Seroprevalence of Measles-, Mumps-, and Rubella-Specific Antibodies in Future Healthcare Workers in Serbia: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Serological Testing—MMR IgG ELISA
2.3. Sample Size
2.4. Statistical Analysis
3. Results
3.1. Seroprevalence of Measles, Mumps, and Rubella IgG Antibodies in Serbian Future HCWs
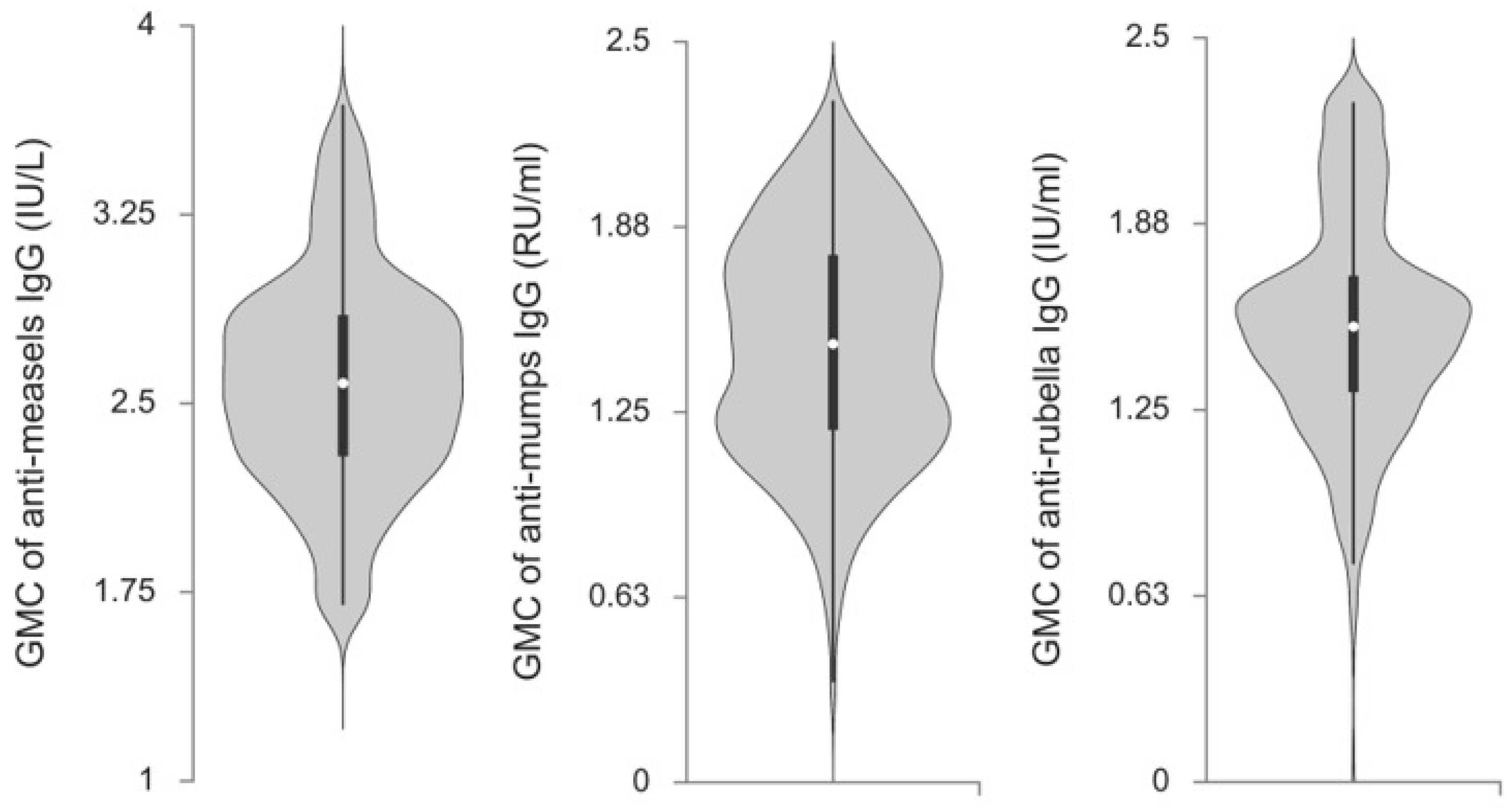
3.2. Anti-Measles, Anti-Mumps, and Anti-Rubella IgG Antibody Titers in Serbian Future Healthcare Workers
3.3. Factors Associated with Measles, Mumps, and Rubella Seronegativity in Future Serbian HCWs
3.4. Combined Seroprevalence Against Measles and Rubella or Measles, Mumps, and Rubella as a Proxy for the Vaccination Status
4. Discussion
Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MMR | Morbilli-Mumps-Rubella |
| HCW | Healthcare workers |
| IgG | Immunoglobulin G |
| GMC | Geometric Mean Concentration |
| WHO | World Health Organisation |
| CRS | Congenital Rubella Syndrome |
| SAGE | Strategic Advisory Group of Experts |
| EU/EEA | European Union and European Economic Area |
| MM | Morbilli-Mumps |
| ECDC | European Centre for Disease Prevention and Control |
| AP | Autonomous Province |
| ELISA | Enzyme linked immunosorbent assay |
References
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, March 2024: Conclusions and Recommendations. Wkly. Epidemiol. Rec. 2024, 99, 285–306. [Google Scholar]
- World Health Organization. Global Measles and Rubella Strategic Plan: 2012–2020; World Health Organization: Geneva, Switzerland, 2012; pp. 1–44. [Google Scholar]
- European Centre for Disease Prevention and Control (ECDC). Measles on the Rise Again in Europe: Time to Check Your Vaccination Status. Available online: https://www.ecdc.europa.eu/en/news-events/measles-rise-again-europe-time-check-your-vaccination-status (accessed on 13 May 2025).
- Institute of Public Health “Dr Milan Jovanovic Batut”. Report on Communicable Diseases in the Republic of Serbia for the Year 2022; Institute of Public Health “Dr Milan Jovanovic Batut”: Belgrade, Serbia, 2023. [Google Scholar]
- IZJZS-Batut. Available online: https://www.batut.org.rs/index.php?content=2776 (accessed on 28 December 2024).
- Patić, A.; Štrbac, M.; Petrović, V.; Milošević, V.; Ristić, M.; Cvjetković, I.H.; Medić, S. Seroepidemiological Study of Rubella in Vojvodina, Serbia: 24 Years after the Introduction of the MMR Vaccine in the National Immunization Programme. PLoS ONE 2020, 15, e0227413. [Google Scholar] [CrossRef]
- Institute of Public Health of Serbia “Dr Milan Jovanović Batut”. Report on the Implementation of Immunization in the Territory of the Republic of Serbia in 2022; Institute of Public Health “Dr Milan Jovanovic Batut”: Belgrade, Serbia, 2023. [Google Scholar]
- UNICEF. Knowledge, Attitudes and Practices in Relation to Immunization of Children In Serbia; UNICEF Belgrade: Belgrade, Serbia, 2018; ISBN 978-86-80902-07-4. [Google Scholar]
- Veljkovic, M.; Loncarevic, G.; Kanazir, M.; Kisic-Tepavcevic, D.; Gazibara, T. Trend in Mandatory Immunisation Coverage: Linear and Joinpoint Regression Approach, Serbia, 2000 to 2017. Eurosurveillance 2021, 26, 2000417. [Google Scholar] [CrossRef]
- Institute of Public Health of Serbia “Dr Milan Jovanovic Batut”. The Current Epidemiological Situation of Measles (Morbilli) in the Republic of Serbia; Institute of Public Health “Dr Milan Jovanovic Batut”: Belgarde, Serbia, 2024. [Google Scholar]
- Ristić, M.; Milošević, V.; Medić, S.; Malbaša, J.D.; Rajčević, S.; Boban, J.; Petrović, V. Sero-Epidemiological Study in Prediction of the Risk Groups for Measles Outbreaks in Vojvodina, Serbia. PLoS ONE 2019, 14, e0216219. [Google Scholar] [CrossRef]
- Institute of Public Helath of Serbia “Dr Milan Jovanovic Batut”. Mandatory Immunization Schedule in the Republic of Serbia; Institut of Public Health “Dr Milan Jovanovic Batut”: Belgarde, Serbia, 2025. [Google Scholar]
- Official Gazette of the Republic of Serbia Rulebook on Immunization and Chemoprophylaxis: 11/2006-114, 25/2013-36, 63/2013-264, 99/2013-18, 118/2013-130, 65/2014-38, 32/2015-105. Available online: https://pravno-informacioni-sistem.rs/SlGlasnikPortal/reg/viewAct/7648144d-9226-46bc-90f5-16fe5dc2201d (accessed on 14 May 2025).
- Weissgerber, T.L.; Savic, M.; Winham, S.J.; Stanisavljevic, D.; Garovic, V.D.; Milic, N.M. Data Visualization, Bar Naked: A Free Tool for Creating Interactive Graphics. J. Biol. Chem. 2017, 292, 20592–20598. [Google Scholar] [CrossRef]
- Springer, D.; Borsodi, C.; Camp, J.; Redlberger-Fritz, M.; Holzmann, H.; Kundi, M.; Aberle, J.; Stiasny, K.; Weseslindtner, L. Seroprevalence against Measles, Austria, Stratified by Birth Years 1922 to 2024. Eurosurveillance 2025, 30, 2400684. [Google Scholar] [CrossRef]
- Sá Machado, R.; Perez Duque, M.; Almeida, S.; Cruz, I.; Sottomayor, A.; Almeida, I.; R Oliveira, J.; Antunes, D. Measles Outbreak in a Tertiary Level Hospital, Porto, Portugal, 2018: Challenges in the Post-Elimination Era. Eurosurveillance 2018, 23, 18-00224. [Google Scholar] [CrossRef]
- Hiller, U.; Mankertz, A.; Köneke, N.; Wicker, S. Hospital Outbreak of Measles—Evaluation and Costs of 10 Occupational Cases among Healthcare Worker in Germany, February to March 2017. Vaccine 2019, 37, 1905–1909. [Google Scholar] [CrossRef]
- Pérez-Alba, E.; García-Ortiz, A.; Salazar-Montalvo, R.G.; Hernández-Guedea, M.A.; Camacho-Ortiz, A. Mumps Outbreak with High Complication Rates among Residents in a University Teaching Hospital. Am. J. Infect. Control. 2019, 47, 337–339. [Google Scholar] [CrossRef]
- Sundell, N.; Dotevall, L.; Sansone, M.; Andersson, M.; Lindh, M.; Wahlberg, T.; Tyrberg, T.; Westin, J.; Liljeqvist, J.Å.; Bergström, T.; et al. Measles Outbreak in Gothenburg Urban Area, Sweden, 2017 to 2018: Low Viral Load in Breakthrough Infections. Eurosurveillance 2019, 24, 1900114. [Google Scholar] [CrossRef]
- Koivisto, K.; Puhakka, L.; Lappalainen, M.; Blomqvist, S.; Saxén, H.; Nieminen, T. Immunity against Vaccine-Preventable Diseases in Finnish Pediatric Healthcare Workers in 2015. Vaccine 2017, 35, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- von Linstow, M.; Yde Nielsen, A.; Kirkby, N.; Eltvedt, A.; Nordmann Winther, T.; Bybeck Nielsen, A.; Bang, D.; Poulsen, A. Immunity to Vaccine-Preventable Diseases among Paediatric Healthcare Workers in Denmark, 2019. Eurosurveillance 2019, 26, 2001167. [Google Scholar] [CrossRef]
- Basu, S.; Giri, P.; Adisesh, A.; McNaught, R. Healthcare Workers and Measles-Mumps-Rubella (MMR) Status: How Worried Should We Be about Further Outbreaks? Epidemiol. Infect. 2014, 142, 1688–1694. [Google Scholar] [CrossRef]
- Fedeli, U.; Zanetti, C.; Saia, B. Susceptibility of Healthcare Workers to Measles, Mumps Rubella and Varicella. J. Hosp. Infect. 2002, 51, 133–135. [Google Scholar] [CrossRef]
- Schenk, J.; Abrams, S.; Theeten, H.; Van Damme, P.; Beutels, P.; Hens, N. Immunogenicity and Persistence of Trivalent Measles, Mumps, and Rubella Vaccines: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2021, 21, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, N.; Poethko-Müller, C.; Kuhnert, R.; Matysiak-Klose, D.; Koch, J.; Wichmann, O.; Santibanez, S.; Mankertz, A. Seroprevalence of Measles-, Mumps-, and Rubella-Specific Antibodies in the German Adult Population—Cross-Sectional Analysis of the German Health Interview and Examination Survey for Adults (DEGS1). Lancet Reg. Health-Eur. 2021, 7, 100128. [Google Scholar] [CrossRef]
- Perfetto, B.; Paduano, G.; Grimaldi, E.; Sansone, V.; Donnarumma, G.; Di Giuseppe, G. Seroprevalence for Measles, Varicella, Mumps and Rubella in the Trainee Obstetric Population: A Survey in Southern Italy. Vaccines 2024, 12, 335. [Google Scholar] [CrossRef]
- Di Pietrantonj, C.; Rivetti, A.; Marchione, P.; Debalini, M.G.; Demicheli, V. Vaccines for Measles, Mumps, Rubella, and Varicella in Children. Cochrane Database Syst. Rev. 2021, 11, CD004407. [Google Scholar] [CrossRef]
- Kontio, M.; Jokinen, S.; Paunio, M.; Peltola, H.; Davidkin, I. Waning Antibody Levels and Avidity: Implications for MMR Vaccine-Induced Protection. J. Infect. Dis. 2012, 206, 1542–1548. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Mascipinto, S.; Stefanizzi, P.; De Nitto, S.; Germinario, C.; Tafuri, S. Long-Term Immunogenicity after Measles Vaccine vs. Wild Infection: An Italian Retrospective Cohort Study. Hum. Vaccines Immunother. 2021, 17, 2078–2084. [Google Scholar] [CrossRef]
- Porteous, G.H.; Hanson, N.A.; Sueda, L.A.A.; Hoaglan, C.D.; Dahl, A.B.; Ohlson, B.B.; Schmidt, B.E.; Wang, C.C.; Fagley, R.E. Resurgence of Vaccine-Preventable Diseases in the United States: Anesthetic and Critical Care Implications. Anesth. Analg. 2016, 122, 1450–1473. [Google Scholar] [CrossRef]
- Mahallawi, W.H.; Ibrahim, N.A. Seroprevalence against the Measles Virus after Vaccination or Natural Infection in an Adult Population in Madinah, Saudi Arabia. Hum. Vaccines Immunother. 2021, 17, 2522–2529. [Google Scholar] [CrossRef]
- Hahné, S.J.M.; Lochlainn, L.M.N.; Van Burgel, N.D.; Kerkhof, J.; Sane, J.; Yap, K.B.; Van Binnendijk, R.S. Measles Outbreak among Previously Immunized Healthcare Workers, The Netherlands, 2014. J. Infect. Dis. 2016, 214, 1980–1986. [Google Scholar] [CrossRef]
- Vaccine Scheduler|ECDC Vaccine Scheduler. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=3&SelectedCountryIdByDisease=-1 (accessed on 28 December 2024).
- Poethko-Müller, C.; Mankertz, A. Sero-Epidemiology of Measles-Specific IgG Antibodies and Predictive Factors for Low or Missing Titres in a German Population-Based Cross-Sectional Study in Children and Adolescents (KiGGS). Vaccine 2011, 29, 7949–7959. [Google Scholar] [CrossRef]
- Veneti, L.; Borgen, K.; Borge, K.S.; Danis, K.; Greve-Isdahl, M.; Konsmo, K.; Njølstad, G.; Nordbø, S.A.; Øystese, K.S.; Rykkvin, R.; et al. Large Outbreak of Mumps Virus Genotype g among Vaccinated Students in Norway, 2015 to 2016. Eurosurveillance 2018, 23, 1700642. [Google Scholar] [CrossRef]
- Sanyaolu, A.; Okorie, C.; Marinkovic, A.; Ayodele, O.; Abbasi, A.F.; Prakash, S.; Gosse, J.; Younis, S.; Mangat, J.; Chan, H. Measles Outbreak in Unvaccinated and Partially Vaccinated Children and Adults in the United States and Canada (2018–2019): A Narrative Review of Cases. Inq. J. Health Care Organ. Provis. Financ. 2019, 56, 46958019894098. [Google Scholar] [CrossRef]
- Hyle, E.P.; Rao, S.R.; Bangs, A.C.; Gastañaduy, P.; Fiebelkorn, A.P.; Hagmann, S.H.F.; Walker, A.T.; Walensky, R.P.; Ryan, E.T.; Larocque, R.C. Clinical Practices for Measles-Mumps-Rubella Vaccination among US Pediatric International Travelers. JAMA Pediatr. 2020, 174, e194515. [Google Scholar] [CrossRef]
- Medić, S.; Petrović, V.; Lonèarević, G.; Kanazir, M.; Lazarević, I.B.; Adrović, S.R.; Banèević, M.; Muller, C.P.; Hübschen, J.M. Epidemiological, Clinical and Laboratory Characteristics of the Measles Resurgence in the Republic of Serbia in 2014-2015. PLoS ONE 2019, 14, e0224009. [Google Scholar] [CrossRef]
- Robert, A.; Suffel, A.M.; Kucharski, A.J. Long-Term Waning of Vaccine-Induced Immunity to Measles in England: A Mathematical Modelling Study. Lancet Public Health 2024, 9, e766–e775. [Google Scholar] [CrossRef]
- Moss, W.J. Measles. Lancet 2017, 390, 2490–2502. [Google Scholar] [CrossRef]
- Hadaye, R.; Chandanshive, P.D.; Khan, N. Seroprevalence of Measles Antibodies in Women Born between 1985 and 1999 in Metropolitan City in India: A Cross-Sectional Study. BMJ Public Health 2024, 2, e001417. [Google Scholar] [CrossRef]
- Waaijenborg, S.; Hahné, S.J.M.; Mollema, L.; Smits, G.P.; Berbers, G.A.M.; Van Der Klis, F.R.M.; De Melker, H.E.; Wallinga, J. Waning of Maternal Antibodies against Measles, Mumps, Rubella, and Varicella in Communities with Contrasting Vaccination Coverage. J. Infect. Dis. 2013, 208, 10–16. [Google Scholar] [CrossRef]
- White, S.J.; Boldt, K.L.; Holditch, S.J.; Poland, G.A.; Jacobson, R.M. Measles, Mumps, and Rubella. Clin. Obstet. Gynecol. 2012, 55, 550–559. [Google Scholar] [CrossRef]
- Reef, S.E.; Plotkin, S.; Cordero, J.F.; Katz, M.; Cooper, L.; Schwartz, B.; Zimmerman-Swain, L.; Danovaro-Holliday, M.C.; Wharton, M. Preparing for Elimination of Congenital Rubella Syndrome (CRS): Summary of a Workshop on CRS Elimination in the United States. Clin. Infect. Dis. 2000, 31, 85–95. [Google Scholar] [CrossRef]
- Bergløv, A.; Hallager, S.; Panum, I.; Weis, N. Prevalence of Herpes-, Measles Morbillivirus-, Parvovirus B19- and Rubella Viruses Immunoglobulin G among Women with Chronic Hepatitis B of Reproductive Age in Denmark: A Cross-Sectional Study. Int. J. Infect. Dis. 2020, 101, 269–275. [Google Scholar] [CrossRef]
- Marchi, S.; Monti, M.; Viviani, S.; Montomoli, E.; Trombetta, C.M. Measles in Pregnancy: A Threat for Italian Women? Hum. Vaccines Immunother. 2019, 15, 2851–2853. [Google Scholar] [CrossRef]
- Fine, P.E.M. Herd Immunity: History, Theory, Practice. Epidemiol. Rev. 1993, 15, 265–302. [Google Scholar] [CrossRef]
- Official Gazette of the Republic of Serbia Rulebook on the Program of Mandatory and Recommended Immunization of the Population Against Certain Infectious Diseases No 88/2018, 11/2018, 14/2018, 45/2018, 48/2018, 58/2018, 104/2018, 6/2021, 52/2021 i 66/2022. Available online: https://www.paragraf.rs/propisi/pravilnik-o-programu-obavezne-i-preporucene-imunizacije-stanovnistva-protiv-odredjenih-zaraznih-bolesti.html (accessed on 13 May 2025).
- Official Gazette of the Republic of Serbia Rulebook on the Program of Mandatory and Recommended Immunization of the Population Against Certain Communicable Diseases No 23/2023. Available online: https://www.paragraf.rs/propisi/pravilnik-o-programu-obavezne-i-preporucene-imunizacije-stanovnistva-protiv-odredjenih-zaraznih-bolesti.html (accessed on 13 May 2025).
- Alp, E.; Cevahir, F.; Gökahmetoglu, S.; Demiraslan, H.; Doganay, M. Prevaccination Screening of Health-Care Workers for Immunity to Measles, Rubella, Mumps, and Varicella in a Developing Country: What Do We Save? J. Infect. Public Health 2012, 5, 127–132. [Google Scholar] [CrossRef]

| Characteristics | Number of Samples | Measles | p * | Mumps | p * | Rubella | p * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative % (95% CI) | Equivocal % (95% CI) | Positive % (95% CI) | Negative % (95% CI) | Equivocal % (95% CI) | Positive % (95% CI) | Negative % (95% CI) | Equivocal % (95% CI) | Positive % (95% CI) | |||||
| Total | 1296 | 25.6 (23.3–28.1) | 11.3 (9.7–13.2) | 63.0 (60.3–65.7) | 26.5 (24.1–29.0) | 13.3 (11.5–15.3) | 60.2 (57.5–62.9) | 4.4 (3.4–5.7) | 3.4 (2.5–4.5) | 92.2 (90.6–93.6) | |||
| Gender | Valid Number | ||||||||||||
| Male | 264 | 29.2 (23.8–35.1) | 9.8 (6.5–14.1) | 61.0 (54.8–66.9) | 0.315 | 31.1 (25.5–37.0) | 13.3 (9.4–18.0) | 55.7 (49.5–61.8) | 0.162 | 3.8 (1.8–6.9) | 1.9 (0.6–4.4) | 94.3 (90.8–96.8) | 0.248 |
| Female | 1026 | 24.9 (22.2–27.6) | 11.6 (9.7–13.7) | 63.5 (60.5–66.5) | 25.4 (22.7–28.1) | 13.2 (11.2–15.4) | 61.5 (58.4–64.5) | 4.6 (3.4–6.1) | 3.8 (2.7–5.2) | 91.6 (89.7–93.2) | |||
| Age groups | Valid Number | ||||||||||||
| 19–20 | 159 | 25.2 (18.6–32.6) | 11.3 (6.8–17.3) | 63.5 (55.5–71.0) | 0.065 | 18.9 (13.1–25.8) | 13.2 (8.4–19.5) | 67.9 (60.1–75.1) | 0.035 | 1.9 (0.4–5.4) | 2.5 (0.7–6.3) | 95.6 (91.1–98.2) | 0.182 |
| 21–23 | 681 | 24.1 (20.9–27.5) | 11.0 (8.8–13.6) | 64.9 (61.2–68.5) | 26.1 (22.9–29.6) | 14.1 (11.6–16.9) | 59.8 (56.0–63.5) | 5.6 (0.4–7.6) | 2.8 (1.7–4.3) | 91.6 (89.3–93.6) | |||
| 24–26 | 355 | 25.6 (21.2–30.5) | 12.4 (9.2–16.3) | 62.0 (56.7–67.0) | 28.7 (24.1–33.7) | 10.7 (7.7–14.4) | 60.6 (55.3–65.7) | 3.9 (2.2–6.5) | 5.1 (0.3–7.9) | 91.0 (87.5–93.8) | |||
| 27–29 | 86 | 40.7 (30.2–51.8) | 10.5 (4.9–18.9) | 48.8 (37.9–59.9) | 34.9 (24.9–45.9) | 17.4 (10.1–27.1) | 47.7 (36.8–58.7) | 3.5 (0.7–9.9) | 3.5 (0.7–9.9) | 93.0 (85.4–97.4) | |||
| Region of origin | Valid Number | ||||||||||||
| Major urban | 632 | 25.0 (21.7–28.6) | 11.9 (9.4–14.6) | 63.1 (59.2–66.9) | 0.785 | 26.7 (23.3–30.4) | 12.8 (10.3–15.7) | 60.4 (56.6–64.3) | 0.894 | 5.2 (3.6–7.3) | 4.0 (2.6–5.8) | 90.8 (88.3–93.0) | 0.234 |
| Other urban and rural | 664 | 26.2 (22.9–29.7) | 10.8 (8.6–13.5) | 63.0 (59.2–66.6) | 26.4 (23.0–29.9) | 13.7 (11.2–16.6) | 59.9 (56.1–63.7) | 3.8 (2.5–5.5) | 2.9 (1.7–4.4) | 93.4 (91.2–95.1) | |||
| Attended nursery/kindergarten | Valid Number | ||||||||||||
| Yes | 1140 | 25.7 (23.2–28.3) | 11.4 (9.6–13.4) | 62.9 (60.0–65.7) | 0.962 | 27.3 (24.7–30.0) | 13.6 (11.7–15.7) | 59.1 (56.2–62.0) | 0.072 | 4.7 (3.6–6.1) | 3.2 (2.2–4.3) | 92.1 (90.4–93.6) | 0.124 |
| No | 153 | 24.8 (18.2–32.5) | 11.1 (6.6–17.2) | 64.1 (55.9–71.6) | 20.3 (14.2–27.5) | 11.1 (6.6–17.2) | 68.6 (60.6–75.9) | 2.0 (0.4–5.6) | 5.2 (2.3–10.0) | 92.8 (87.5–96.4) | |||
| Migration | Valid Number | ||||||||||||
| Yes | 551 | 25.4 (21.8–29.3) | 11.1 (8.6–14.0) | 63.5 (59.3–67.5) | 0.944 | 26.0 (22.4–29.9) | 14.0 (11.2–17.2) | 60.0 (55.8–64.1) | 0.816 | 3.4 (2.1–5.3) | 3.6 (2.2–5.6) | 92.9 (90.5–94.9) | 0.282 |
| No | 741 | 25.6 (22.5–28.9) | 11.6 (9.4–14.1) | 62.8 (59.2–66.2) | 27.0 (23.8–30.3) | 12.8 (10.5–15.4) | 60.2 (56.6–63.7) | 5.1 (3.7–7.0) | 3.2 (2.1–4.8) | 91.6 (89.4–93.5) | |||
| Self-reported vaccination status | Valid Number | ||||||||||||
| Yes | 1138 | 24.1 (21.6–26.7) | 11.7 (9.9–13.7) | 64.2 (61.4–67.0) | 0.025 | 25.5 (23.0–28.1) | 12.9 (11.0–15.0) | 61.6 (58.7–64.4) | 0.017 | 4.1 (3.1–5.5) | 3.4 (2.5–4.7) | 92.4 (90.7–93.9) | 0.513 |
| No | 152 | 34.8 (26.8–43.5) | 9.6 (5.2–15.9) | 55.6 (46.8–64.1) | 34.8 (26.8–43.5) | 16.3 (10.5–23.6) | 48.9 (40.2–57.6) | 5.9 (2.6–11.3) | 3.4 (0.5–6.4) | 91.9 (85.9–95.9) | |||
| Characteristics | GMCs of IgG Antibodies | |||||
|---|---|---|---|---|---|---|
| Anti-Measles (IU/L) | p * | Anti-Mumps (RU/mL) | p * | Anti-Rubella (IU/mL) | p * | |
| Gender | ||||||
| Male | 359.75 ± 2.60 | 0.225 | 27.23 ± 2.43 | 0.050 | 34.04 ± 2.29 | 0.987 |
| Female | 389.94 ± 2.67 | 30.69 ± 2.39 | 34.04 ± 2.35 | |||
| Age groups | ||||||
| 19–20 | 427.56 ± 2.79 | 0.056 | 38.90 ± 2.39 | <0.001 | 38.37 ± 2.26 | 0.067 |
| 21–23 | 384.59 ± 2.55 | 29.51 ± 2.33 | 33.11 ± 2.36 | |||
| 24–26 | 384.59 ± 2.73 | 29.04 ± 2.48 | 34.51 ± 2.34 | |||
| 27–29 | 299.23 ± 2.96 | 23.99 ± 2.43 | 28.84 ± 2.11 | |||
| Region of origin | ||||||
| Major urban | 384.59 ± 2.70 | 0.989 | 30.20 ± 2.44 | 0.755 | 34.36 ± 2.40 | 0.743 |
| Other urban and rural | 384.59 ± 2.62 | 29.72 ± 2.36 | 33.81 ± 2.28 | |||
| Attended nursery/kindergarten | ||||||
| Yes | 380.19 ± 2.67 | 0.210 | 29.38 ± 2.40 | 0.020 | 33.57 ± 2.33 | 0.144 |
| No | 422.67 ± 2.59 | 34.99 ± 2.38 | 37.41 ± 2.36 | |||
| Migration | ||||||
| Yes | 390.84 ± 2.59 | 0.621 | 28.97 ± 2.39 | 0.276 | 34.20 ± 2.34 | 0.881 |
| No | 380.19 ± 2.70 | 30.62 ± 2.41 | 33.96 ± 2.34 | |||
| Self-reported vaccination status | ||||||
| Yes | 397.19 ± 2.64 | 0.003 | 30.62 ± 2.38 | 0.005 | 34.12 ± 2.32 | 0.771 |
| No | 306.20 ± 2.67 | 24.55 ± 2.49 | 33.34 ± 2.45 | |||
| Virus | Characteristics | Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI OR | p * | OR | 95% CI OR | p * | OR a | p *a | |||
| Measles | Gender | Female | Ref. | |||||||
| Male | 1.245 | 0.92–1.68 | 0.153 | |||||||
| Age groups | 19–20 | 1.060 | 0.71–1.58 | 0.776 | 1.054 | 0.70–1.58 | 0.800 | 1.071 | 0.748 | |
| 21–23 | Ref. | Ref. | Ref. | |||||||
| 24–26 | 1.087 | 0.81–1.46 | 0.582 | 1.138 | 0.84–1.54 | 0.398 | 1.157 | 0.361 | ||
| 27–29 | 2.163 | 1.36–3.44 | 0.001 | 2.151 | 1.34–3.45 | 0.001 | 2.155 | 0.002 | ||
| Region of origin | Major urban | Ref. | ||||||||
| Other urban or rural | 1.065 | 0.83–1.37 | 0.619 | |||||||
| Nursery/kindergarten attendance | No | Ref. | ||||||||
| Yes | 1.047 | 0.71–1.55 | 0.818 | |||||||
| Migration | Yes | Ref. | ||||||||
| No | 1.012 | 0.79–1.30 | 0.924 | |||||||
| Self-reported vaccination status | Yes | Ref. | Ref. | Ref. | ||||||
| No | 1.684 | 1.15–2.46 | 0.007 | 1.730 | 1.18–2.54 | 0.005 | 1.701 | 0.007 | ||
| Mumps | Gender | Female | Ref. | |||||||
| Male | 1.321 | 0.98–1.78 | 0.066 | |||||||
| Age groups | 19–20 | Ref. | Ref. | Ref. | ||||||
| 21–23 | 1.522 | 0.99–2.35 | 0.057 | 1.525 | 0.99–2.36 | 0.058 | 1.496 | 0.053 | ||
| 24–26 | 1.734 | 1.10–2.74 | 0.019 | 1.796 | 1.13–2.85 | 0.013 | 1.771 | 0.009 | ||
| 27–29 | 2.304 | 1.27–4.18 | 0.006 | 2.217 | 1.21–4.05 | 0.010 | 2.179 | 0.006 | ||
| Region of origin | Major urban | Ref. | ||||||||
| Other urban or rural | 0.980 | 0.77–1.26 | 0.875 | |||||||
| Nursery/kindergarten attendance | No | Ref. | ||||||||
| Yes | 1.483 | 0.98–2.25 | 0.063 | |||||||
| Migration | Yes | Ref. | ||||||||
| Self-reported vaccination status | No | 1.045 | 0.81–1.34 | 0.731 | ||||||
| Yes | Ref. | Ref. | Ref. | |||||||
| No | 1.555 | 1.07–2.27 | 0.022 | 1.675 | 1.14–2.46 | 0.009 | 1.646 | 0.011 | ||
| Rubella | Gender | Male | Ref. | |||||||
| Female | 1.247 | 0.62–2.50 | 0.534 | |||||||
| Age groups | 19–20 | Ref. | ||||||||
| 21–23 | 3.073 | 0.94–10.09 | 0.064 | |||||||
| 24–26 | 2.135 | 0.61–7.54 | 0.239 | |||||||
| 27–29 | 1.880 | 0.37–9.52 | 0.446 | |||||||
| Region of origin | Other urban or rural | Ref. | ||||||||
| Major urban | 1.408 | 0.83–2.40 | 0.207 | |||||||
| Nursery/kindergarten attendance | No | Ref. | ||||||||
| Yes | 2.535 | 0.78–8.20 | 0.121 | |||||||
| Migration | Yes | Ref. | ||||||||
| No | 0.643 | 0.37–1.13 | 0.122 | |||||||
| Self-reported vaccination status | Yes | Ref. | ||||||||
| No | 1.430 | 0.66–3.09 | 0.363 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banko, A.; Cirkovic, A.; Petrovic, V.; Ristic, M.; Vukovic, V.; Stankovic-Djordjevic, D.; Miljanovic, D. Seroprevalence of Measles-, Mumps-, and Rubella-Specific Antibodies in Future Healthcare Workers in Serbia: A Cross-Sectional Study. Vaccines 2025, 13, 700. https://doi.org/10.3390/vaccines13070700
Banko A, Cirkovic A, Petrovic V, Ristic M, Vukovic V, Stankovic-Djordjevic D, Miljanovic D. Seroprevalence of Measles-, Mumps-, and Rubella-Specific Antibodies in Future Healthcare Workers in Serbia: A Cross-Sectional Study. Vaccines. 2025; 13(7):700. https://doi.org/10.3390/vaccines13070700
Chicago/Turabian StyleBanko, Ana, Andja Cirkovic, Vladimir Petrovic, Mioljub Ristic, Vladimir Vukovic, Dobrila Stankovic-Djordjevic, and Danijela Miljanovic. 2025. "Seroprevalence of Measles-, Mumps-, and Rubella-Specific Antibodies in Future Healthcare Workers in Serbia: A Cross-Sectional Study" Vaccines 13, no. 7: 700. https://doi.org/10.3390/vaccines13070700
APA StyleBanko, A., Cirkovic, A., Petrovic, V., Ristic, M., Vukovic, V., Stankovic-Djordjevic, D., & Miljanovic, D. (2025). Seroprevalence of Measles-, Mumps-, and Rubella-Specific Antibodies in Future Healthcare Workers in Serbia: A Cross-Sectional Study. Vaccines, 13(7), 700. https://doi.org/10.3390/vaccines13070700







