Nasopharyngeal Carriage, Serotype Distribution, and Antimicrobial Susceptibility of Streptococcus pneumoniae Among PCV13-Vaccinated and -Unvaccinated Children in Iran
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Sample Size Calculations
2.4. Sample Collection and Culture Methods
2.5. DNA Extraction and Real-Time PCR
2.6. PCR and cpsB Serotyping
2.7. Minimum Inhibitory Concentrations (MICs)
2.8. Statistical Analysis
3. Results
3.1. Demographic Characteristics of the Study Population
3.2. Pneumococcal Prevalence and Serotype Distribution
3.3. PCV13 Coverage
3.4. Distribution of S. pneumoniae Serotypes in Children Based on the Number of PCV13 Doses
3.5. Antimicrobial Resistance Profile of S. pneumoniae Carriage Isolates
3.6. The Association of S. pneumoniae Serotypes with Antibiotic Resistance
3.7. Risk Factors for Pneumococcal Carriage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef]
- CDC. Pneumococcal Disease Surveillance and Trends. 2024. Available online: https://www.cdc.gov/pneumococcal/php/surveillance/index.html#:~:text=Global%20trends,deaths%20occur%20in%20developing%20countries (accessed on 9 September 2024).
- Simell, B.; Auranen, K.; Käyhty, H.; Goldblatt, D.; Dagan, R.; O’bRien, K.L.; for the Pneumococcal Carriage Group (PneumoCarr). The fundamental link between pneumococcal carriage and disease. Expert Rev. Vaccines 2012, 11, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Werren, J.P.; Ortika, B.D.; Bellich, B.; Pell, C.L.; Nikolaou, E.; Gjuroski, I.; Lo, S.; Hinds, J.; Tundev, O.; et al. Streptococcus pneumoniae serotype 33G: Genetic, serological, and structural analysis of a new capsule type. Microbiol. Spectr. 2024, 12, e03579-23. [Google Scholar] [CrossRef]
- Hackel, M.; Lascols, C.; Bouchillon, S.; Hilton, B.; Morgenstern, D.; Purdy, J. Serotype prevalence and antibiotic resistance in Streptococcus pneumoniae clinical isolates among global populations. Vaccine 2013, 31, 4881–4887. [Google Scholar] [CrossRef]
- Zahari, N.I.N.; Engku Abd Rahman, E.N.S.; Irekeola, A.A.; Ahmed, N.; Rabaan, A.A.; Alotaibi, J.; Alqahtani, S.A.; Halawi, M.Y.; Alamri, I.A.; Almogbel, M.S.; et al. A review of the resistance mechanisms for β-lactams, macrolides and fluoroquinolones among Streptococcus pneumoniae. Medicina 2023, 59, 1927. [Google Scholar] [CrossRef] [PubMed]
- Son, B.A.; Hai, T.X.; Van Cuong, T.; Chinh, D.D.; Le, T.-H.; Dung, N.M.; Dinh, V.N.; Anh, D.N. Serotype distribution and antibiotic resistance of Streptococcus pneumoniae isolates collected from unvaccinated children with pneumonia at a province in central Vietnam. Iran. J. Microbiol. 2022, 14, 653. [Google Scholar] [CrossRef]
- Eichler, N.; Reynolds, E.; Jackson, C.; Thornley, S.; Peters, J. Invasive pneumococcal disease and serotype emergence in the Auckland region during the vaccine era 2009–16. J. Prim. Health Care 2019, 11, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Gergova, R.; Boyanov, V.; Muhtarova, A.; Alexandrova, A. A review of the impact of streptococcal infections and antimicrobial resistance on human health. Antibiotics 2024, 13, 360. [Google Scholar] [CrossRef]
- Saeed, U.; Insaf, R.A.; Piracha, Z.Z.; Tariq, M.N.; Sohail, A.; Abbasi, U.A.; Fida Rana, M.S.; Gilani, S.S.; Noor, S.; Noor, E.; et al. Crisis averted: A world united against the menace of multiple drug-resistant superbugs-pioneering anti-AMR vaccines, RNA interference, nanomedicine, CRISPR-based antimicrobials, bacteriophage therapies, and clinical artificial intelligence strategies to safeguard global antimicrobial arsenal. Front. Microbiol. 2023, 14, 1270018. [Google Scholar]
- CDC. Chapter 17: Pneumococcal Disease. 2024. Available online: https://www.cdc.gov/pinkbook/hcp/table-of-contents/chapter-17-pneumococcal-disease.html (accessed on 1 May 2024).
- Chang, B.; Akeda, H.; Nakamura, Y.; Hamabata, H.; Ameku, K.; Toma, T.; Miyagi, M.; Ohnishi, M. Impact of thirteen-valent pneumococcal conjugate vaccine on nasopharyngeal carriage in healthy children under 24 months in Okinawa, Japan. J. Infect. Chemother. 2020, 26, 465–470. [Google Scholar] [CrossRef]
- W Satzke, C.; Turner, P.; Virolainen-Julkunen, A.; Adrian, P.V.; Antonio, M.; Hare, K.M.; Henao-Restrepo, A.M.; Leach, A.J.; Klugman, K.P.; Porter, B.D.; et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: Updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine 2013, 32, 165–179. [Google Scholar] [CrossRef] [PubMed]
- CDC. Streptococcus Laboratory Protocols. 2024. Available online: https://www.cdc.gov/strep-lab/media/pcr-pneumo-carriage.pdf (accessed on 8 April 2024).
- Wu, H.M.; Cordeiro, S.M.; Harcourt, B.H.; Carvalho, M.d.G.S.; Azevedo, J.; Oliveira, T.Q.; Leite, M.C.; Salgado, K.; Reis, M.G.; Plikaytis, B.D.; et al. Accuracy of real-time PCR, Gram stain and culture for Streptococcus pneumoniae, Neisseria meningitidis and Haemophilus influenzae meningitis diagnosis. BMC Infect. Dis. 2013, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tavares, D.A.; Handem, S.; Carvalho, R.J.; Paulo, A.C.; de Lencastre, H.; Hinds, J.; Sá-Leão, R. Identification of Streptococcus pneumoniae by a real-time PCR assay targeting SP2020. Sci. Rep. 2019, 9, 3285. [Google Scholar] [CrossRef]
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing, 32nd Edition. Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2022.
- Bondarchuk, C.P.; Grobman, B.; Mansur, A.; Lu, C.Y. National trends in pneumonia-related mortality in the United States, 1999–2019. Infect. Dis. 2025, 57, 56–65. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Bacterial Priority. Pathogens List, 2024. 17 May 2024. Available online: https://iris.who.int/bitstream/handle/10665/376776/9789240093461-eng.pdf?sequence=1 (accessed on 17 May 2024).
- Beissegulova, G.; Ramazanova, B.; Mustafina, K.; Begadilova, T.; Koloskova, Y.; Seitkhanova, B.; Mamatova, A.; Iskakova, U.; Sailaubekuly, R.; Seiitbay, Z.; et al. Prevalence of nasopharyngeal Streptococcus Pneumoniae carriage in infants: A systematic review and meta-analysis of cohort studies and randomized controlled trials. PLoS ONE 2024, 19, e0315461. [Google Scholar] [CrossRef]
- Ghahfarokhi, S.H.; Mosadegh, M.; Ahmadi, A.; Pourmand, M.R.; Azarsa, M.; Rahbar, M.; Nikmanesh, B. Serotype distribution and antibiotic susceptibility of Streptococcus pneumoniae isolates in Tehran, Iran: A surveillance study. Infect. Drug Resist. 2020, 13, 333–340. [Google Scholar] [CrossRef]
- Gupta, P.; Awasthi, S.; Gupta, U.; Verma, N.; Rastogi, T.; Pandey, A.K.; Naziat, H.; Rahman, H.; Islam, M.; Saha, S. Nasopharyngeal carriage of Streptococcus pneumoniae serotypes among healthy children in Northern India. Curr. Microbiol. 2023, 80, 41. [Google Scholar] [CrossRef]
- Walekhwa, M.; Muturi, M.; Gunturu, R.; Kenya, E.; Kabera, B. Streptococcus pneumoniae serotype epidemiology among PCV-10 vaccinated and unvaccinated children at Gertrude’s Children’s Hospital, Nairobi County: A cross-sectional study. F1000Research 2019, 31, 879. [Google Scholar] [CrossRef]
- Kandasamy, R.; Voysey, M.; Collins, S.; Berbers, G.; Robinson, H.; Noel, I.; Hughes, H.; Ndimah, S.; Gould, K.; Fry, N.; et al. Persistent circulation of vaccine serotypes and serotype replacement after 5 years of infant immunization with 13-valent pneumococcal conjugate vaccine in the United Kingdom. J. Infect. Dis. 2020, 221, 1361–1370. [Google Scholar] [CrossRef]
- Yue, Y.; Wu, D.; Zeng, Q.; Li, Y.; Yang, C.; Lv, X.; Wang, L. Changes in children respiratory infections pre and post COVID-19 pandemic. Front. Cell. Infect. Microbiol. 2025, 15, 1549497. [Google Scholar]
- Li, Y.; Guo, Y.; Duan, Y. Changes in Streptococcus pneumoniae infection in children before and after the COVID-19 pandemic in Zhengzhou, China. J. Infect. 2022, 85, e80. [Google Scholar] [CrossRef]
- Pérez-García, C.; Sempere, J.; de Miguel, S.; Hita, S.; Úbeda, A.; Vidal, E.J.; Llorente, J.; Limia, A.; Gil de Miguel, A.; Sanz, J.C.; et al. Surveillance of invasive pneumococcal disease in Spain exploring the impact of the COVID-19 pandemic (2019–2023). J. Infect. 2024, 89, 106204. [Google Scholar] [CrossRef]
- Almeida, S.C.; de Lemos, A.P.S.; Bierrenbach, A.L.; de Moraes, J.C.; Brandileone, M.C.d.C. Serotype Distribution and Antimicrobial Susceptibility Pattern of Streptococcus pneumoniae in COVID-19 Pandemic Era in Brazil. Microorganisms 2024, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Mohammadi, M.; Afshar, D.; Nazari-Alam, A. Evaluation of frequency, drug resistance and serotyping of streptococcus pneumoniae in Iran: A systematic review. J. Babol Univ. Med. Sci. 2021, 23, 189. [Google Scholar]
- Mamishi, S.; Pourakbari, B.; Bahador, A.; Sadeghi, R.H.; Pourhajibagher, M. Monitoring Over a Decade in the Serotype Prevalence of Streptococcus pneumoniae in Iran: A Systematic Review and Meta-analysis. Infect. Disord.-Drug Targets 2024, 24, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Cleary, D.W.; Jones, J.; Gladstone, R.A.; Osman, K.L.; Devine, V.T.; Jefferies, J.M.; Bentley, S.D.; Faust, S.N.; Clarke, S.C. Changes in serotype prevalence of Streptococcus pneumoniae in Southampton, UK between 2006 and 2018. Sci. Rep. 2022, 12, 13332. [Google Scholar] [CrossRef]
- Sayyahfar, S.; Esteghamati, A.; Fahimzad, S.A.; Hajisadeghi-Isfahani, S.; Nazari-Alam, A.; Azimi, L. Serotype Distribution of Streptococcus pneumoniae Carriage in Six-Month-Old Infants: A Cross-sectional Study During 2017–18, Tehran, Iran. Arch. Pediatr. Infect. Dis. 2021, 10. [Google Scholar] [CrossRef]
- Kielbik, K.; Pietras, A.; Jablonska, J.; Bakiera, A.; Borek, A.; Niedzielska, G.; Grzegorczyk, M.; Grywalska, E.; Korona-Glowniak, I. Impact of pneumococcal vaccination on nasopharyngeal carriage of Streptococcus pneumoniae and microbiota profiles in preschool children in South East Poland. Vaccines 2022, 10, 791. [Google Scholar] [CrossRef]
- Huo, X.; Tan, Z.; Qian, H.; Qin, Y.; Dong, C.; Li, C.; Kong, X.; Hong, J. Serotypes and Genotypes of Streptococcus pneumoniae in an Unvaccinated Population in Suzhou, China. Infect. Drug Resist. 2024, 17, 4001–4009. [Google Scholar] [CrossRef]
- Sidorenko, S.; Rennert, W.; Lobzin, Y.; Briko, N.; Kozlov, R.; Namazova-Baranova, L.; Tsvetkova, I.; Ageevets, V.; Nikitina, E.; Ardysheva, A.; et al. Multicenter study of serotype distribution of Streptococcus pneumoniae nasopharyngeal isolates from healthy children in the Russian Federation after introduction of PCV13 into the National Vaccination Calendar. Diagn. Microbiol. Infect. Dis. 2020, 96, 114914. [Google Scholar] [CrossRef]
- Watkins, E.R.; Kalizang’oMa, A.; Gori, A.; Gupta, S.; Heyderman, R.S. Factors affecting antimicrobial resistance in Streptococcus pneumoniae following vaccination introduction. Trends Microbiol. 2022, 30, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Obolski, U.; Lourenço, J.; Thompson, C.; Thompson, R.; Gori, A.; Gupta, S. Vaccination can drive an increase in frequencies of antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae. Proc. Natl. Acad. Sci. USA 2018, 115, 3102–3107. [Google Scholar] [CrossRef] [PubMed]
- Opavski, N.; Jovićević, M.; Kabić, J.; Kekić, D.; Gajić, I. Effect of Childhood Pneumococcal Conjugate Vaccination on Invasive Disease Serotypes in Serbia. Vaccines 2024, 12, 940. [Google Scholar] [CrossRef] [PubMed]
- Zuccotti, G.; Mameli, C.; Daprai, L.; Garlaschi, M.L.; Dilillo, D.; Bedogni, G.; Faccini, M.; Gramegna, M.; Torresani, E.; Emanuela, B.; et al. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine 2014, 32, 527–534. [Google Scholar] [CrossRef]
- Ben Ayed, N.; Ktari, S.; Jdidi, J.; Gargouri, O.; Smaoui, F.; Hachicha, H.; Ksibi, B.; Mezghani, S.; Mnif, B.; Mahjoubi, F.; et al. Nasopharyngeal Carriage of Streptococcus pneumoniae in Tunisian Healthy under-Five Children during a Three-Year Survey Period (2020 to 2022). Vaccines 2024, 12, 393. [Google Scholar] [CrossRef]

| Variable | Category | Vaccinated (n (%)) | Unvaccinated (n (%)) | p-Value | Adjusted p-Value # |
|---|---|---|---|---|---|
| Gender | Girl | 44 (43.1) | 32 (31.4) | 0.082 | 1.000 |
| Boy | 58 (56.9) | 70 (68.6) | |||
| Underlying disease | No | 67 (65.7) | 59 (57.8) | 0.249 | 1.000 |
| Yes | 35 (34.3) | 43 (42.2) | |||
| Geographic area | Urban | 98 (96.1) | 54 (57.4) | <0.001 | <0.001 |
| Suburban/rural | 4 (3.9) | 40 (42.6) | |||
| Area (Tehran) | Yes | 90 (90.9) | 28 (29.8) | <0.001 | <0.001 |
| No | 9 (9.1) | 66 (70.2) | |||
| Mother’s education | Academic education | 60 (58.8) | 29 (30.2) | <0.001 | 0.001 |
| Non-academic education | 42 (41.2) | 67 (69.8) | |||
| Father’s education | Academic education | 79 (80.6) | 31 (32.6) | <0.001 | <0.001 |
| Non-academic education | 19 (19.4) | 64 (67.4) | |||
| Previous hospitalization | Yes | 50 (49.0) | 31 (30.4) | 0.007 | 0.140 |
| No | 52 (51.0) | 71 (69.6) | |||
| Cause of hospitalization | Non-infectious | 28 (58.3) | 20 (76.9) | 0.247 | 1.000 |
| Infectious | 19 (39.6) | 6 (23.1) | |||
| Antibiotic treatment within preceding 3 months | Yes | 44 (43.1) | 11 (10.8) | <0.001 | <0.001 |
| No | 58 (56.9) | 91 (89.2) | |||
| Previous respiratory infections | Yes | 17 (16.7) | 5 (4.9) | 0.007 | 0.140 |
| No | 85 (83.3) | 97 (95.1) | |||
| Type of daycare | At home | 70 (72.2) | 93 (95.9) | <0.001 | <0.001 |
| Private household care | 6 (6.2) | 3 (3.1) | |||
| Daycare attendance | 21 (21.6) | 1 (1.0) | |||
| Sharing bedroom with >2 persons | Yes | 48 (47.1) | 76 (74.5) | <0.001 | 0.001 |
| No | 54 (52.9) | 26 (25.5) | |||
| Sharing bedroom with parents | Yes | 46 (45.1) | 72 (70.6) | <0.001 | 0.005 |
| No | 56 (54.9) | 30 (29.4) | |||
| Exposure to cigarette smoke | Yes | 18 (17.6) | 39 (38.2) | 0.001 | 0.020 |
| No | 84 (82.4) | 63 (61.8) | |||
| Presence of person > 60 years old | Yes | 5 (4.9) | 14 (13.7) | 0.03 | 0.600 |
| No | 97 (95.1) | 88 (86.3) | |||
| Sibling | Yes | 41 (40.2) | 68 (66.7) | <0.001 | 0.003 |
| No | 61 (59.8) | 34 (33.3) | |||
| Number of siblings | 0 | 61 (59.8) | 34 (33.3) | <0.001 * | 0.001 |
| 1–2 | 41 (40.2) | 62 (60.8) | |||
| ≥3 | 0 (0.0) | 6 (5.9) | |||
| Presence of older sibling | Yes | 26 (66.7) | 43 (82.7) | 0.077 | 1.000 |
| No | 13 (33.3) | 9 (17.3) | |||
| Presence of both older and younger siblings | Yes | 2 (5.1) | 5 (9.6) | 0.694 ** | 1.000 |
| No | 37 (94.9) | 47 (90.4) | |||
| Sibling younger than 5 years | Yes | 16 (40.0) | 24 (46.2) | 0.555 | 1.000 |
| No | 24 (60.0) | 28 (53.8) |
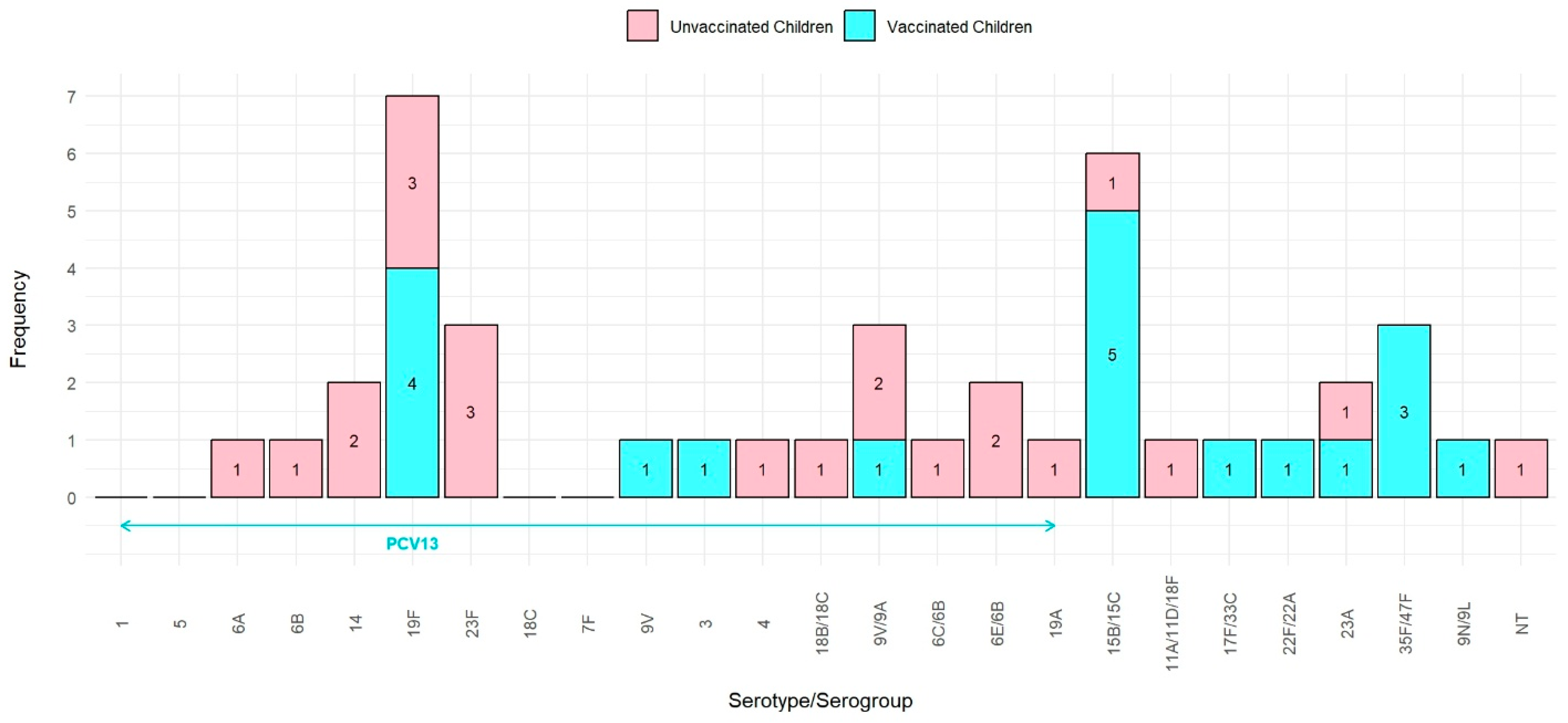
| Serogroup/Serogroup | Unvaccinated | PCV13-Vaccinated | Total |
|---|---|---|---|
| N = 22 | N = 19 | N = 41 | |
| 1 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 |
| 6A | 1 | 0 | 1 |
| 6B | 1 | 0 | 1 |
| 14 | 2 | 0 | 2 |
| 19F | 3 | 4 | 7 |
| 23F | 3 | 0 | 3 |
| 18C | 0 | 0 | 0 |
| 7F | 0 | 0 | 0 |
| 9V | 0 | 1 | 1 |
| 3 | 0 | 1 | 1 |
| 4 | 1 | 0 | 1 |
| 18B/18C | 1 | 0 | 1 |
| 9V/9A | 2 | 1 | 3 |
| 6C/6B | 1 | 0 | 1 |
| 6E/6B | 2 | 0 | 2 |
| 19A | 1 | 0 | 1 |
| N | 18 | 7 | 25 |
| PCV13 coverage (95% CI) | 81.8 (61.5, 92.7) | 36.8 (19.2, 59.0) | 61.0 (45.7, 74.3) |
| Unvaccinated | Vaccinated | p-Value * | Total | ||
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Penicillin | Sensitive | 18 (81.8) | 20 (100.0) | 38 (90.5) | |
| Intermediate | 2 (9.1) | 0 (0.0) | 0.239 | 2 (4.8) | |
| Resistant | 2 (9.1) | 0 (0.0) | 2 (4.8) | ||
| Ceftriaxone | Sensitive | 18 (81.8) | 18 (90.0) | 36 (85.7) | |
| Intermediate | 3 (13.6) | 1 (5.0) | 0.799 | 4 (9.5) | |
| Resistant | 1 (4.5) | 1 (5.0) | 2 (4.8) | ||
| Erythromycin | Sensitive | 5 (22.7) | 3 (15.0) | 8 (19.0) | |
| Intermediate | 1 (4.5) | 2 (10.0) | 0.769 | 3 (7.1) | |
| Resistant | 16 (72.7) | 15 (75.0) | 31 (73.8) | ||
| Chloramphenicol | Sensitive | 19 (86.4) | 19 (95.0) | 38 (90.5) | |
| Intermediate | 0 (0.0) | 0 (0.0) | 0.608 | 0 (0.0) | |
| Resistant | 3 (13.6) | 1 (5.0) | 4 (9.5) | ||
| Trimethoprim/Sulphamethoxazole | Sensitive | 5 (22.7) | 4 (20.0) | 9 (21.4) | |
| Intermediate | 3 (13.6) | 2 (10.0) | 1.000 | 5 (11.9) | |
| Resistant | 14 (63.6) | 14 (70.0) | 28 (66.7) | ||
| Variable | Univariate Models | Multivariable Model | ||
|---|---|---|---|---|
| Crude OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| Age (months) | 1.01 (0.99, 1.03) | 0.455 | 1.00 (0.97, 1.03) | 0.938 |
| Gender (Ref: Boy) | 1 | - | 1 | - |
| Girl | 1.63 (0.82, 3.22) | 0.16 | 1.52 (0.65, 3.55) | 0.332 |
| Underlying disease (Ref: No) | 1 | - | 1 | - |
| Yes | 0.64 (0.31, 1.32) | 0.226 | 1.04 (0.36, 2.98) | 0.938 |
| Geographic area (Ref: Urban) | 1 | - | 1 | - |
| Suburban/rural | 1.00 (0.44, 2.31) | 0.993 | 1.68 (0.32, 8.79) | 0.536 |
| Area (Ref: Tehran) | 1 | - | 1 | - |
| Other | 0.53 (0.25, 1.13) | 0.101 | 0.16 (0.03, 0.82) | 0.028 |
| Mother’s education (Ref: Non-academic) | 1 | - | 1 | - |
| Academic | 0.95 (0.47, 1.89) | 0.88 | 0.81 (0.31, 2.14) | 0.672 |
| Father’s education (Ref: Non-academic) | 1 | - | 1 | - |
| Academic | 1.08 (0.54, 2.18) | 0.822 | 0.77 (0.27, 2.20) | 0.625 |
| Previous hospitalization (Ref: No) | 1 | - | 1 | - |
| Yes | 1.12 (0.57, 2.22) | 0.745 | 1.20 (0.46, 3.09) | 0.713 |
| Antibiotic treatment within the preceding 3 months (Ref: No) | 1 | - | 1 | - |
| Yes | 2.12 (1.04, 4.31) | 0.039 | 1.28 (0.46, 3.58) | 0.638 |
| Previous respiratory infections (Ref: No) | 1 | - | 1 | - |
| Yes | 3.01 (1.19, 7.62) | 0.02 | 3.11 (1.00, 9.62) | 0.05 |
| Type of day care (Ref: At home) | 1 | - | ||
| Private household care | 1.22 (0.24, 6.14) | 0.812 | 0.83 (0.14, 5.13) | 0.845 |
| Daycare attendance | 2.95 (1.16, 7.51) | 0.024 | 4.49 (1.20, 16.80) | 0.026 |
| Sharing bedroom with more than 2 persons (Ref: No) | 1 | - | 1 | - |
| Yes | 0.98 (0.49, 1.96) | 0.962 | 1.34 (0.46, 3.91) | 0.593 |
| Sharing bedroom with parents (Ref: No) | 1 | - | ||
| Yes | 0.71 (0.36, 1.39) | 0.319 | ||
| Exposure to cigarette smoke (Ref: No) | 1 | - | 1 | - |
| Yes | 1.15 (0.55, 2.41) | 0.706 | 1.50 (0.58, 3.86) | 0.401 |
| Presence of person > 60 years (Ref: No) | 1 | - | 1 | - |
| Yes | 1.00 (0.31, 3.19) | 0.998 | 0.62 (0.11, 3.38) | 0.583 |
| Sibling (Ref: No) | 1 | - | 1 | - |
| Yes | 1.84 (0.92, 3.71) | 0.086 | 1.53 (0.66, 3.57) | 0.323 |
| Number of siblings (Ref: None) | 1 | - | ||
| 1–2 | 1.71 (0.85, 3.44) | 0.131 | ||
| >=3 | 1.00 (0.11, 9.14) | 1.000 | ||
| Presence of older sibling (Ref: No) | 1 | - | ||
| Yes | 0.78 (0.30, 2.03) | 0.617 | ||
| Sibling younger than 5 years (Ref: No) | 1 | - | ||
| Yes | 0.53 (0.20, 1.36) | 0.184 | ||
| Vaccinated (Ref: No) | 1 | - | 1 | - |
| Yes | 0.94 (0.48, 1.85) | 0.864 | 0.42 (0.12, 1.48) | 0.179 |
| Number of vaccine doses (Ref: None) | 1 | - | ||
| 1–2 | 0.53 (0.19, 1.51) | 0.235 | ||
| ≥3 | 1.49 (0.71, 3.14) | 0.294 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashrafian, F.; Sadat Larijani, M.; Haji Maghsoudi, S.; Doroud, D.; Fahimzad, A.; Pournasiri, Z.; Jafari, E.; Parzadeh, M.; Abdollahi, S.; Haj Agha Gholizadeh Khiavi, E.; et al. Nasopharyngeal Carriage, Serotype Distribution, and Antimicrobial Susceptibility of Streptococcus pneumoniae Among PCV13-Vaccinated and -Unvaccinated Children in Iran. Vaccines 2025, 13, 707. https://doi.org/10.3390/vaccines13070707
Ashrafian F, Sadat Larijani M, Haji Maghsoudi S, Doroud D, Fahimzad A, Pournasiri Z, Jafari E, Parzadeh M, Abdollahi S, Haj Agha Gholizadeh Khiavi E, et al. Nasopharyngeal Carriage, Serotype Distribution, and Antimicrobial Susceptibility of Streptococcus pneumoniae Among PCV13-Vaccinated and -Unvaccinated Children in Iran. Vaccines. 2025; 13(7):707. https://doi.org/10.3390/vaccines13070707
Chicago/Turabian StyleAshrafian, Fatemeh, Mona Sadat Larijani, Saiedeh Haji Maghsoudi, Delaram Doroud, Alireza Fahimzad, Zahra Pournasiri, Elham Jafari, Masoumeh Parzadeh, Sara Abdollahi, Elham Haj Agha Gholizadeh Khiavi, and et al. 2025. "Nasopharyngeal Carriage, Serotype Distribution, and Antimicrobial Susceptibility of Streptococcus pneumoniae Among PCV13-Vaccinated and -Unvaccinated Children in Iran" Vaccines 13, no. 7: 707. https://doi.org/10.3390/vaccines13070707
APA StyleAshrafian, F., Sadat Larijani, M., Haji Maghsoudi, S., Doroud, D., Fahimzad, A., Pournasiri, Z., Jafari, E., Parzadeh, M., Abdollahi, S., Haj Agha Gholizadeh Khiavi, E., Bavand, A., Shafiei, M., Rohani, M., & Ramezani, A. (2025). Nasopharyngeal Carriage, Serotype Distribution, and Antimicrobial Susceptibility of Streptococcus pneumoniae Among PCV13-Vaccinated and -Unvaccinated Children in Iran. Vaccines, 13(7), 707. https://doi.org/10.3390/vaccines13070707





