A Novel, Safe, Non-Adjuvanted Alphavirus Replicon-Based Vaccine Expressing the Feline Leukemia Virus Envelope Protein Protects Against Virulent FeLV Challenge
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Formulations
2.2. Challenge Virus
2.3. Sample Collection
2.4. FeLV p27 ELISA
2.5. PCR
2.6. RT-PCR
2.7. Total Protein Assay
2.8. Bovine Serum Albumin Assay
2.9. Data Analysis
2.10. Dose-Ranging Study Design
2.11. Comparison Study Design
2.12. Field Safety Study Design
3. Results
3.1. Dose-Ranging Study
3.2. Comparison Study
3.3. Field Safety Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martano, M.; Morello, E.; Buracco, P. Feline Injection-Site Sarcoma: Past, Present and Future Perspectives. Vet. J. 2011, 188, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.K.; Scott, H.M.; Lachtara, J.L.; Crawford, P.C. Seroprevalence of Feline Leukemia Virus and Feline Immunodeficiency virus Infection Among Cats in North America and Risk Factors for Seropositivity. J. Am. Vet. Med. Assoc. 2006, 228, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Little, S.; Sears, W.; Lachtara, J.; Bienzle, D. Seroprevalence of Feline Leukemia Virus and Feline Immunodeficiency Virus Infection Among Cats in Canada. Can. Vet. J. 2009, 50, 644–648. [Google Scholar]
- Burling, A.N.; Levy, J.K.; Scott, H.M.; Crandall, M.M.; Tucker, S.J.; Wood, E.G.; Foster, J.D. Seroprevalences of Feline Leukemia Virus and Feline Immunodeficiency Virus Infection in Cats in the United States and Canada and Risk Factors for Seropositivity. J. Am. Vet. Med. Assoc. 2017, 251, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Studer, N.; Lutz, H.; Saegerman, C.; Gönczi, E.; Meli, M.L.; Boo, G.; Hartmann, K.; Hosie, M.J.; Moestl, K.; Tasker, S.; et al. Pan-European Study on the Prevalence of the Feline Leukaemia Virus Infection—Reported by the European Advisory Board on Cat Diseases (ABCD Europe). Viruses 2019, 11, 993. [Google Scholar] [CrossRef]
- Hartmann, K.; Hofmann-Lehmann, R. What’s New in Feline Leukemia Virus Infection. Vet. Clin. Small Anim. 2020, 50, 1013–1036. [Google Scholar] [CrossRef]
- Torres, A.N.; Mathiason, C.K.; Hoover, E.A. Re-examination of Feline Leukemia Virus: Host Relationships Using Real-Time PCR. Virology 2005, 332, 272–283. [Google Scholar] [CrossRef]
- Beall, M.J.; Buch, J.; Clark, G.; Estrada, M.; Rakitin, A.; Hamman, N.T.; Frenden, M.K.; Jefferson, E.P.; Amirian, E.S.; Levy, J.K. Feline Leukemia Virus p27 Antigen Concentration and Proviral DNA Load are Associated with Survival in Naturally Infected Cats. Viruses 2021, 13, 302. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Cattori, V.; Tandon, R.; Boretti, F.S.; Meli, M.L.; Riond, B.; Lutz, H. How Molecular Methods Change our Views of FeLV Infection and Vaccination. Vet. Immunol. Immunopathol. 2008, 123, 119–123. [Google Scholar] [CrossRef]
- Patel, M.; Carritt, K.; Lane, J.; Jayappa, H.; Stahl, M.; Bourgeois, M. Comparative Efficacy of Feline Leukemia Virus (FeLV) Inactivated Whole-Virus Vaccine and Canarypox Virus-Vectored Vaccine During Virulent FeLV Challenge and Immunosuppression. Clin. Vaccine Immunol. 2015, 22, 798–805. [Google Scholar] [CrossRef]
- Sparkes, A.H. Feline Leukaemia Virus: A Review of Immunity and Vaccination. J. Small Anim. Pract. 1997, 38, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Stuke, K.; King, V.; Southwick, K.; Stoeva, M.I.; Thomas, A.; Winkler, M.T.C. Efficacy of an Inactivated FeLV Vaccine Compared to a Recombinant FeLV Vaccine in Minimum Age Cats Following Virulent FeLV Challenge. Vaccine 2014, 32, 2599–2603. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.N.; O’Halloran, K.P.; Larson, L.J.; Schultz, R.D.; Hoover, E.A. Feline Leukemia Virus Immunity Induced by Whole Inactivated Virus Vaccination. Vet. Immunol. Immunopathol. 2010, 134, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Tizard, I.R. Adjuvants and Adjuvanticity. In Vaccines for Veterinarians, 1st ed.; Tizard, I.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 75–86. [Google Scholar] [CrossRef]
- Jirjis, F.F.; Davis, T.; Lane, J.; Carritt, K.; Sweeney, D.; Williams, J.; Wasmoen, T. Protection Against Feline Leukemia Virus Challenge for at Least 2 Years After Vaccination with an Inactivated Feline Leukemia Virus Vaccine. Vet. Ther. 2010, 11, E1–E6. [Google Scholar]
- Hartmann, K.; Egberink, H.; Möstl, K.; Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Frymus, T.; Lloret, A.; Hofmann-Lehmann, R.; Marsilio, F.; et al. Feline Injection-Site Sarcoma and Other Adverse Reactions to Vaccination in Cats. Viruses 2023, 15, 1708. [Google Scholar] [CrossRef]
- Stone, A.E.; Brummet, G.O.; Carozza, E.M.; Kass, P.H.; Petersen, E.P.; Sykes, J.; Westman, M.E. 2020 AAHA/AAFP Feline Vaccination Guidelines. J. Feline Med. Surg. 2020, 22, 813–830. [Google Scholar] [CrossRef]
- Squires, R.A.; Crawford, C.; Marcondes, M.; Whitley, N. 2024 Guidelines for the Vaccination of Dogs and Cats—Compiled by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association (WSAVA). J. Small Anim. Pract. 2024, 65, 277–316. [Google Scholar] [CrossRef]
- Tartaglia, J.; Jarrett, O.; Neil, J.C.; Desmettre, P.; Paoletti, E. Protection of Cats Against Feline Leukemia Virus by Vaccination with a Canarypox Virus Recombinant, ALVAC-FL. J. Virol. 1993, 67, 2370–2375. [Google Scholar] [CrossRef]
- Grosenbaugh, D.A.; Frances-Duvert, V.; Abedi, S.; Feilmeier, B.; Ru, H.; Poulet, H. Efficacy of a Nonadjuvanted Recombinant FeLV Vaccine and Two Inactivated FeLV Vaccines When Subject to Consistent Virulent FeLV Challenge Conditions. Biologicals 2017, 49, 76–80. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Tasker, S.; Thibault, J.C.; Poulet, H. A Comparative Study of the Efficacy of a Canarypox Based Recombinant Leukaemia Vaccine Against a Natural Contact FeLV Challenge in Cats. J. Vaccines Vaccin. 2016, 6, 300. [Google Scholar] [CrossRef]
- Kamrud, K.I.; Custer, M.; Dudek, J.M.; Owens, G.; Alterson, K.D.; Lee, J.S.; Groebner, J.L.; Smith, J.F. Alphavirus Replicon Approach to Promoterless Analysis of IRES Elements. Virology 2007, 360, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Vander Veen, R.L.; Loynachan, A.T.; Mogler, M.A.; Russell, B.J.; Harris, D.L.H.; Kamrud, K.I. Safety, Immunogenicity, and Efficacy of an Alphavirus Replicon-Based Swine Influenza Virus Hemagglutinin Vaccine. Vaccine 2012, 30, 1944–1950. [Google Scholar] [CrossRef]
- Langereis, M.A.; Albulescu, I.C.; Stammen-Vogelzangs, J.; Lambregts, M.; Stachura, K.; Miller, S.; Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Allen, M.; et al. Alphavirus Replicon-Based Vaccine Expressing a Stabilized Spike Antigen Induces Protective Immunity and Prevents Transmission of SARS-CoV-2 Between Cats. npj Vaccine 2021, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Vander Veen, R.L.; Harris, D.L.H.; Kamrud, K.I. Alphavirus Replicon Vaccines. Anim. Health Res. Rev. 2012, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Parker, M.; Ludwig, G.V.; Davis, N.L.; Johnston, R.E.; Smith, J.F. Replicon-Helper Systems from Attenuated Venezuelan Equine Encephalitis Virus: Expression of Heterologous Genes In Vitro and Immunization Against Heterologous Pathogens In Vivo. Virology 1997, 239, 389–401. [Google Scholar] [CrossRef]
- Mogler, M.A.; Kamrud, K.I. RNA-Based Viral Vectors. Expert Rev. Vaccines 2015, 14, 283–312. [Google Scholar] [CrossRef]
- Cattori, V.; Hofmann-Lehmann, R. Absolute Quantitation of Feline Leukemia Virus Proviral DNA and Viral RNA Loads by TaqMan® Real-Time PCR and RT-PCR. In Molecular Beacons: Signalling Nucleic Acid Probes, Methods and Protocols; Marx, A., Seitz, O., Eds.; Human Press: Totowa, NJ, USA, 2008; Volume 429, pp. 73–87. [Google Scholar]
- Shibley, G.P.; Tanner, J.E.; Hanna, S.A. United States Department of Agriculture Licensing Requirements for Feline Leukemia Virus Vaccines. J. Am. Vet. Med. Assoc. 1991, 199, 1402–1406. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Huder, J.B.; Gruber, S.; Boretti, F.; Sigrist, B.; Lutz, H. Feline Leukemia Provirus Load During the Course of Experimental Infection and in Naturally Infected Cats. J. Gen. Virol. 2001, 82, 1589–1596. [Google Scholar] [CrossRef]
- Helfer-Hungerbuehler, A.K.; Spiri, A.M.; Riond, B.; Grest, P.; Boretti, F.S.; Hofmann-Lehmann, R. No Benefit of Therapeutic Vaccination in Clinically Healthy Cats Persistently Infected with Feline Leukemia Virus. Vaccine 2015, 13, 1578–1585. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Cattori, V.; Tandon, R.; Boretti, F.S.; Meli, M.L.; Riond, B.; Pepin, A.C.; Willi, B.; Ossent, P.; Lutz, H. Vaccination Against the Feline Leukemia Virus: Outcome and Response Categories and Long-Term Follow-Up. Vaccine 2007, 25, 5531–5539. [Google Scholar] [CrossRef]
- Hofmann-Lehmann, R.; Levy, L.S.; Willett, B.J. Comparing the Efficacy of FeLV Vaccines: Comment on: Stuke, K. et al. Efficacy of an Inactivated FeLV Vaccine Compared to a Recombinant FeLV Vaccine in Minimum Age Cats Following Virulent FeLV Challenge Vaccine 2014;32(22):2599-603. Vaccine 2015, 33, 2737–2738. [Google Scholar] [CrossRef]
- Poulet, H.; Thibault, J.C.; Masias, A. Immunosuppression in a Comparative Study of Feline Leukemia Virus Vaccines. Clin. Vaccine Immunol. 2015, 22, 1294–1295. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.M.C.; Petrovajová, D.; Horňáková, T. Viral Vaccine Stabilizers: Status and Trends. Acta Virol. 2017, 61, 231–239. [Google Scholar] [CrossRef]
- Ohmori, K.; Masuda, K.; Maeda, S.; Kaburagi, Y.; Kurata, K.; Ohno, K.; DeBoer, D.J.; Tsujimoto, H.; Sakaguchi, M. IgE Reactivity to Vaccine Components in Dogs that Developed Immediate-Type Allergic Reactions After Vaccination. Vet. Immunol. Immunopathol. 2005, 104, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Mizukami, K.; Hisasue, M.; Imanishi, I.; Kurata, K.; Ochiai, M.; Itoh, M.; Nasukawa, T.; Uchiyama, J.; Tsujimoto, H.; et al. Anaphylaxis After Vaccination for Cats in Japan. J. Vet. Med. Sci. 2022, 84, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Osterhaus, A.; Weijer, K.; Uytdehaag, F.; Jarrett, O.; Sundquist, B.; Morein, B. Induction of Protective Immune Response in Cats by Vaccination with Feline Leukemia Virus ISCOM. J. Immunol. 1985, 135, 591–596. [Google Scholar] [CrossRef]
- Weijer, K.; Pfauth, A.; van Herwijnen, R.; Jarrett, O.; Meloen, R.H.; Tomee, C.; Osterhaus, A.D.M.E. Induction of Feline Leukemia Virus-Neutralizing Antibodies by Immunization with Synthetic Peptides Derived from the FeLV env Gene. Vaccine 1993, 11, 946–956. [Google Scholar] [CrossRef]
- White, C.N. The Perilous P Value. J. Am. Vet. Med. Assoc. 2025, 263, 267–270. [Google Scholar] [CrossRef]
- Moore, G.E.; DeSantis-Kerr, A.C.; Guptill, L.F.; Glickman, N.W.; Lewis, H.D.; Glickman, L.T. Adverse Events After Vaccine Administration in Cats: 2,560 Cases (2002–2005). J. Am. Vet. Med. Assoc. 2007, 231, 94–100. [Google Scholar] [CrossRef]
- Animal and Plant Health Inspection Service, United States Department of Agriculture, Veterinary Biologics, Product Summaries. Available online: https://www.aphis.usda.gov/165a-1555r8 (accessed on 17 June 2025).
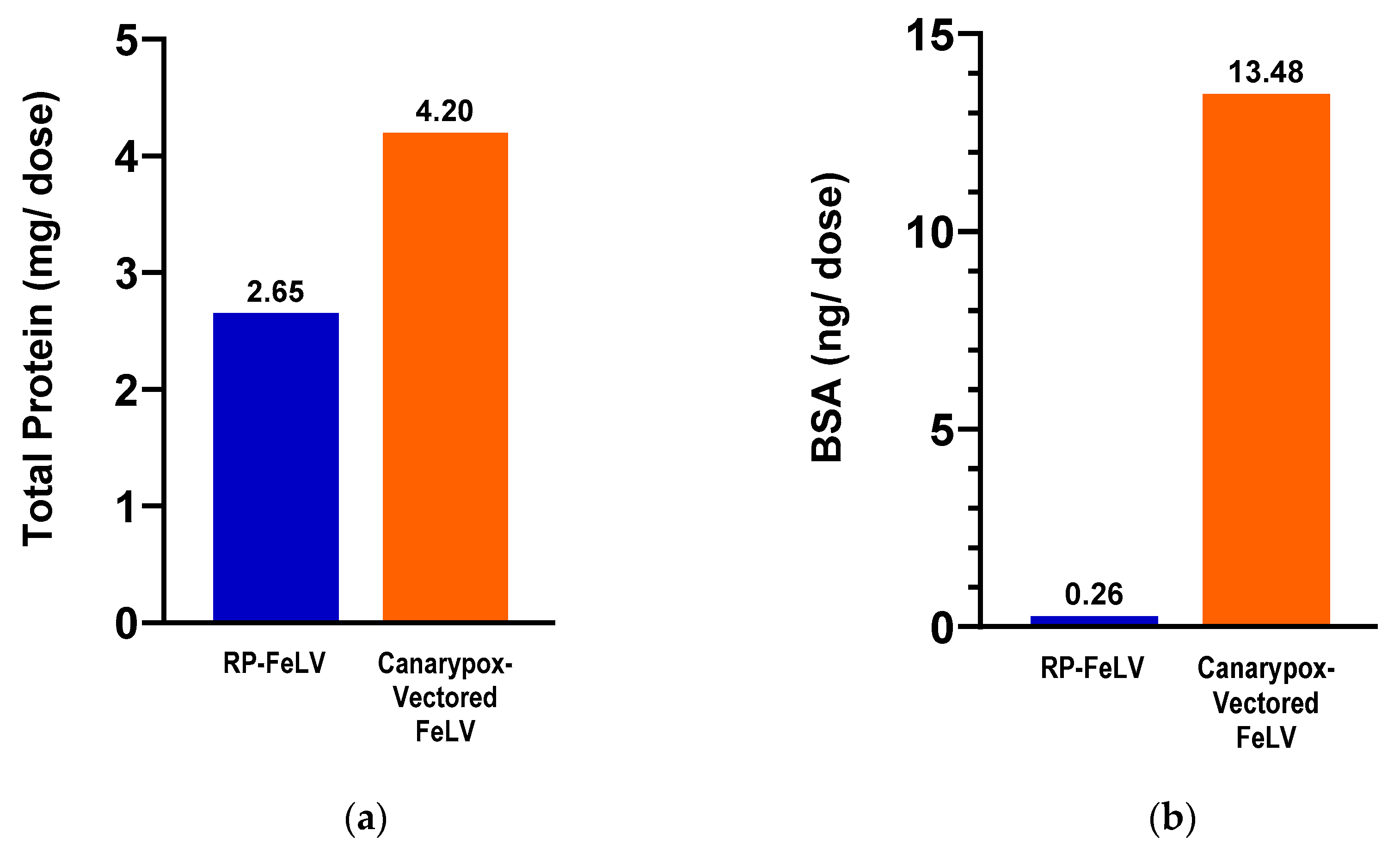
| Treatment Group | Number of Cats | Vaccine | Vaccination Days |
|---|---|---|---|
| 1 | 8 | RP-FeLV 2.5 × 108 RP/dose | 0, 21 |
| 2 | 8 | RP-FeLV 3.6 × 107 RP/dose | 0, 21 |
| 3 | 8 | RP-FeLV 1.5 × 108 RP/dose | 21 |
| 4 | 8 | Canarypox-vectored FeLV | 0, 21 |
| 5 | 8 | Placebo | 0, 21 |
| Treatment Group | Vaccine | Cats Antigenemic | Preventable Fraction 95% Confidence Interval [LL, UL] |
|---|---|---|---|
| 1 | RP-FeLV 2.5 × 108 RP/dose | 0/8 | 100% [60%, 100%] |
| 2 | RP-FeLV 3.6 × 107 RP/dose | 0/8 | 100% [60%, 100%] |
| 3 | RP-FeLV 1.5 × 108 RP/dose | 1/8 | 86% [33%, 99%] |
| 4 | Canarypox-vectored FeLV | 3/7 | 51% [−11%, 92%] |
| 5 | Placebo | 7/8 | - |
| Treatment Group | Vaccine | Cats Antigenemic | Preventable Fraction 95% Confidence Interval [LL, UL] |
|---|---|---|---|
| A | RP-FeLV | 0/10 | 100% [69%, 100%] |
| B | Canarypox-vectored FeLV | 3/10 | 70% [35%, 93%] |
| C | Placebo | 10/10 | - |
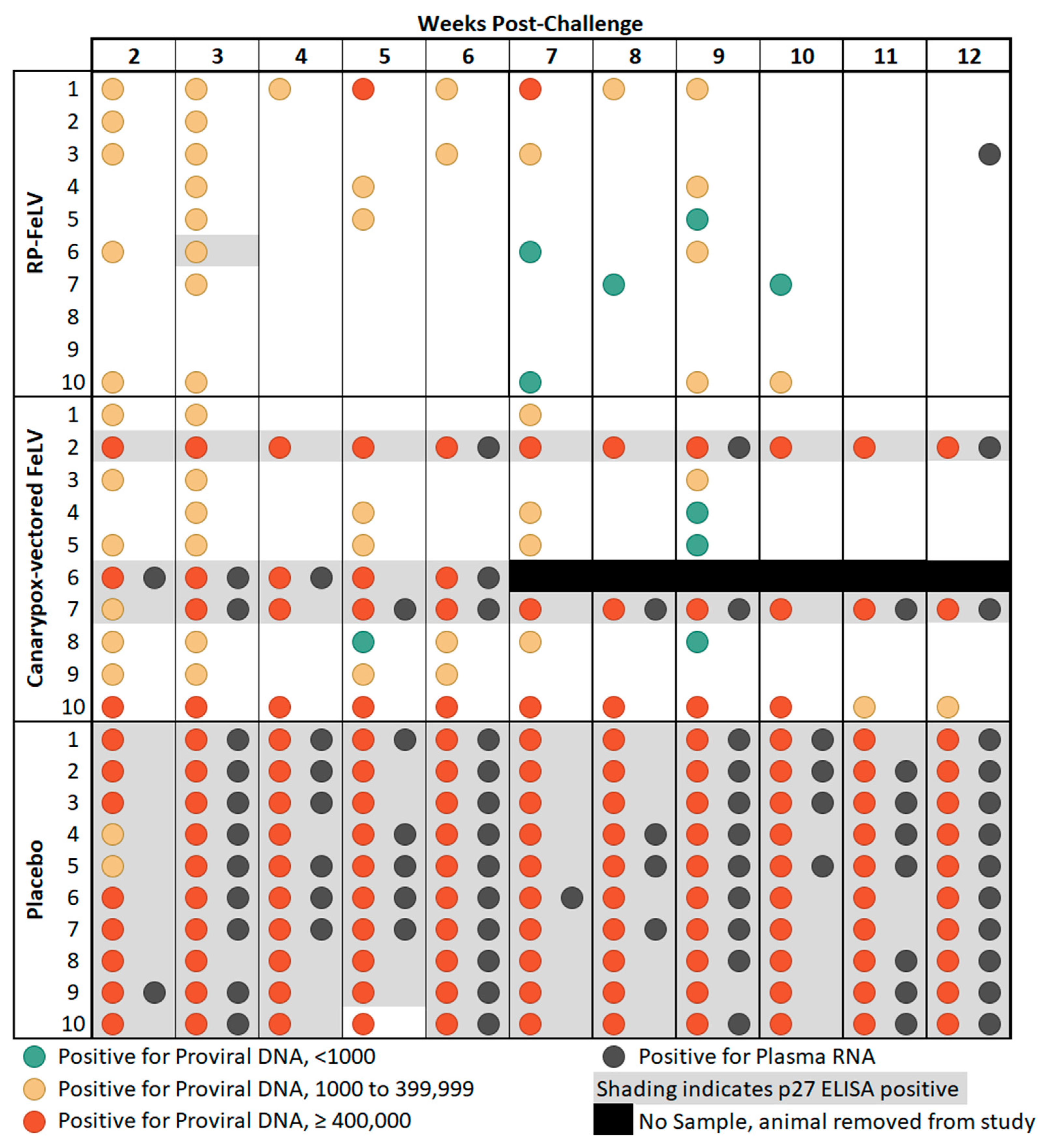
| Weeks Post-Challenge | Proportion of Group Positive for Proviral DNA (p-Value) | Proportion of Group Positive for RNA (p-Value) | ||
|---|---|---|---|---|
| RP-FeLV vs. Placebo | Canarypox- Vectored FeLV vs. Placebo | RP-FeLV vs. Placebo | Canarypox- Vectored FeLV vs. Placebo | |
| 2 | 5 vs. 10 (0.0325) | 9 vs. 10 (>0.9999) | 0 vs. 1 (>0.9999) | 1 vs. 1 (>0.9999) |
| 3 | 8 vs. 10 (0.4737) | 10 vs. 10 (>0.9999) | 0 vs. 9 (0.0001) | 2 vs. 9 (0.0055) |
| 4 | 1 vs. 10 (0.0001) | 4 vs. 10 (0.0108) | 0 vs. 6 (0.0108) | 1 vs. 6 (0.0573) |
| 5 | 3 vs. 10 (0.0031) | 8 vs. 10 (0.4737) | 0 vs. 5 (0.0325) | 1 vs. 5 (0.1409) |
| 6 | 2 vs. 10 (0.0007) | 6 vs. 10 (0.0867 | 0 vs. 10 (0.0001) | 3 vs. 10 (0.0031) |
| 7 | 4 vs. 10 (0.0108) | 9 vs. 10 (0.2105) | 0 vs. 1 (>0.9999) | 0 vs. 1 (>0.9999) |
| 8 | 2 vs. 10 (0.0007) | 3 vs. 10 (0.0031) | 0 vs. 3 (0.2105) | 1 vs. 3 (0.5820) |
| 9 | 5 vs. 10 (0.0325) | 7 vs. 10 (0.2105) | 0 vs. 9 (0.0001) | 2 vs. 9 (0.0055) |
| 10 | 2 vs. 10 (0.0007) | 3 vs. 10 (0.0031) | 0 vs. 4 (0.0867) | 0 vs. 4 (0.0867) |
| 11 | 0 vs. 10 (<0.0001) | 3 vs. 10 (0.0031) | 0 vs. 7 (0.0031) | 1 vs. 7 (0.0198) |
| 12 | 0 vs. 10 (<0.0001) | 3 vs. 10 (0.0031) | 1 vs. 10 (0.0001) | 2 vs. 10 (0.0007) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carritt, K.; Davis, R.; Stachura, K.; Crumley, P.; Mogler, M.; Stahl, M.; Deng, L.; Xu, Z.; Tarpey, I. A Novel, Safe, Non-Adjuvanted Alphavirus Replicon-Based Vaccine Expressing the Feline Leukemia Virus Envelope Protein Protects Against Virulent FeLV Challenge. Vaccines 2025, 13, 697. https://doi.org/10.3390/vaccines13070697
Carritt K, Davis R, Stachura K, Crumley P, Mogler M, Stahl M, Deng L, Xu Z, Tarpey I. A Novel, Safe, Non-Adjuvanted Alphavirus Replicon-Based Vaccine Expressing the Feline Leukemia Virus Envelope Protein Protects Against Virulent FeLV Challenge. Vaccines. 2025; 13(7):697. https://doi.org/10.3390/vaccines13070697
Chicago/Turabian StyleCarritt, Kari, Randall Davis, Ken Stachura, Paige Crumley, Mark Mogler, Madeleine Stahl, Lijuan Deng, Zach Xu, and Ian Tarpey. 2025. "A Novel, Safe, Non-Adjuvanted Alphavirus Replicon-Based Vaccine Expressing the Feline Leukemia Virus Envelope Protein Protects Against Virulent FeLV Challenge" Vaccines 13, no. 7: 697. https://doi.org/10.3390/vaccines13070697
APA StyleCarritt, K., Davis, R., Stachura, K., Crumley, P., Mogler, M., Stahl, M., Deng, L., Xu, Z., & Tarpey, I. (2025). A Novel, Safe, Non-Adjuvanted Alphavirus Replicon-Based Vaccine Expressing the Feline Leukemia Virus Envelope Protein Protects Against Virulent FeLV Challenge. Vaccines, 13(7), 697. https://doi.org/10.3390/vaccines13070697





