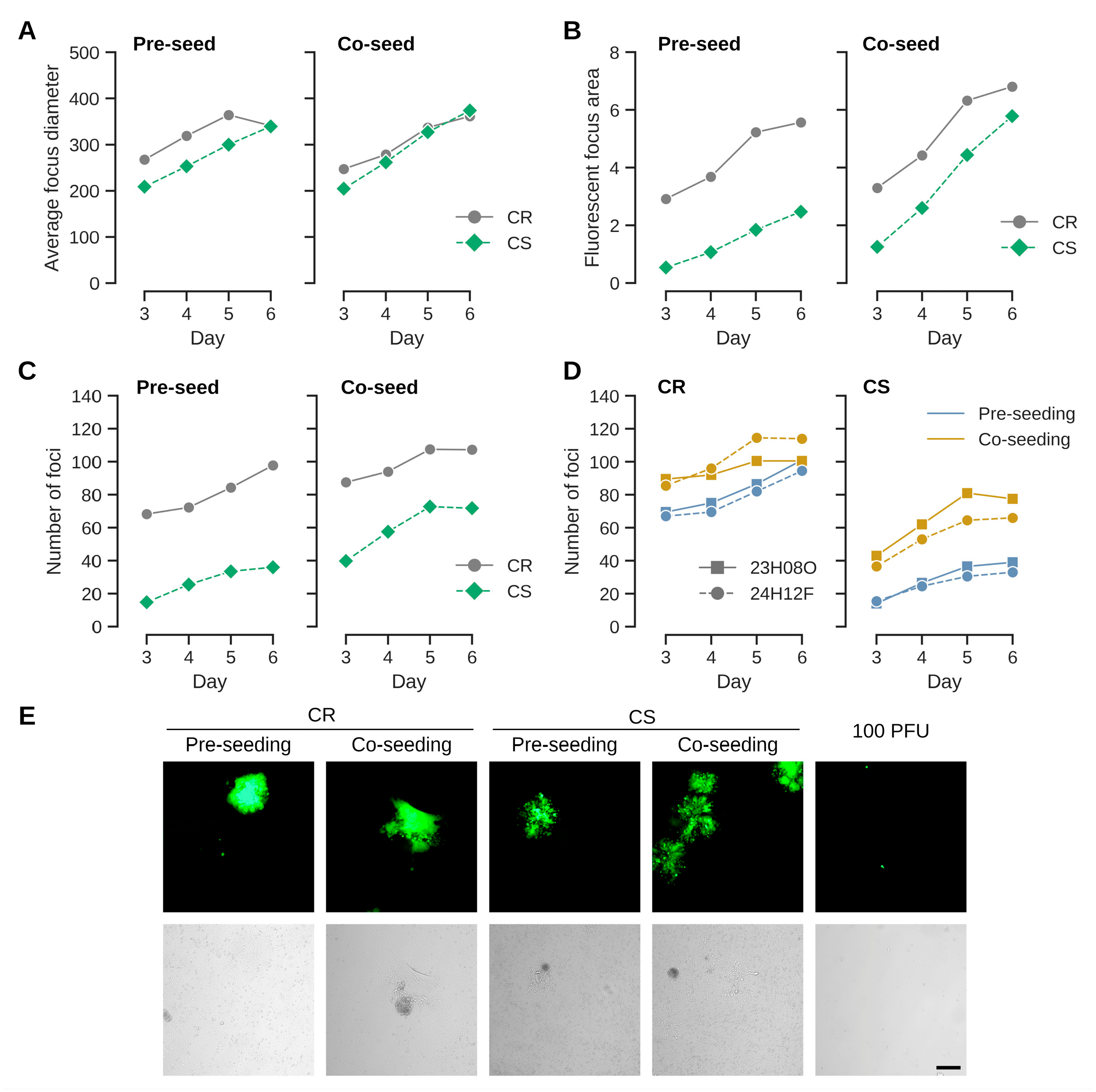
3.1. Co-Seeding (As Opposed to Overlay Infection) Appears to Improve Virus Transfer
3.2. HVT Infects and Replicates in Adherent Cairina Moschata Cell Lines from Different Lineages
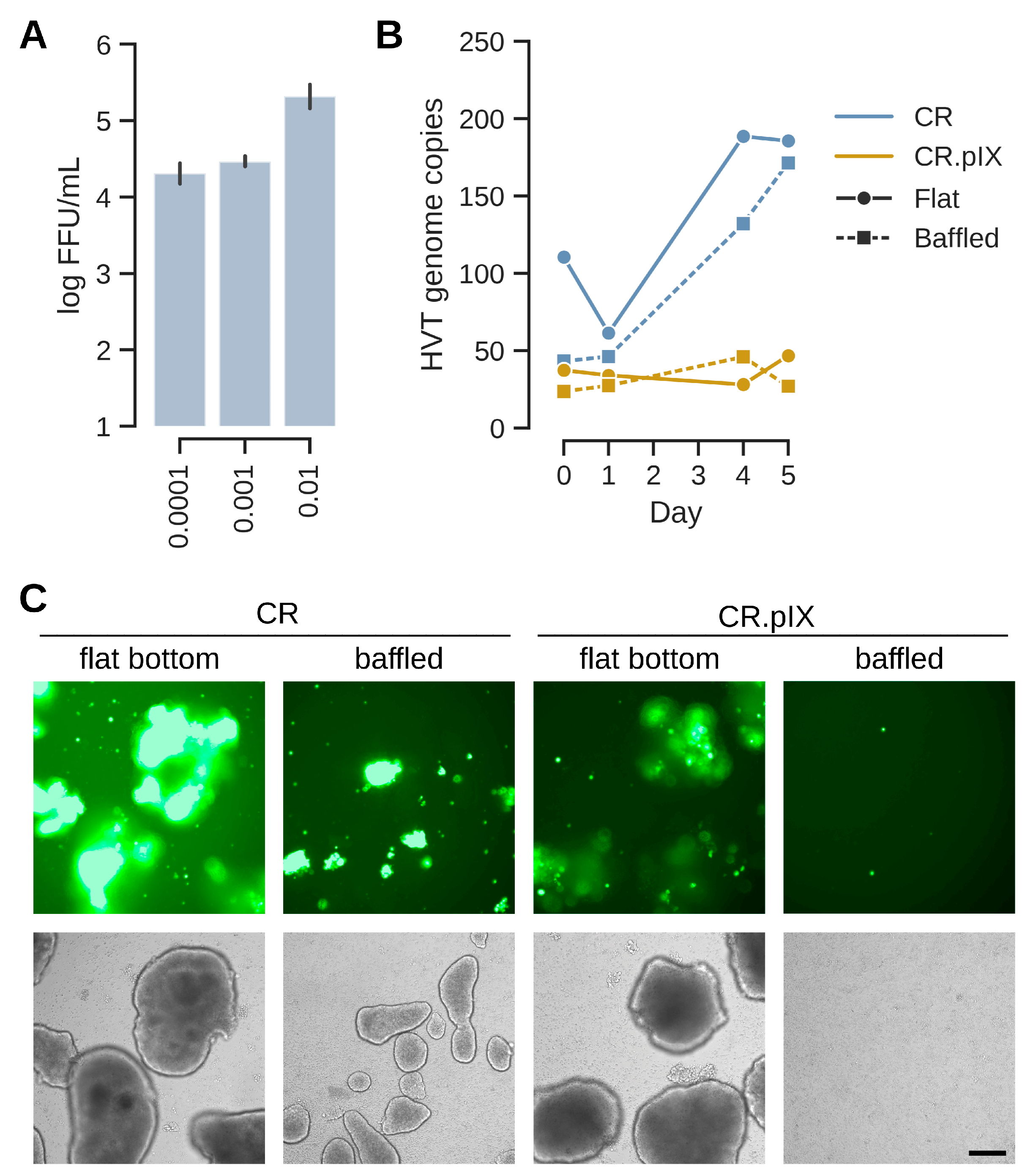
3.3. HVT Can Be Propagated in Microcarrier-Independent Suspension Cells
3.4. Metabolic Stressor Improves Titers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheppard, M. Viral Vectors for Veterinary Vaccines. Adv. Vet. Med. 1999, 41, 145. [Google Scholar] [PubMed]
- Okura, T.; Taneno, A.; Oishi, E. Cell-to-Cell Transmission of Turkey Herpesvirus in Chicken Embryo Cells via Tunneling Nanotubes. Avian Dis. 2021, 65, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Okura, T.; Otomo, H.; Taneno, A.; Oishi, E. Replication Kinetics of Turkey Herpesvirus in Lymphoid Organs and Feather Follicle Epithelium in Chickens. J. Vet. Med. Sci. 2021, 83, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Palya, V.; Tatár-Kis, T.; Mató, T.; Felföldi, B.; Kovács, E.; Gardin, Y. Onset and Long-Term Duration of Immunity Provided by a Single Vaccination with a Turkey Herpesvirus Vector ND Vaccine in Commercial Layers. Vet. Immunol. Immunopathol. 2014, 158, 105–115. [Google Scholar] [CrossRef]
- Fiddaman, S.R.; Dimopoulos, E.A.; Lebrasseur, O.; du Plessis, L.; Vrancken, B.; Charlton, S.; Haruda, A.F.; Tabbada, K.; Flammer, P.G.; Dascalu, S.; et al. Ancient Chicken Remains Reveal the Origins of Virulence in Marek’s Disease Virus. Science 2023, 382, 1276–1281. [Google Scholar] [CrossRef]
- Trapp, S.; Osterrieder, N. Herpesviruses of Birds. In Desk Encyclopedia of Animal and Bacterial Virology; Elsevier: Amsterdam, The Netherlands, 2010; p. 415. [Google Scholar]
- Reemers, S.; Verstegen, I.; Basten, S.; Hubers, W.; van de Zande, S. A Broad Spectrum HVT-H5 Avian Influenza Vector Vaccine Which Induces a Rapid Onset of Immunity. Vaccine 2021, 39, 1072–1079. [Google Scholar] [CrossRef]
- Read, A.F.; Baigent, S.J.; Powers, C.; Kgosana, L.B.; Blackwell, L.; Smith, L.P.; Kennedy, D.A.; Walkden-Brown, S.W.; Nair, V.K. Imperfect Vaccination Can Enhance the Transmission of Highly Virulent Pathogens. PLoS Biol. 2015, 13, e1002198. [Google Scholar] [CrossRef]
- Uscher-Pines, L.; Barnett, D.J.; Sapsin, J.W.; Bishai, D.M.; Balicer, R.D. A Systematic Analysis of Influenza Vaccine Shortage Policies. Public Health 2008, 122, 183–191. [Google Scholar] [CrossRef]
- Geerligs, H.; Quanz, S.; Suurland, B.; Spijkers, I.E.M.; Rodenberg, J.; Davelaar, F.G.; Jongsma, B.; Kumar, M. Efficacy and Safety of Cell Associated Vaccines against Marek’s Disease Virus Grown in a Continuous Cell Line from Chickens. Vaccine 2008, 26, 5595–5600. [Google Scholar] [CrossRef]
- Rong, S.; Wheeler, D.; Weber, F. Efficient Marek’s Disease Virus (MDV) and Herpesvirus of Turkey Infection of the QM7 Cell Line That Does Not Contain Latent MDV Genome. Avian Pathol. J. WVPA 2014, 43, 414–419. [Google Scholar] [CrossRef]
- Vautherot, J.-F.; Jean, C.; Fragnet-Trapp, L.; Rémy, S.; Chabanne-Vautherot, D.; Montillet, G.; Fuet, A.; Denesvre, C.; Pain, B. ESCDL-1, a New Cell Line Derived from Chicken Embryonic Stem Cells, Supports Efficient Replication of Mardiviruses. PLoS ONE 2017, 12, e0175259. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhang, A.; Li, Y.; Lai, H.; Li, H.; Luo, Q.; Jin, S.; Chen, R. Suspension Culture of Marek’s Disease Virus and Evaluation of Its Immunological Effects. Avian Pathol. J. WVPA 2019, 48, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Vautherot, J.-F.; Pain, B.; Denesvre, C.; Fragnet-Trapp, L. Method for Selecting a Permissive Cell Line for Replicating Avian Viruses. U.S. Patent No. 10,053,672, 21 August 2018. [Google Scholar]
- Geerligs, H.; Spijkers, I.; Rodenberg, J. Efficacy and Safety of Cell-Associated Vaccines against Marek’s Disease Virus Grown in QT35 Cells or JBJ-1 Cells. Avian Dis. 2013, 57, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Kaplan, S.L.; Wakenell, P.; Schat, K.A. Transactivation of Latent Marek’s Disease Herpesvirus Genes in QT35, a Quail Fibroblast Cell Line, by Herpesvirus of Turkeys. J. Virol. 2000, 74, 10176–10186. [Google Scholar] [CrossRef]
- Briggs, D.J. The Role of Vaccination in Rabies Prevention. Curr. Opin. Virol. 2012, 2, 309–314. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.Y.; Ryu, K.-H.; Kim, A.-Y.; Kim, J.; Ko, Y.-J.; Lee, E.G. Production of a Foot-and-Mouth Disease Vaccine Antigen Using Suspension-Adapted BHK-21 Cells in a Bioreactor. Vaccines 2021, 9, 505. [Google Scholar] [CrossRef]
- Sanders, B.P.; Edo-Matas, D.; Custers, J.H.H.V.; Koldijk, M.H.; Klaren, V.; Turk, M.; Luitjens, A.; Bakker, W.A.M.; Uytdehaag, F.; Goudsmit, J.; et al. PER.C6® Cells as a Serum-Free Suspension Cell Platform for the Production of High Titer Poliovirus: A Potential Low Cost of Goods Option for World Supply of Inactivated Poliovirus Vaccine. Vaccine 2013, 31, 850–856. [Google Scholar] [CrossRef]
- Shittu, I.; Zhu, Z.; Lu, Y.; Hutcheson, J.M.; Stice, S.L.; West, F.D.; Donadeu, M.; Dungu, B.; Fadly, A.M.; Zavala, G. Development, Characterization and Optimization of a New Suspension Chicken-Induced Pluripotent Cell Line for the Production of Newcastle Disease Vaccine. Biologicals 2016, 44, 24–32. [Google Scholar] [CrossRef]
- Jordan, I.; Vos, A.; Beilfuss, S.; Neubert, A.; Breul, S.; Sandig, V. An Avian Cell Line Designed for Production of Highly Attenuated Viruses. Vaccine 2009, 27, 748–756. [Google Scholar] [CrossRef]
- Parks, R.J. Adenovirus Protein IX: A New Look at an Old Protein. Mol. Ther. J. Am. Soc. Gene Ther. 2005, 11, 19–25. [Google Scholar] [CrossRef]
- Baigent, S.J.; Petherbridge, L.J.; Smith, L.P.; Zhao, Y.; Chesters, P.M.; Nair, V.K. Herpesvirus of Turkey Reconstituted from Bacterial Artificial Chromosome Clones Induces Protection against Marek’s Disease. J. Gen. Virol. 2006, 87, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Denesvre, C.; You, Y.; Rémy, S.; Vychodil, T.; Courvoisier, K.; Penzes, Z.; Bertzbach, L.D.; Kheimar, A.; Kaufer, B.B. Impact of Viral Telomeric Repeat Sequences on Herpesvirus Vector Vaccine Integration and Persistence. PLoS Pathog. 2024, 20, e1012261. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; Kohn, M.; You, Y.; Kossak, L.; Sabsabi, M.A.; Kheimar, A.; Härtle, S.; Kaufer, B.B. In Vitro Infection of Primary Chicken Lymphocytes with Marek’s Disease Virus. STAR Protoc. 2023, 4, 102343. [Google Scholar] [CrossRef] [PubMed]
- Jordan, I.; John, K.; Höwing, K.; Lohr, V.; Penzes, Z.; Gubucz-Sombor, E.; Fu, Y.; Gao, P.; Harder, T.; Zádori, Z.; et al. Continuous Cell Lines from the Muscovy Duck as Potential Replacement for Primary Cells in the Production of Avian Vaccines. Avian Pathol. J. WVPA 2016, 45, 137–155. [Google Scholar] [CrossRef]
- Jordan, I.; Northoff, S.; Thiele, M.; Hartmann, S.; Horn, D.; Höwing, K.; Bernhardt, H.; Oehmke, S.; von Horsten, H.; Rebeski, D.; et al. A Chemically Defined Production Process for Highly Attenuated Poxviruses. Biol. J. Int. Assoc. Biol. Stand. 2011, 39, 50–58. [Google Scholar] [CrossRef]
- Wood, M.L.; Neumann, R.; Roy, P.; Nair, V.; Royle, N.J. Characterization of Integrated Marek’s Disease Virus Genomes Supports a Model of Integration by Homology-Directed Recombination and Telomere-Loop-Driven Excision. J. Virol. 2023, 97, e00716-23. [Google Scholar] [CrossRef]
- Islam, A.; Harrison, B.; Cheetham, B.F.; Mahony, T.J.; Young, P.L.; Walkden-Brown, S.W. Differential Amplification and Quantitation of Marek’s Disease Viruses Using Real-Time Polymerase Chain Reaction. J. Virol. Methods 2004, 119, 103–113. [Google Scholar] [CrossRef]
- de Boer, G.F.; Groenendal, J.E.; Boerrigter, H.M.; Kok, G.L.; Pol, J.M. Protective Efficacy of Marek’s Disease Virus (MDV) CVI-988 CEF65 Clone C against Challenge Infection with Three Very Virulent MDV Strains. Avian Dis. 1986, 30, 276–283. [Google Scholar] [CrossRef]
- Rispens, B.H.; van Vloten, H.; Mastenbroek, N.; Maas, H.J.; Schat, K.A. Control of Marek’s Disease in the Netherlands. I. Isolation of an Avirulent Marek’s Disease Virus (Strain CVI 988) and Its Use in Laboratory Vaccination Trials. Avian Dis. 1972, 16, 108–125. [Google Scholar] [CrossRef]
- Handberg, K.J.; Nielsen, O.L.; Jørgensen, P.H. The Use of Serotype 1- and Serotype 3-Specific Polymerase Chain Reaction for the Detection of Marek’s Disease Virus in Chickens. Avian Pathol. 2001, 30, 243–249. [Google Scholar] [CrossRef]
- Delecluse, H.J.; Schüller, S.; Hammerschmidt, W. Latent Marek’s Disease Virus Can Be Activated from Its Chromosomally Integrated State in Herpesvirus-Transformed Lymphoma Cells. EMBO J. 1993, 12, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Bertzbach, L.D.; You, Y.; Vychodil, T.; Kheimar, A.; Kossak, L.; Sabsabi, M.A.; Conradie, A.M.; Kaufer, B.B. Role of the Multiple Telomeric Repeat Arrays in Integration, Persistence, and Efficacy of the Commercial CVI988 Vaccine. mSphere 2025, 10, e0014225. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Kheimar, A.M.; Vychodil, T.; Kossak, L.; Sabsabi, M.A.; Conradie, A.M.; Reddy, S.M.; Bertzbach, L.D.; Kaufer, B.B. Telomeric Repeats in the Commercial SB-1 Vaccine Facilitate Viral Integration and Contribute to Vaccine Efficacy. NPJ Vaccines 2024, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, W.N.; Vasoya, D.; Kgosana, L.B.; Watson, M.; Nair, V. Differentially Expressed Genes during Spontaneous Lytic Switch of Marek’s Disease Virus in Lymphoblastoid Cell Lines Determined by Global Gene Expression Profiling. J. Gen. Virol. 2017, 98, 779–790. [Google Scholar] [CrossRef]
- Robinson, C.M.; Cheng, H.H.; Delany, M.E. Temporal Kinetics of Marek’s Disease Herpesvirus: Integration Occurs Early after Infection in Both B and T Cells. Cytogenet. Genome Res. 2014, 144, 142–154. [Google Scholar] [CrossRef]
- Li, H.; Zhu, J.; He, M.; Luo, Q.; Liu, F.; Chen, R. Marek’s Disease Virus Activates the PI3K/Akt Pathway Through Interaction of Its Protein Meq With the P85 Subunit of PI3K to Promote Viral Replication. Front. Microbiol. 2018, 9, 2547. [Google Scholar] [CrossRef]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like Growth Factors and Neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef]
- Baigent, S.J.; Smith, L.P.; Currie, R.J.W.; Nair, V.K. Replication Kinetics of Marek’s Disease Vaccine Virus in Feathers and Lymphoid Tissues Using PCR and Virus Isolation. J. Gen. Virol. 2005, 86, 2989–2998. [Google Scholar] [CrossRef]
- Palya, V.; Kiss, I.; Tatár-Kis, T.; Mató, T.; Felföldi, B.; Gardin, Y. Advancement in Vaccination against Newcastle Disease: Recombinant HVT NDV Provides High Clinical Protection and Reduces Challenge Virus Shedding with the Absence of Vaccine Reactions. Avian Dis. 2012, 56, 282–287. [Google Scholar] [CrossRef]
- Zinnecker, T.; Reichl, U.; Genzel, Y. Innovations in Cell Culture-Based Influenza Vaccine Manufacturing—From Static Cultures to High Cell Density Cultivations. Hum. Vaccines Immunother. 2024, 20, 2373521. [Google Scholar] [CrossRef]
- Bodiou, V.; Moutsatsou, P.; Post, M.J. Microcarriers for Upscaling Cultured Meat Production. Front. Nutr. 2020, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Denesvre, C. Marek’s Disease Virus Morphogenesis. Avian Dis. 2013, 57, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.M.; Graham, C.K. Influence of Maternal Antibody on Efficacy of Embryo Vaccination with Cell-Associated and Cell-Free Marek’s Disease Vaccine. Avian Dis. 1982, 26, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Witter, R.L.; Burmester, B.R. Differential Effect of Maternal Antibodies on Efficacy of Cellular and Cell-Free Marek’s Disease Vaccines. Avian Pathol. J. WVPA 1979, 8, 145–156. [Google Scholar] [CrossRef]
- Witter, R.L. Protection by Attenuated and Polyvalent Vaccines against Highly Virulent Strains of Marek’s Disease Virus. Avian Pathol. 1982, 11, 49–62. [Google Scholar] [CrossRef]




| Target | 5′→3′ |
|---|---|
| sORF-1 forward | GGCAGACACCGCGTTGTAT |
| sORF-1 reverse | TGTCCACGCTCGAGACTATCC |
| sORF-1 probe | HEX-AACCCGGGCTTGTGGACGTCTTC-BHQ1 |
| GFP forward | GCACAAGCTGGAGTACAACTA |
| GFP reverse | TGTTGTGGCGGATCTTGAA |
| GFP probe | HEX-CAAGCAGAAGAACGGCATCAAGGC-BHQ1 |
| E1A forward | TGACTCCGGTCCTTCTAACACA |
| E1A reverse | TCACGGCAACTGGTTTAATGG |
| E1A probe | YAK-CCCGGTGGTCCCGCTGTGC-BHQ1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mähl, K.; Horn, D.; Abidi, S.; Kaufer, B.B.; Sandig, V.; Karlas, A.; Jordan, I. Replication of Vectored Herpesvirus of Turkey (HVT) in a Continuous, Microcarrier-Independent Suspension Cell Line from Muscovy Duck. Vaccines 2025, 13, 714. https://doi.org/10.3390/vaccines13070714
Mähl K, Horn D, Abidi S, Kaufer BB, Sandig V, Karlas A, Jordan I. Replication of Vectored Herpesvirus of Turkey (HVT) in a Continuous, Microcarrier-Independent Suspension Cell Line from Muscovy Duck. Vaccines. 2025; 13(7):714. https://doi.org/10.3390/vaccines13070714
Chicago/Turabian StyleMähl, Karoline, Deborah Horn, Sirine Abidi, Benedikt B. Kaufer, Volker Sandig, Alexander Karlas, and Ingo Jordan. 2025. "Replication of Vectored Herpesvirus of Turkey (HVT) in a Continuous, Microcarrier-Independent Suspension Cell Line from Muscovy Duck" Vaccines 13, no. 7: 714. https://doi.org/10.3390/vaccines13070714
APA StyleMähl, K., Horn, D., Abidi, S., Kaufer, B. B., Sandig, V., Karlas, A., & Jordan, I. (2025). Replication of Vectored Herpesvirus of Turkey (HVT) in a Continuous, Microcarrier-Independent Suspension Cell Line from Muscovy Duck. Vaccines, 13(7), 714. https://doi.org/10.3390/vaccines13070714






