BCG Vaccination Potentially Modulates the Transcriptome of Infant CD4 T Cells in Addition to Age-Dependent Immune Ontogeny-Associated Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Infant Study Subjects and Sample Collection
2.2. PBMC Isolation
2.3. CD4 T Cell and Monocyte Isolation by Fluorescence-Activated Cell Sorting (FACS)
2.4. RNA Isolation, Library Preparation, and RNA Sequencing
2.5. RNA Sequencing Data Analysis
3. Results
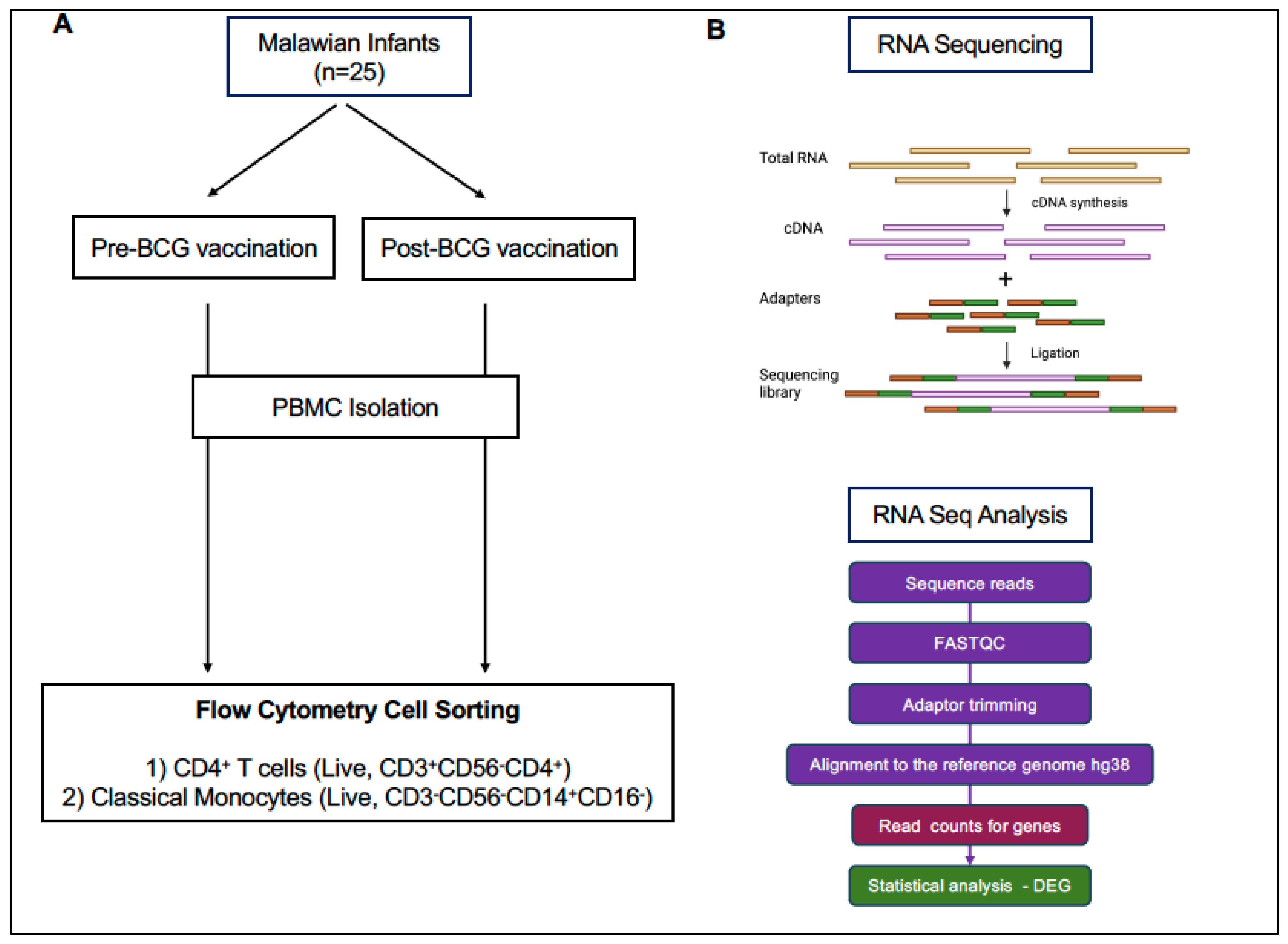
3.1. Study Design, Sample Collection, and Data Analysis
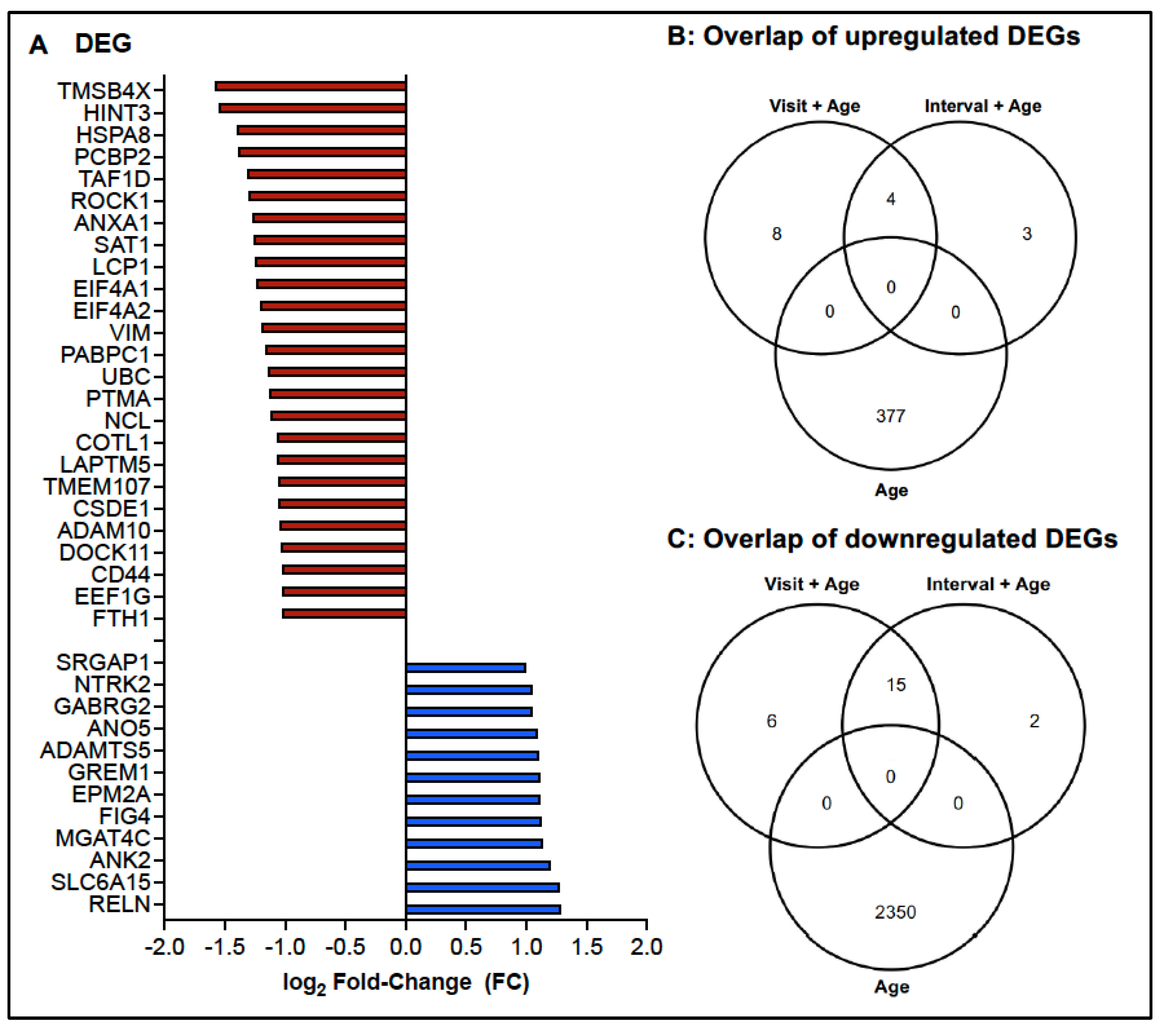
3.2. Age-Dependent Changes in the Transcriptome of CD4 T Cells
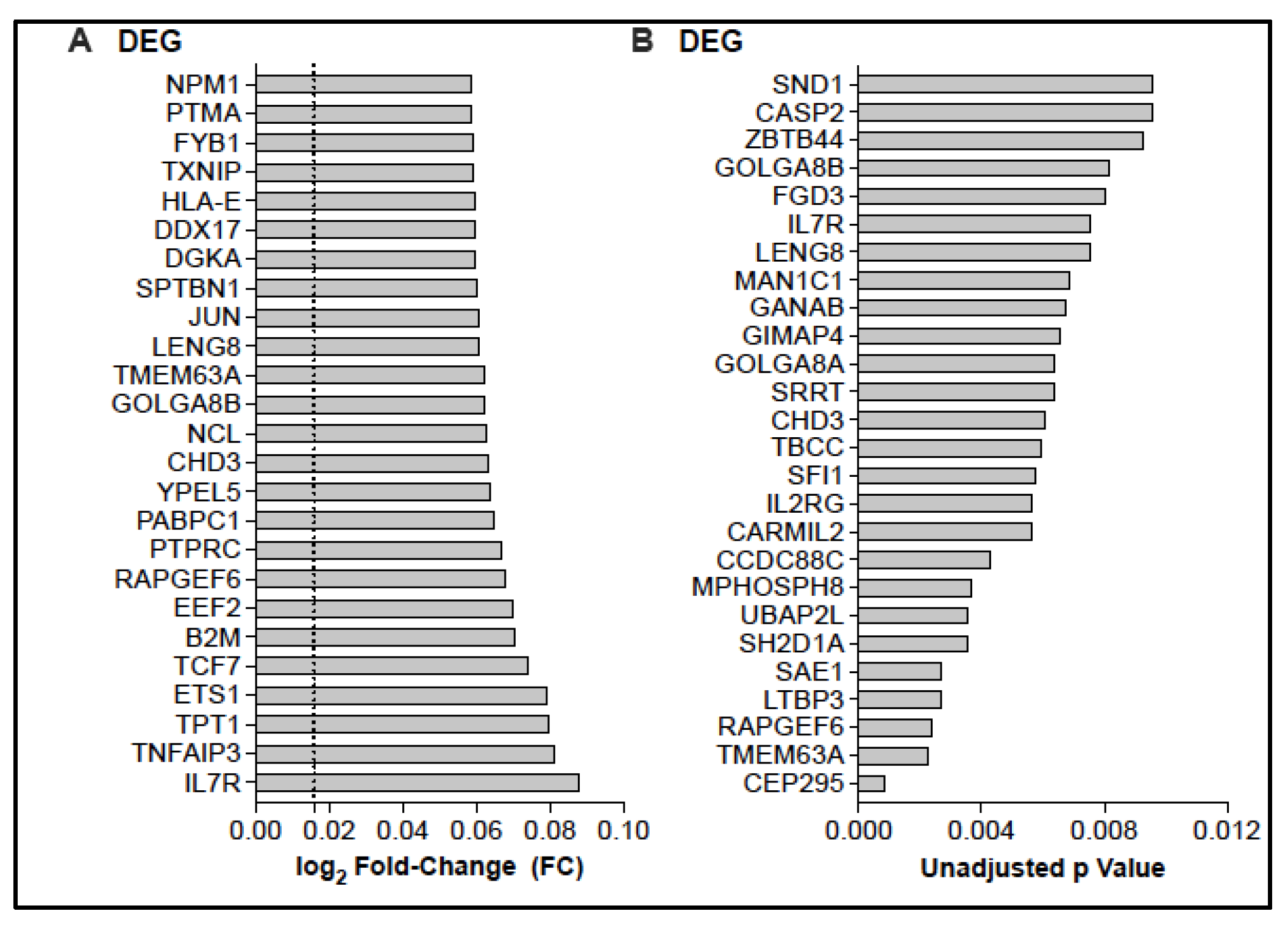
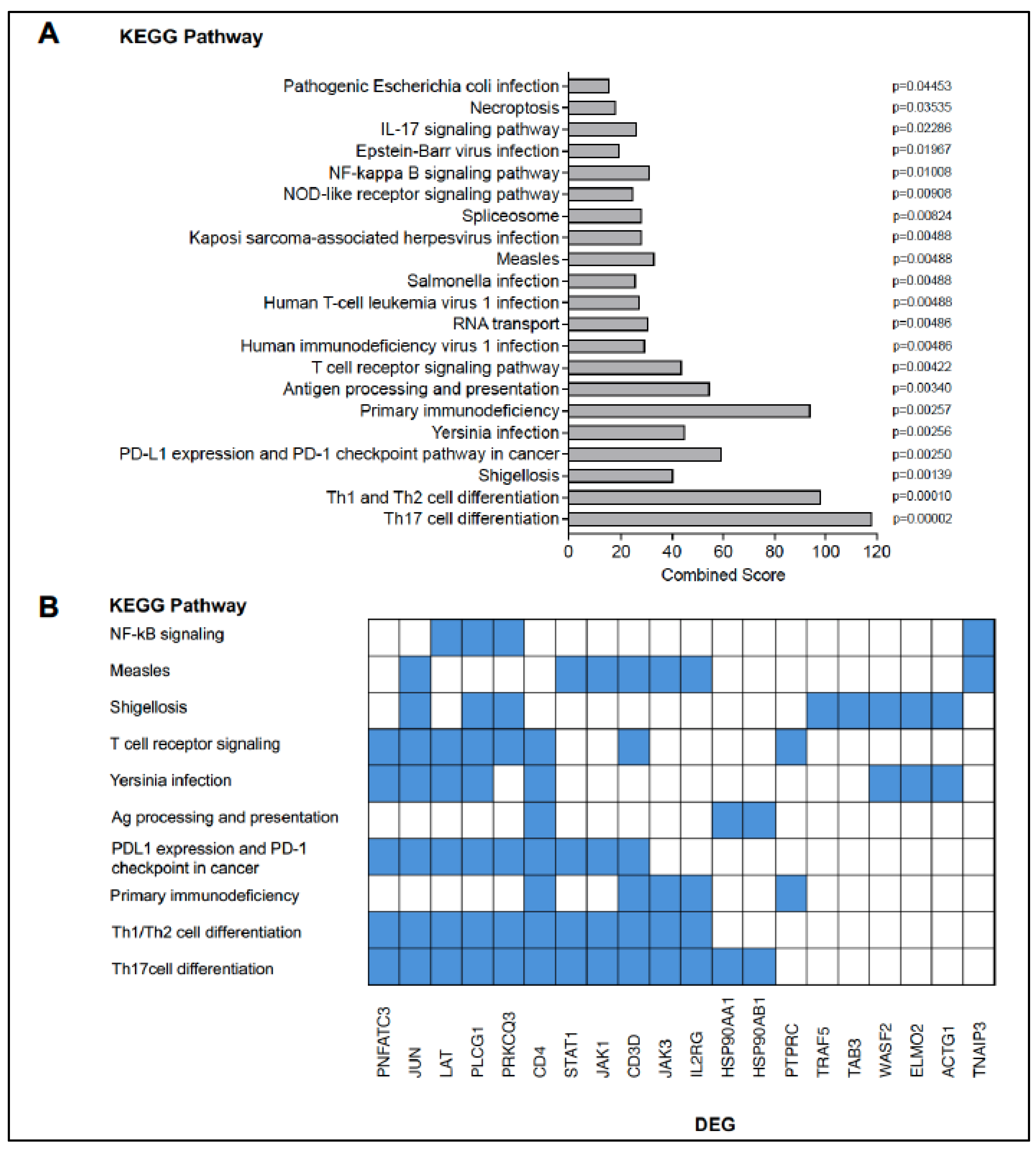
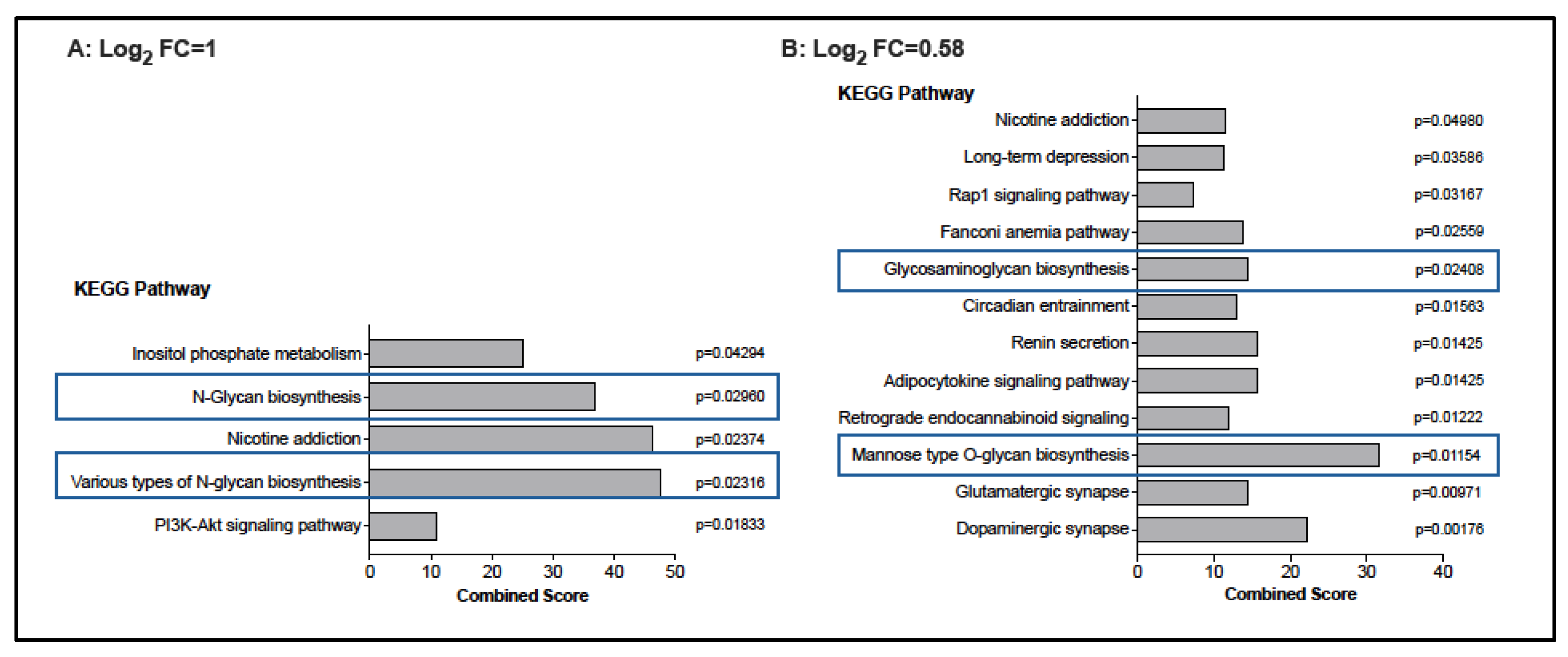
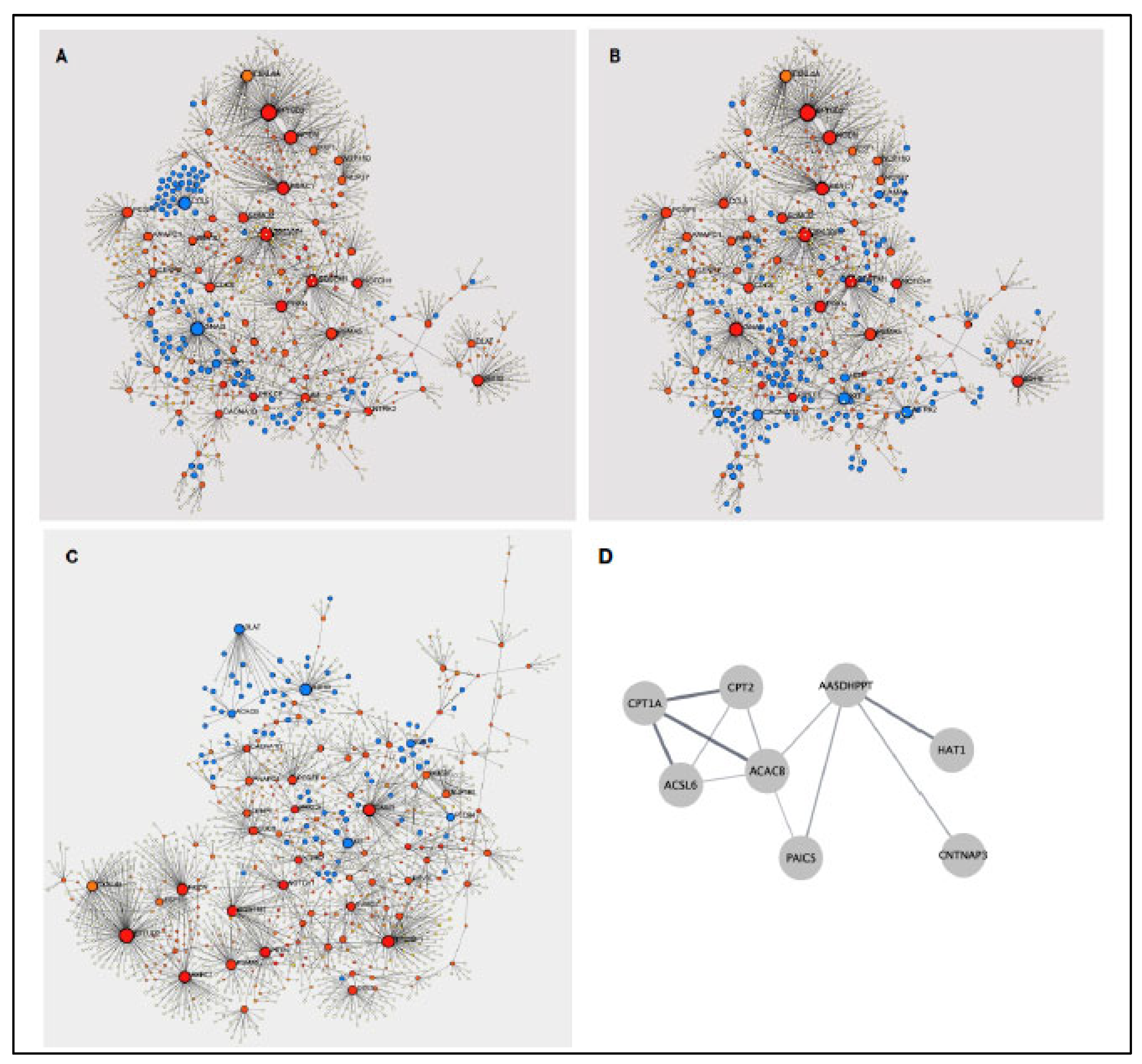
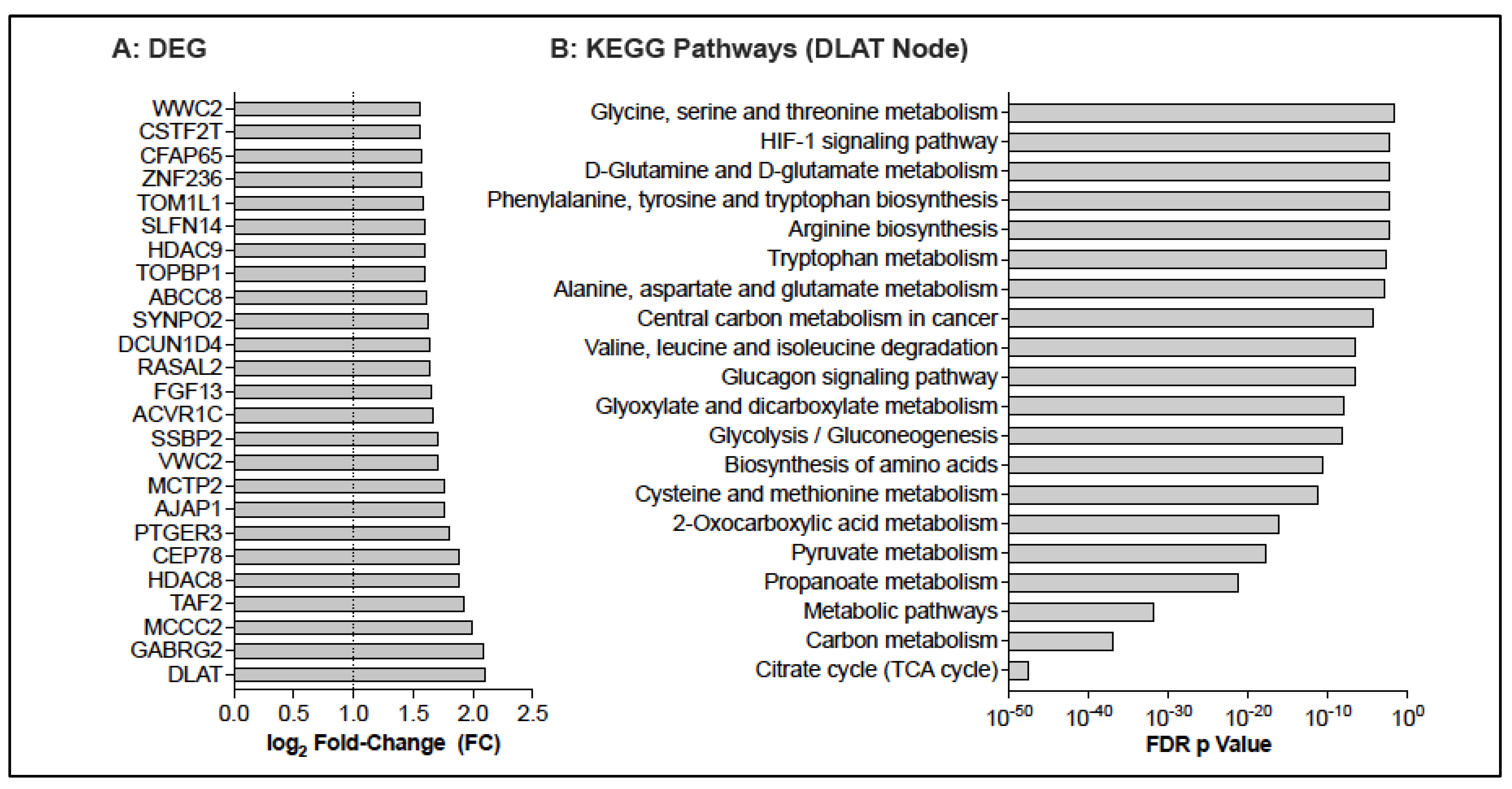
3.3. Potential Changes in the CD4 T Cell Transcriptome Associated with BCG Vaccination
3.4. Transcriptome Changes in Infant Peripheral Blood Monocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tagliabue, A.; Boraschi, D.; Leite, L.C.C.; Kaufmann, S.H.E. 100 Years of BCG Immunization: Past, Present, and Future. Vaccines 2022, 10, 1743. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.I.; Sackett, M.F. Results of BCG immunization in New York City. Am. Rev. Tuberc. 1946, 53, 517–532. [Google Scholar] [PubMed]
- Benn, C.S.; Netea, M.G.; Selin, L.K.; Aaby, P. A small jab-a big effect: Nonspecific immunomodulation by vaccines. Trends Immunol. 2013, 34, 431–439. [Google Scholar] [CrossRef]
- Cirovic, B.; de Bree, L.C.J.; Groh, L.; Blok, B.A.; Chan, J.; van der Velden, W.; Bremmers, M.E.J.; van Crevel, R.; Handler, K.; Picelli, S.; et al. BCG Vaccination in Humans Elicits Trained Immunity via the Hematopoietic Progenitor Compartment. Cell Host Microbe 2020, 28, 322–334.e5. [Google Scholar] [CrossRef]
- Soto, J.A.; Galvez, N.M.S.; Andrade, C.A.; Ramirez, M.A.; Riedel, C.A.; Kalergis, A.M.; Bueno, S.M. BCG vaccination induces cross-protective immunity against pathogenic microorganisms. Trends Immunol. 2022, 43, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Gustafson, P.; Nhaga, A.; Djana, Q.; Poulsen, A.; Garly, M.L.; Jensen, H.; Sodemann, M.; Rodriques, A.; Aaby, P. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int. J. Epidemiol. 2005, 34, 540–547. [Google Scholar] [CrossRef]
- Bates, M.N.; Herron, T.J.; Lwi, S.J.; Baldo, J.V. BCG vaccination at birth and COVID-19: A case-control study among U.S. military Veterans. Hum. Vaccines Immunother. 2022, 18, 1981084. [Google Scholar] [CrossRef]
- Chowdhury, U.N.; Faruqe, M.O.; Mehedy, M.; Ahmad, S.; Islam, M.B.; Shoombuatong, W.; Azad, A.K.M.; Moni, M.A. Effects of Bacille Calmette Guerin (BCG) vaccination during COVID-19 infection. Comput. Biol. Med. 2021, 138, 104891. [Google Scholar] [CrossRef]
- Negi, K.; Bhaskar, A.; Dwivedi, V.P. Progressive Host-Directed Strategies to Potentiate BCG Vaccination Against Tuberculosis. Front. Immunol. 2022, 13, 944183. [Google Scholar] [CrossRef]
- Liu, C.H.; Liu, H.; Ge, B. Innate immunity in tuberculosis: Host defense vs pathogen evasion. Cell Mol. Immunol. 2017, 14, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef]
- Diray-Arce, J.; Angelidou, A.; Jensen, K.J.; Conti, M.G.; Kelly, R.S.; Pettengill, M.A.; Liu, M.; van Haren, S.D.; McCulloch, S.D.; Michelloti, G.; et al. Bacille Calmette-Guerin vaccine reprograms human neonatal lipid metabolism in vivo and in vitro. Cell Rep. 2022, 39, 110772. [Google Scholar] [CrossRef]
- Kumar, R.; Kolloli, A.; Singh, P.; Shi, L.; Kupz, A.; Subbian, S. The innate memory response of macrophages to Mycobacterium tuberculosis is shaped by the nature of the antigenic stimuli. Microbiol. Spectr. 2024, 12, e0047324. [Google Scholar] [CrossRef]
- Bekkering, S.; Blok, B.A.; Joosten, L.A.; Riksen, N.P.; van Crevel, R.; Netea, M.G. In Vitro Experimental Model of Trained Innate Immunity in Human Primary Monocytes. Clin. Vaccine Immunol. 2016, 23, 926–933. [Google Scholar] [CrossRef]
- Butkeviciute, E.; Jones, C.E.; Smith, S.G. Heterologous effects of infant BCG vaccination: Potential mechanisms of immunity. Future Microbiol. 2018, 13, 1193–1208. [Google Scholar] [CrossRef] [PubMed]
- McCoy, K.D.; Ignacio, A.; Geuking, M.B. Microbiota and Type 2 immune responses. Curr. Opin. Immunol. 2018, 54, 20–27. [Google Scholar] [CrossRef]
- Frenkel, L.D. The global burden of vaccine-preventable infectious diseases in children less than 5 years of age: Implications for COVID-19 vaccination. How can we do better? Allergy Asthma Proc. 2021, 42, 378–385. [Google Scholar] [CrossRef]
- Kelly, M.S.; Cataldi, J.R.; Schlaudecker, E.P.; Shah, S.S.; Vinci, R.J.; Myers, A.L. Child Health Needs and the Pediatric Infectious Diseases Workforce: 2020–2040. Pediatrics 2024, 153 (Suppl. 2), e2023063678N. [Google Scholar] [CrossRef]
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000-19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child. Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Black, R.E. Global maternal, newborn, and child health—So near and yet so far. N. Engl. J. Med. 2013, 369, 2226–2235. [Google Scholar] [CrossRef]
- Moorlag, S.; Arts, R.J.W.; van Crevel, R.; Netea, M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019, 25, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Dela Pena-Ponce, M.G.; Rodriguez-Nieves, J.; Bernhardt, J.; Tuck, R.; Choudhary, N.; Mengual, M.; Mollan, K.R.; Hudgens, M.G.; Peter-Wohl, S.; De Paris, K. Increasing JAK/STAT Signaling Function of Infant CD4+ T Cells during the First Year of Life. Front. Pediatr. 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.D., 2nd; Dennis, M.; Eudailey, J.; Walter, K.L.; Cronin, K.; Alam, S.M.; Choudhary, N.; Tuck, R.H.; Hudgens, M.; Kozlowski, P.A.; et al. HIV Env-Specific IgG Antibodies Induced by Vaccination of Neonatal Rhesus Macaques Persist and Can Be Augmented by a Late Booster Immunization in Infancy. mSphere 2020, 5, e00162-20. [Google Scholar] [CrossRef]
- Jensen, K.; Dela Pena-Ponce, M.G.; Piatak, M., Jr.; Shoemaker, R.; Oswald, K.; Jacobs, W.R., Jr.; Fennelly, G.; Lucero, C.; Mollan, K.R.; Hudgens, M.G.; et al. Balancing trained immunity with persistent immune activation and the risk of SIV infection in infant macaques vaccinated with attenuated Mycobacterium tuberculosis or BCG vaccines. Clin. Vaccine Immunol. 2017, 24, e00360-16. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence dataFastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc (accessed on 5 May 2025).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Xia, J.; Fjell, C.D.; Mayer, M.L.; Pena, O.M.; Wishart, D.S.; Hancock, R.E. INMEX—A web-based tool for integrative meta-analysis of expression data. Nucleic Acids Res. 2013, 41, W63–W70. [Google Scholar] [CrossRef]
- Xia, J.; Lyle, N.H.; Mayer, M.L.; Pena, O.M.; Hancock, R.E. INVEX—A web-based tool for integrative visualization of expression data. Bioinformatics 2013, 29, 3232–3234. [Google Scholar] [CrossRef]
- Xia, J.; Benner, M.J.; Hancock, R.E. NetworkAnalyst—Integrative approaches for protein-protein interaction network analysis and visual exploration. Nucleic Acids Res. 2014, 42, W167–W174. [Google Scholar] [CrossRef]
- Xia, J.; Gill, E.E.; Hancock, R.E. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat. Protoc. 2015, 10, 823–844. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Soufan, O.; Ewald, J.; Hancock, R.E.W.; Basu, N.; Xia, J. NetworkAnalyst 3.0: A visual analytics platform for comprehensive gene expression profiling and meta-analysis. Nucleic Acids Res. 2019, 47, W234–W241. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Gao, Y.; Li, Y.; Feng, C.; Song, C.; Ning, Z.; Zhou, X.; Zhao, J.; Feng, M.; et al. LncSEA: A platform for long non-coding RNA related sets and enrichment analysis. Nucleic Acids Res. 2021, 49, D969–D980. [Google Scholar] [CrossRef]
- Yao, R.W.; Wang, Y.; Chen, L.L. Cellular functions of long noncoding RNAs. Nat. Cell Biol. 2019, 21, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, E.; Sanz, J.; Dunn, J.L.; Khan, N.; Mendonca, L.E.; Pacis, A.; Tzelepis, F.; Pernet, E.; Dumaine, A.; Grenier, J.C.; et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell 2018, 172, 176–190.e19. [Google Scholar] [CrossRef]
- Saeed, S.; Quintin, J.; Kerstens, H.H.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef]
- Moorlag, S.; Folkman, L.; Ter Horst, R.; Krausgruber, T.; Barreca, D.; Schuster, L.C.; Fife, V.; Matzaraki, V.; Li, W.; Reichl, S.; et al. Multi-omics analysis of innate and adaptive responses to BCG vaccination reveals epigenetic cell states that predict trained immunity. Immunity 2024, 57, 171–187.e14. [Google Scholar] [CrossRef]
- Mourits, V.P.; van Puffelen, J.H.; Novakovic, B.; Bruno, M.; Ferreira, A.V.; Arts, R.J.; Groh, L.; Crisan, T.O.; Zwaag, J.; Jentho, E.; et al. Lysine methyltransferase G9a is an important modulator of trained immunity. Clin. Transl. Immunol. 2021, 10, e1253. [Google Scholar] [CrossRef]
- Arts, R.J.W.; Moorlag, S.; Novakovic, B.; Li, Y.; Wang, S.Y.; Oosting, M.; Kumar, V.; Xavier, R.J.; Wijmenga, C.; Joosten, L.A.B.; et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe 2018, 23, 89–100.e5. [Google Scholar] [CrossRef]
- Sun, S.J.; Aguirre-Gamboa, R.; de Bree, L.C.J.; Sanz, J.; Dumaine, A.; Joosten, L.A.B.; Divangahi, M.; Netea, M.G.; Barreiro, L.B. BCG vaccination impacts the epigenetic landscape of progenitor cells in human bone marrow. bioRxiv 2023. [Google Scholar] [CrossRef]
- Dias, H.F.; Kuhtreiber, W.M.; Nelson, K.J.; Ng, N.C.; Zheng, H.; Faustman, D.L. Epigenetic changes related to glucose metabolism in type 1 diabetes after BCG vaccinations: A vital role for KDM2B. Vaccine 2022, 40, 1540–1554. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, H.A.; Keyser, A.; Bowmaker, M.; Sayles, P.C.; Kaplan, G.; Hussey, G.; Hill, A.V.; Hanekom, W.A. Transcriptional profiling of mycobacterial antigen-induced responses in infants vaccinated with BCG at birth. BMC Med. Genom. 2009, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Bannister, S.; Kim, B.; Dominguez-Andres, J.; Kilic, G.; Ansell, B.R.E.; Neeland, M.R.; Moorlag, S.; Matzaraki, V.; Vlahos, A.; Shepherd, R.; et al. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci. Adv. 2022, 8, eabn4002. [Google Scholar] [CrossRef] [PubMed]
- Covian, C.; Fernandez-Fierro, A.; Retamal-Diaz, A.; Diaz, F.E.; Vasquez, A.E.; Lay, M.K.; Riedel, C.A.; Gonzalez, P.A.; Bueno, S.M.; Kalergis, A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019, 10, 2806. [Google Scholar] [CrossRef]
- Daskalaki, M.G.; Tsatsanis, C.; Kampranis, S.C. Histone methylation and acetylation in macrophages as a mechanism for regulation of inflammatory responses. J. Cell Physiol. 2018, 233, 6495–6507. [Google Scholar] [CrossRef]
- Soares, A.P.; Kwong Chung, C.K.; Choice, T.; Hughes, E.J.; Jacobs, G.; van Rensburg, E.J.; Khomba, G.; de Kock, M.; Lerumo, L.; Makhethe, L.; et al. Longitudinal changes in CD4(+) T-cell memory responses induced by BCG vaccination of newborns. J. Infect. Dis. 2013, 207, 1084–1094. [Google Scholar] [CrossRef]
- Kiravu, A.; Osawe, S.; Happel, A.U.; Nundalall, T.; Wendoh, J.; Beer, S.; Dontsa, N.; Alinde, O.B.; Mohammed, S.; Datong, P.; et al. Bacille Calmette-Guerin Vaccine Strain Modulates the Ontogeny of Both Mycobacterial-Specific and Heterologous T Cell Immunity to Vaccination in Infants. Front. Immunol. 2019, 10, 2307. [Google Scholar] [CrossRef]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Benn, C.S.; Joosten, L.A.; Jacobs, C.; van Loenhout, J.; Xavier, R.J.; Aaby, P.; van der Meer, J.W.; et al. Long-lasting effects of BCG vaccination on both heterologous Th1/Th17 responses and innate trained immunity. J. Innate Immun. 2014, 6, 152–158. [Google Scholar] [CrossRef]
- Lee, A.; Floyd, K.; Wu, S.; Fang, Z.; Tan, T.K.; Froggatt, H.M.; Powers, J.M.; Leist, S.R.; Gully, K.L.; Hubbard, M.L.; et al. BCG vaccination stimulates integrated organ immunity by feedback of the adaptive immune response to imprint prolonged innate antiviral resistance. Nat. Immunol. 2024, 25, 41–53. [Google Scholar] [CrossRef]
- Tran, K.A.; Pernet, E.; Sadeghi, M.; Downey, J.; Chronopoulos, J.; Lapshina, E.; Tsai, O.; Kaufmann, E.; Ding, J.; Divangahi, M. BCG immunization induces CX3CR1(hi) effector memory T cells to provide cross-protection via IFN-gamma-mediated trained immunity. Nat. Immunol. 2024, 25, 418–431. [Google Scholar] [CrossRef]
- Georgountzou, A.; Papadopoulos, N.G. Postnatal Innate Immune Development: From Birth to Adulthood. Front. Immunol. 2017, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.J.; Levy, O. Ontogeny of early life immunity. Trends Immunol. 2014, 35, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Angelidou, A.; Diray-Arce, J.; Conti, M.G.; Netea, M.G.; Blok, B.A.; Liu, M.; Sanchez-Schmitz, G.; Ozonoff, A.; van Haren, S.D.; Levy, O. Human Newborn Monocytes Demonstrate Distinct BCG-Induced Primary and Trained Innate Cytokine Production and Metabolic Activation In Vitro. Front. Immunol. 2021, 12, 674334. [Google Scholar] [CrossRef]
- Scalabre, A.; Jobard, E.; Demede, D.; Gaillard, S.; Pontoizeau, C.; Mouriquand, P.; Elena-Herrmann, B.; Mure, P.Y. Evolution of Newborns’ Urinary Metabolomic Profiles According to Age and Growth. J. Proteome Res. 2017, 16, 3732–3740. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moorlag, S.; Koeken, V.; Roring, R.J.; de Bree, L.C.J.; Mourits, V.P.; Gupta, M.K.; Zhang, B.; Fu, J.; Zhang, Z.; et al. A single-cell view on host immune transcriptional response to in vivo BCG-induced trained immunity. Cell Rep. 2023, 42, 112487. [Google Scholar] [CrossRef]
- Zhang, B.; Moorlag, S.J.; Dominguez-Andres, J.; Bulut, O.; Kilic, G.; Liu, Z.; van Crevel, R.; Xu, C.J.; Joosten, L.A.; Netea, M.G.; et al. Single-cell RNA sequencing reveals induction of distinct trained-immunity programs in human monocytes. J. Clin. Investig. 2022, 132, e147719. [Google Scholar] [CrossRef]
- Subbian, S.; Singh, P.; Kolloli, A.; Nemes, E.; Scriba, T.; Hanekom, W.A.; Kaplan, G. BCG Vaccination of Infants Confers Mycobacterium tuberculosis Strain-Specific Immune Responses by Leukocytes. ACS Infect. Dis. 2020, 6, 3141–3146. [Google Scholar] [CrossRef]
- Anderson, E.J.; Webb, E.L.; Mawa, P.A.; Kizza, M.; Lyadda, N.; Nampijja, M.; Elliott, A.M. The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine 2012, 30, 2083–2089. [Google Scholar] [CrossRef]
- Flores-Concha, M.; Onate, A.A. Long Non-coding RNAs in the Regulation of the Immune Response and Trained Immunity. Front. Genet. 2020, 11, 718. [Google Scholar] [CrossRef]
- Yi, Z.; Li, J.; Gao, K.; Fu, Y. Identifcation of differentially expressed long non-coding RNAs in CD4+ T cells response to latent tuberculosis infection. J. Infect. 2014, 69, 558–568. [Google Scholar] [CrossRef]
| Sample ID | Sex | Age at BCG Vaccination | Age at 2nd Visit | Interval |
|---|---|---|---|---|
| and 1st Blood Collection | Blood Collection | |||
| TBI017 | M | 1 day | 77 days | 76 days |
| TBI019 | F | 1 day | 42 days | 41 days |
| TBI043 | M | 1 day | 70 days | 69 days |
| TBI032 | M | 6 days | 70 days a | 64 days |
| TBI056 | M | 7 days | 84 days | 77 days |
| TBI000 | F | 8 days | 42 days | 34 days |
| TBI015 | F | 8 days | 77 days | 69 days |
| TBI035 | M | 8 days | 84 days | 66 days |
| TBI054 | M | 8 days | 70 days | 62 days |
| TBI016 | F | 9 days | 77 days | 68 days |
| TBI013 | F | 10 days | 77 days a | 67 days |
| TBI049 | M | 10 days | 105 days | 95 days |
| TBI008 | F | 11 days | 42 days | 31 days |
| TBI023 | F | 11 days a | 70 days | 59 days |
| TBI026 | M | 12 days | 77 days | 65 days |
| TBI036 | M | 13 days | 77 days | 64 days |
| TBI053 | M | 13 days | 77 days | 64 days |
| TBI058 | M | 13 days | 77 days | 64 days |
| TBI048 | M | 16 days a | 63 days | 47 days |
| TBI047 | F | 18 days | 56 days | 38 days |
| TBI004 | M | 31 days a | 42 days | 11 days |
| TBI022 | M | 35 days | no sample | |
| TBI025 | F | 47 days | 77 days | 30 days |
| TBI038 | F | 47 days | 77 days | 30 days |
| TBI042 | M | 60 days | 70 days | 10 days |
| TBI024 | F | 74 days a | 112 days | 38 days |
| Histone | Name | FDR p Value | Function | |
|---|---|---|---|---|
| CD4 T Cells | Monocytes | |||
| H3K4me2-3 | histone 3 with demethylation at lysine 2 or 3 | 3.84 × 10−15 | 8.80 × 10−13 | transcription activation; recovery of protein synthesis following DNA damage |
| H3K122ac | histone 3 with acetylation at lysine 122 | 1.83 × 10−10 | 4.85 × 10−7 | transcription activation |
| H3T11P | histone 3 with phosphorylation at threonine 11 | 1.33 × 10−6 | 0.00025 | Response to nutritional stress and metabolic changes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandiyil, V.V.K.; Kang, E.; Coates, E.; Kamthunzi, P.; Tegha, G.; Hosseinipour, M.; Wu, D.; Zou, F.; De Paris, K. BCG Vaccination Potentially Modulates the Transcriptome of Infant CD4 T Cells in Addition to Age-Dependent Immune Ontogeny-Associated Changes. Vaccines 2025, 13, 706. https://doi.org/10.3390/vaccines13070706
Kandiyil VVK, Kang E, Coates E, Kamthunzi P, Tegha G, Hosseinipour M, Wu D, Zou F, De Paris K. BCG Vaccination Potentially Modulates the Transcriptome of Infant CD4 T Cells in Addition to Age-Dependent Immune Ontogeny-Associated Changes. Vaccines. 2025; 13(7):706. https://doi.org/10.3390/vaccines13070706
Chicago/Turabian StyleKandiyil, Vidya Vijayan Karuvan, Eunchong Kang, Emily Coates, Portia Kamthunzi, Gerald Tegha, Mina Hosseinipour, Di Wu, Fei Zou, and Kristina De Paris. 2025. "BCG Vaccination Potentially Modulates the Transcriptome of Infant CD4 T Cells in Addition to Age-Dependent Immune Ontogeny-Associated Changes" Vaccines 13, no. 7: 706. https://doi.org/10.3390/vaccines13070706
APA StyleKandiyil, V. V. K., Kang, E., Coates, E., Kamthunzi, P., Tegha, G., Hosseinipour, M., Wu, D., Zou, F., & De Paris, K. (2025). BCG Vaccination Potentially Modulates the Transcriptome of Infant CD4 T Cells in Addition to Age-Dependent Immune Ontogeny-Associated Changes. Vaccines, 13(7), 706. https://doi.org/10.3390/vaccines13070706






