Childhood Immunization Coverage Before, During and After the COVID-19 Pandemic in Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Joinpoint Regression Analysis of Vaccination Coverage Trends (2014–2021)
2.3. Period Definitions
- Pre- vs. post-mandate phase (2015–2016 vs. 2017–2019): To assess the impact of Law 119/2017 introducing mandatory childhood vaccinations.
- Pandemic phase (2017–2019 vs. 2020–2021): To capture the impact of the COVID-19 pandemic on immunization activities.
- Post-pandemic recovery phase (2020–2021 vs. 2022–2023): To assess the recovery in vaccine coverage following the acute phase of the pandemic.
2.4. Regional Comparisons by Geographic Area
2.5. Correlation Between Baseline Coverage and Variation
3. Results
3.1. Temporal Trends in Vaccination Coverage and Joinpoint Analysis
3.2. Regional Trends Across Key Phases (2015–2023)
3.3. Geographic Differences in Coverage Trends
3.4. Association Between Baseline Coverage and Vaccination Trends
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Causey, K.; Fullman, N.; Sorensen, R.J.D.; Galles, N.C.; Zheng, P.; Aravkin, A.; Danovaro-Holliday, M.C.; Martinez-Piedra, R.; Sodha, S.V.; Velandia-González, M.P.; et al. Estimating global and regional disruptions to routine childhood vaccine coverage during the COVID-19 pandemic in 2020: A modelling study. Lancet 2021, 398, 522–534. [Google Scholar] [CrossRef]
- Chiappini, E.; Parigi, S.; Galli, L.; Licari, A.; Brambilla, I.; Tosca, M.A.; Ciprandi, G.; Marseglia, G. Impact that the COVID-19 pandemic on routine childhood vaccinations and challenges ahead: A narrative review. Acta Paediatr. 2021, 110, 2529–2535. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidance on Routine Immunization Services During COVID-19 Pandemic in the WHO European Region; World Health Organization. Regional Office for Europe: Copenhagen, Denmark, 2020.
- WHO. Guiding Principles for Immunization Activities During the COVID-19 Pandemic: Interim Guidance, 26 March 2020; World Health Organization: Geneva, Switzerland, 2020.
- Law 31 July 217, n. 119. Conversione in Legge, Con Modificazioni, Del Decreto-Legge 7 Giugno 2017, n. 73, Recante Disposizioni Urgenti in Materia di Prevenzione Vaccinale. (17G00132). 2017. Available online: https://www.gazzettaufficiale.it/eli/id/2017/08/5/17G00132/sg (accessed on 23 April 2025).
- Santoro, V.; Morucci, L.; Ciabattini, M.; D’Alò, G.L.; D’Ercole, M.; Gallo, R.; Mastrosanti, R.; Russomanno, A.; Terracciano, E.; Zaratti, L.; et al. The Italian Immunization Plan 2017: Analysis and starting point for the Regional Plans. Ig. Sanita Pubbl. 2017, 73, 185–192. [Google Scholar]
- SItI—Società Italiana di Igiene Medicina Preventiva e Sanità Pubblica. Giunta Nazionale e Gruppi di Lavoro “Vaccini e Politiche Vaccinali” e “Dipartimento di Prevenzione” Buone Pratiche Vaccinali per l’Italia. Available online: https://mondosanita.it/wp-content/uploads/2024/01/21-1-24-Guida-alle-buone-pratiche-vaccinali-SITI.pdf (accessed on 18 December 2024).
- Signorelli, C.; Pennisi, F.; D’Amelio, A.C.; Conversano, M.; Cinquetti, S.; Blandi, L.; Rezza, G. Vaccinating in Different Settings: Best Practices from Italian Regions. Vaccines 2024, 13, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pennisi, F.; Mastrangelo, M.; De Ponti, E.; Cuciniello, R.; Mandelli, A.; Vaia, F.; Signorelli, C. The role of pharmacies in the implementation of vaccination cover- age in Italy. Insights from the preliminary data of the Lombardy Region. Ann. Ig. 2024, 36, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; De Ponti, E.; Mastrangelo, M.; Pennisi, F.; Cereda, D.; Corti, F.; Beretta, D.; Pelissero, G. The contribution of the private healthcare sector during the COVID-19 pandemic: The experience of the Lombardy Region in Northern Italy. Ann. Ig. 2024, 36, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, F.; Minerva, M.; Dalla Valle, Z.; Odone, A.; Signorelli, C. Genesis and prospects of the shortage of specialist physicians in Italy and indicators of the 39 schools of hygiene and preventive medicine. Acta Biomed. 2023, 94, e2023159. [Google Scholar] [CrossRef] [PubMed]
- Bezzini, D.; Schiavetti, I.; Manacorda, T.; Franzone, G.; Battaglia, M.A. First Wave of COVID-19 Pandemic in Italy: Data and Evidence. Adv. Exp. Med. Biol. 2021, 1353, 91–113. [Google Scholar] [CrossRef] [PubMed]
- Beccia, F.; Di Pilla, A.; Causio, F.A.; Federico, B.; Specchia, M.L.; Favaretti, C.; Boccia, S.; Damiani, G. Narrative Review of the COVID-19 Pandemic’s First Two Years in Italy. Int. J. Environ. Res. Public Health 2022, 19, 15443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Masino, S.; Enria, L. Experiences and Implications of the First Wave of the COVID-19 Emergency in Italy: A Social Science Perspective. Int. J. Health. Policy Manag. 2023, 12, 6871. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lugli, G.; Ottaviani, M.M.; Botta, A.; Ascione, G.; Bruschi, A.; Cagnazzo, F.; Zammarchi, L.; Romagnani, P.; Portaluri, T. The Impact of the SARS-CoV-2 Pandemic on Healthcare Provision in Italy to non-COVID Patients: A Systematic Review. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armocida, B.; Formenti, B.; Ussai, S.; Palestra, F.; Missoni, E. The Italian health system and the COVID-19 challenge. Lancet. Public Health. 2020, 5, e253. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Bidino, R.; Cicchetti, A. Impact of SARS-CoV-2 on Provided Healthcare. Evidence from the Emergency Phase in Italy. Front. Public Health 2020, 8, 583583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabbatucci, M.; Odone, A.; Signorelli, C.; Siddu, A.; Maraglino, F.; Rezza, G. Improved Temporal Trends of Vaccination Coverage Rates in Childhood after the Mandatory Vaccination Act, Italy 2014–2019. J. Clin. Med. 2021, 10, 2540. [Google Scholar] [CrossRef]
- Hill, H.; Chen, M.; Elam-Evans, L.D.; Yankey, D.; Singleton, J.A. Vaccination coverage by ag 24 months among children born during 2018-2019-National immunization survey-child, United States, 2019–2020. MMWR 2023, 72, 33–38. [Google Scholar]
- CDC. Chilhood Vaccination Coverage Before and During the COVID-19 Pandemic Among Children Born January 2017-May 2020, National Immunization Survey-Child (NIS-Child), 2018–2021. Available online: https://www.cdc.gov/vaccines/imz-managers/coverage/childvaxview/pubs-presentations/nis-child-pandemic-effects-2018-2021.html (accessed on 12 May 2025).
- Rezza, G. Mandatory vaccination for infants and children: The Italian experience. Pathog. Glob. Health 2019, 113, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Filia, A.; Bella, A.; Del Manso, M.; Baggieri, M.; Magurano, F.; Rota, M.C. Ongoing outbreak with well over 4,000 measles cases in Italy from January to end August 2017-what is making elimination so difficult? Eurosurveillance 2017, 22, 30614. [Google Scholar] [CrossRef]
- Lytras, T.; Di Gregorio, A.A.A.; Apostolopoulos, D.; Naziris, D.; Zingerle, C.; Heraclides, A. Effectiveness of COVID-19 vaccine mandates in raising vaccination rates among the elderly and general population in Europe: Controlled interrupted time series analysis. Vaccine 2024, 42, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, H.; Wiedermann, U. Mandatory vaccination: Suited to enhance vaccination coverage in Europe? Eurosurveillance 2019, 24, 1900376. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Farina, S.; Maio, A.; Gualano, M.R.; Ricciardi, W.; Villani, L. Childhood Mandatory Vaccinations: Current Situation in European Countries and Changes Occurred from 2014 to 2024. Vaccines 2024, 12, 1296. [Google Scholar] [CrossRef]
- Kuznetsova, L.; Cortassa, G.; Trilla, A. Effectiveness of Mandatory and Incentive-Based Routine Childhood Immunization Programs in Europe: A Systematic Review of the Literature. Vaccines 2021, 9, 1173. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Acebo, I.; Barquín-Ruiz, A.; Llorente, S.; Alonso-Molero, J.; Llorca, J.; Cabero-Perez, M.J.; Dierssen-Sotos, T. The impact of the COVID-19 pandemic on childhood vaccination rates and the role of sociodemographic factors: A cohort study. Vaccine 2024, 42, 126207. [Google Scholar] [CrossRef] [PubMed]
- Lassi, Z.S.; Naseem, R.; Salam, R.A.; Siddiqui, F.; Das, J.K. The Impact of the COVID-19 Pandemic on Immunization Campaigns and Programs: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 988. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Kim, S.J.; Lee, J.Y.; Choe, Y.J.; Choi, E.H.; Cho, E.H. Sustained vaccination coverage during the coronavirus disease 2019 epidemic in the Republic of Korea. Vaccines 2021, 9, 2. [Google Scholar] [CrossRef]
- Jarchow-MacDonald, A.A.; Burns, R.; Miller, J.; Kerr, L.; Willocks, L.J. Keeping childhood immunisation rates stable during the COVID-19 pandemic. Lancet Infect. Dis. 2021, 21, 459–460. [Google Scholar] [CrossRef]
- Middeldorp, M.; van Lier, A.; van der Maas, N.; Veldhuijzen, I.; Freudenburg, W.; van Sorge, N.M.; Sanders, E.A.; Knol, M.J.; de Melker, H.E. Short term impact of the COVID-19 pandemic on incidence of vaccine preventable diseases and participation in routine infant vaccinations in the Netherlands in the period March-September 2020. Vaccine 2021, 39, 1039–1043. [Google Scholar] [CrossRef]
- Zhong, Y.; Clapham, H.E.; Aishworiya, R.; Chua, Y.X.; Mathews, J.; Ong, M.; Wang, J.; Murugasu, B.; Chiang, W.C.; Lee, B.W.; et al. Childhood vaccinations: Hidden impact of COVID-19 on children in Singapore. Vaccine 2021, 39, 780–785. [Google Scholar] [CrossRef]
- Masresha, B.G.; Luce RJr Shibeshi, M.E.; Ntsama, B.; N’Diaye, A.; Chakauya, J.; Poy, A.; Mihigo, R. The performance of routine immunization in selected African countries during the first six months of the COVID-19 pandemic. Pan. Afr. Med. J. 2020, 37, 12. [Google Scholar] [CrossRef]
- Santolim, J.M. Effects of the COVID-19 pandemic on routine pediatric vaccine ordering and administration—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 591–593. [Google Scholar] [CrossRef]
- Mansour, Z.; Arab, J.; Said, R.; Rady, A.; Hamadeh, R.; Gerbaka, B.; Bizri, A.R. Impact of COVID-19 pandemic on the utilization of routine immunization services in Lebanon. PLoS ONE 2021, 16, e0246951. [Google Scholar] [CrossRef]
- Chandir, S.; Siddiqi, D.A.; Setayesh, H.; Khan, A.J. Impact of COVID-19 lockdown on routine immunisation in Karachi, Pakistan. Lancet Glob. Health 2020, 8, e1118–e1120. [Google Scholar] [CrossRef] [PubMed]
- McDonald, H.I.; Tessier, E.; White, J.M.; Woodruff, M.; Knowles, C.; Bates, C.; Parry, J.; Walker, J.L.; Scott, J.A.; Smeeth, L.; et al. Early impact of the coronavirus disease (COVID-19) pandemic and physical distancing measures on routine childhood vaccinations in England, January to April 2020. Eurosurveillance 2020, 25, 2000848. [Google Scholar] [CrossRef]
- Piché-Renaud, P.-P.; Ji, C.; Farrar, D.S.; Friedman, J.N.; Science, M.; Kitai, I.; Burey, S.; Feldman, M.; Morris, S.K. Impact of the COVID-19 pandemic on the provision of routine childhood immunizations in Ontario, Canada. Vaccine 2021, 39, 4373–4382. [Google Scholar] [CrossRef]
- Shet, A.; Dhaliwal, B.; Banerjee, P.; Carr, K.; DeLuca, A.; Britto, C.; Seth, R.; Parekh, B.; Basavaraj, G.V.; Shastri, D.; et al. COVID-19-related disruptions to routine vaccination services in India: A survey of paediatric providers. BMJ Paediatr. Open 2021, 5, e001060. [Google Scholar] [CrossRef]
- Harris, R.C.; Chen, Y.; Côte, P.; Ardillon, A.; Nievera, M.C.; Ong-Lim, A.; Aiyamperumal, S.; Chong, C.P.; Kandasamy, K.V.; Mahenthiran, K.; et al. Impact of COVID-19 on routine immunisation in South-East Asia and Western Pacific: Disruptions and solutions. Lancet. Reg. Health West. Pac. 2021, 10, 100140. [Google Scholar] [CrossRef] [PubMed]
- Ackerson, B.K.; Sy, L.S.; Glenn, S.C.; Qian, L.; Park, C.H.; Riewerts, R.J.; Jacobsen, S.J. Pediatric Vaccination During the COVID-19 Pandemic. Pediatrics 2021, 148, e2020047092. [Google Scholar] [CrossRef]
- Baloch, A.A.; Baig, N.; Baloch, F.; Suhag, Z. Impact on the Utilization of Reproductive, Maternal, Newborn and Child Health Care Services at Primary Health Care Level During First Wave of COVID-19 Outbreak in Pakistan. Cureus 2021, 13, e17430. [Google Scholar] [CrossRef] [PubMed]
- Shet, A.; Carr, K.; Danovaro-Holliday, M.C.; Sodha, S.V.; Prosperi, C.; Wunderlich, J.; Wonodi, C.; Reynolds, H.W.; Mirza, I.; Gacic-Dobo, M.; et al. Impact of the SARS-CoV-2 pandemic on routine immunisation services: Evidence of disruption and recovery from 170 countries and territories. Lancet Glob. Health 2022, 10, e186–e194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gambrell, A.; Sundaram, M.; Bednarczyk, R.A. Estimating the number of US children susceptible to measles resulting from COVID-19-related vaccination coverage declines. Vaccine 2022, 40, 4574–4579. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hill, H.A.; Yankey, D.; Elam-Evans, L.D.; Mu, Y.; Chen, M.; Peacock, G.; Singleton, J.A. Decline in Vaccination Coverage by Age 24 Months and Vaccination Inequities Among Children Born in 2020 and 2021—National Immunization Survey-Child, United States, 2021–2023. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 844–853. [Google Scholar] [CrossRef]
- Ota, M.O.C.; Badur, S.; Romano-Mazzotti, L.; Friedland, R.L. Impact of COIVID-19 pandemic on routine immunization. Ann. Med. 2021, 53, 2286–2297. [Google Scholar] [CrossRef]
- Ji, C.; Piché-Renaud, P.P.; Apajee, J.; Stephenson, E.; Forte, M.; Friedman, J.N.; Science, M.; Zlotkin, S.; Morris, S.K.; Tu, K. Impact of the COVID-19 pandemic on routine immunization coverage in children under 2 years old in Ontario, Canada: A retrospective cohort study. Vaccine 2022, 40, 1790–1798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sabbatucci, M.; Odone, A.; Signorelli, C.; Siddu, A.; Silenzi, A.; Maraglino, F.P.; Rezza, G. Childhood Immunisation Coverage during the COVID-19 Epidemic in Italy. Vaccines 2022, 10, 120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Centre for Disease Prevention and Control. Facilitating COVID-19 Vaccination Acceptance and Uptake in the EU/EEA; ECDC: Stockholm, Sweden, 2021.
- Pennisi, F.; Genovese, C.; Gianfredi, V. Lessons from the COVID-19 Pandemic: Promoting Vaccination and Public Health Resilience, a Narrative Review. Vaccines 2024, 12, 891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filip, R.; Gheorghita Puscaselu, R.; Anchidin-Norocel, L.; Dimian, M.; Savage, W.K. Global Challenges to Public Health Care Systems during the COVID-19 Pandemic: A Review of Pandemic Measures and Problems. J. Pers. Med. 2022, 12, 1295. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Zhang, K.; Chen, S.; Chan, P.S.-F.; Fang, Y.; Cao, H.; Chen, H.; Hu, T.; Chen, Y.; et al. Changes in Parents’ COVID-19 Vaccine Hesitancy for Children Aged 3–17 Years before and after the Rollout of the National Childhood COVID-19 Vaccination Program in China: Repeated Cross-Sectional Surveys. Vaccines 2022, 10, 1478. [Google Scholar] [CrossRef]
- Cao, H.; Chen, S.; Liu, Y.; Zhang, K.; Fang, Y.; Chen, H.; Hu, T.; Zhong, R.; Zhou, X.; Wang, Z. Parental Hesitancy toward Seasonal Influenza Vaccination for Children under the Age of 18 Years and Its Determinants in the Post-Pandemic Era: A Cross-Sectional Survey among 1175 Parents in China. Vaccines 2024, 12, 988. [Google Scholar] [CrossRef]
- Ghislandi, S.; Muttarak, R.; Sauerberg, M.; Scotti, B. News from the front: Estimation of excess mortality and life expectancy in the major epicenters of the COVID-19 pandemic in Italy. MedRxiv 2020, 2020, 20084335. [Google Scholar] [CrossRef]
- Kargi, B.; Cihan Uçkaç, B.; Coccia, M. Regional Responses to COVID-19 in Italy: A Comparative Analysis of Public Health Interventions and Their Impact. In Post-COVID-19 Society and Profound Societal Shifts; IntechOpen: London, UK, 2025. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Board on Children, Youth, and Families; Committee on Addressing the Long-Term Impact of the COVID-19 Pandemic on Children and Families. Addressing the Long-Term Effects of the COVID-19 Pandemic on Children and Families; Backes, E.P., Gootman, J.A., Coker, T.R., Eds.; National Academies Press: Washington, WA, USA, 2023. [PubMed]
- Lebrun-Harris, L.A.; Sappenfield, O.R.; Warren, M.D. Missed and Delayed Preventive Health Care Visits Among US Children Due to the COVID-19 Pandemic. Public Health Rep. 2022, 137, 336–343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Contoli, B.; Tosti, M.E.; Asta, F.; Minardi, V.; Marchetti, G.; Casigliani, V.; Scarso, S.; Declich, S.; Masocco, M. Exploring COVID-19 Vaccination Willingness in Italy: A Focus on Resident Foreigners and Italians Using Data from PASSI and PASSI d’Argento Surveillance Systems. Vaccines 2024, 12, 124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
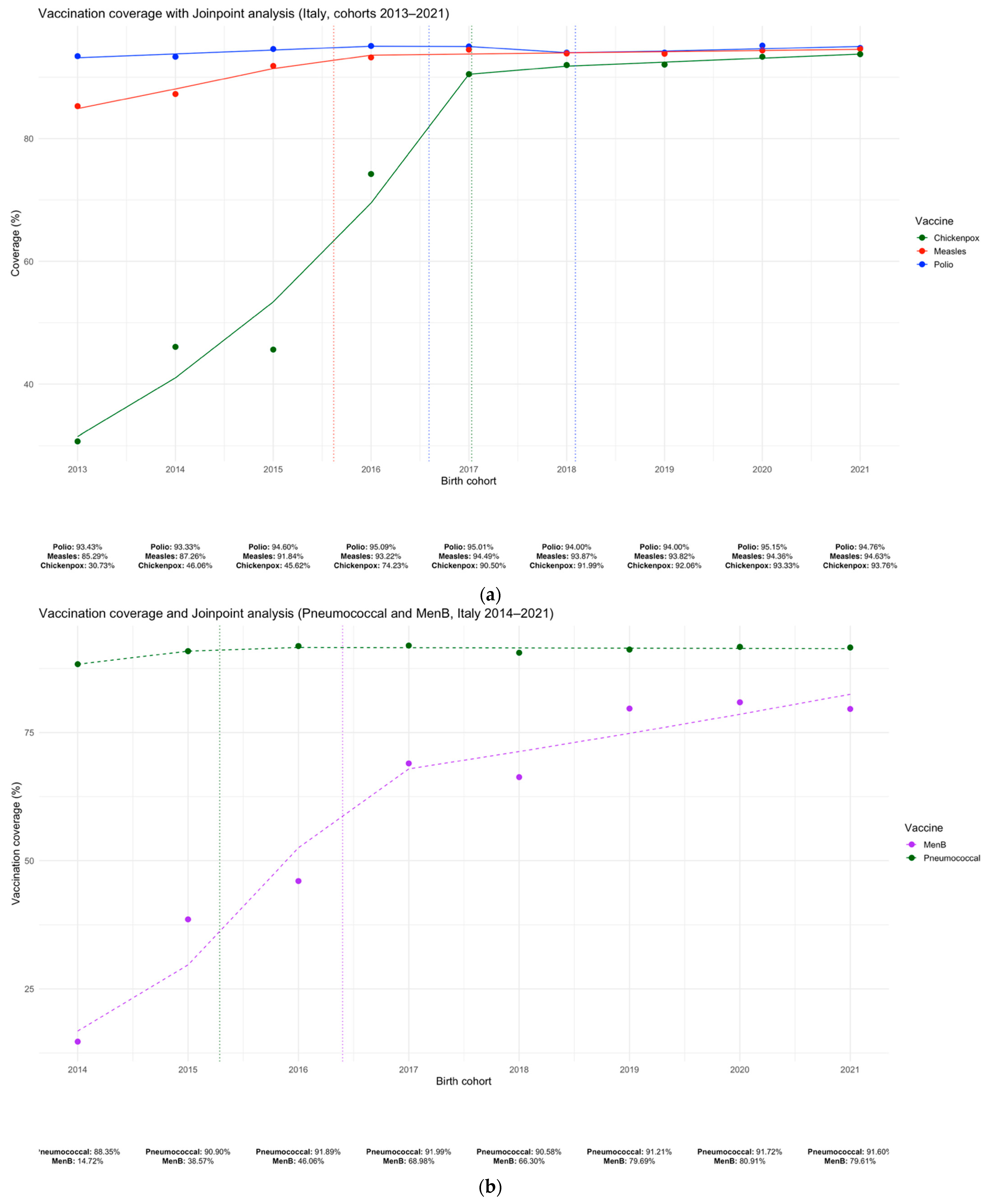

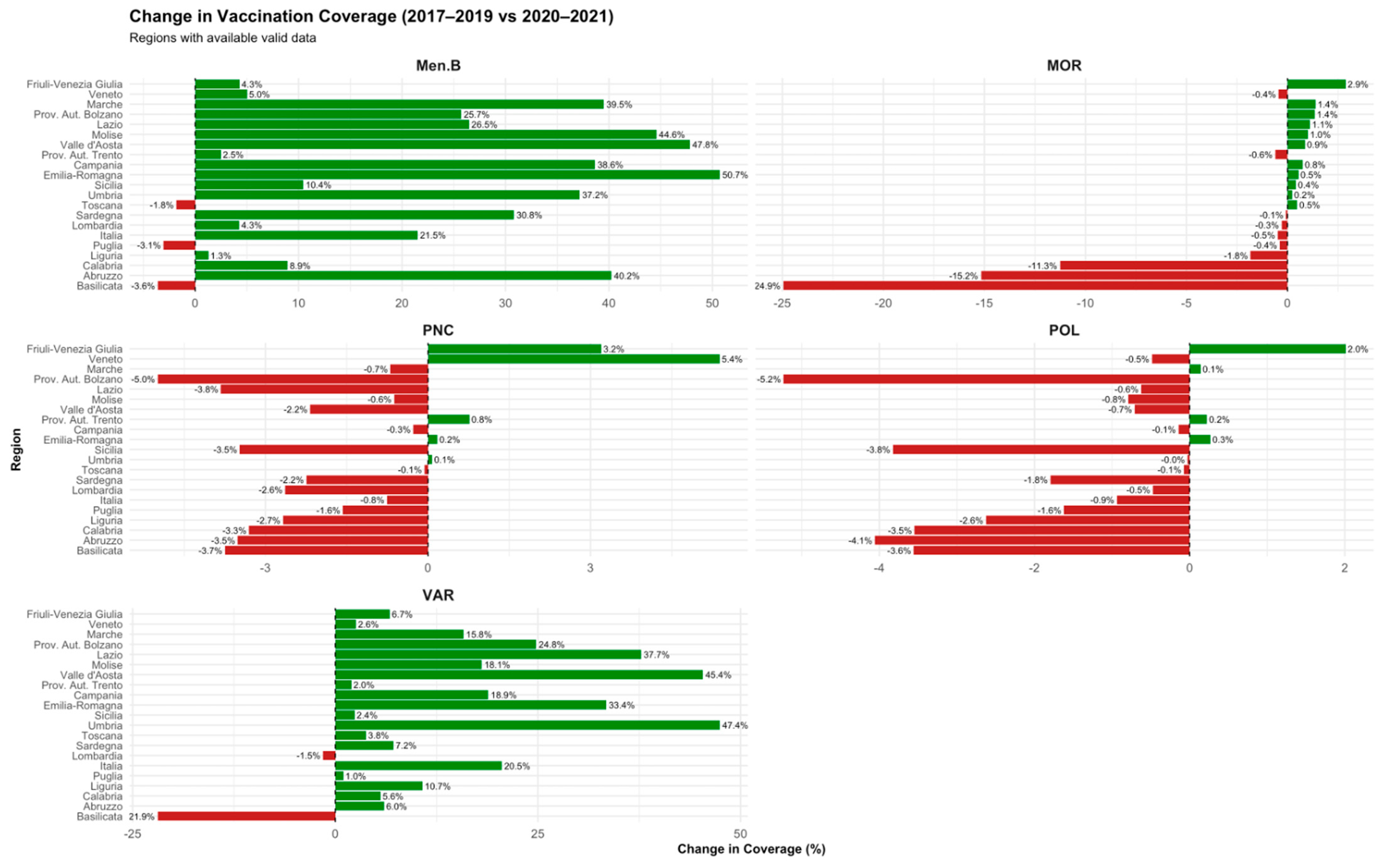
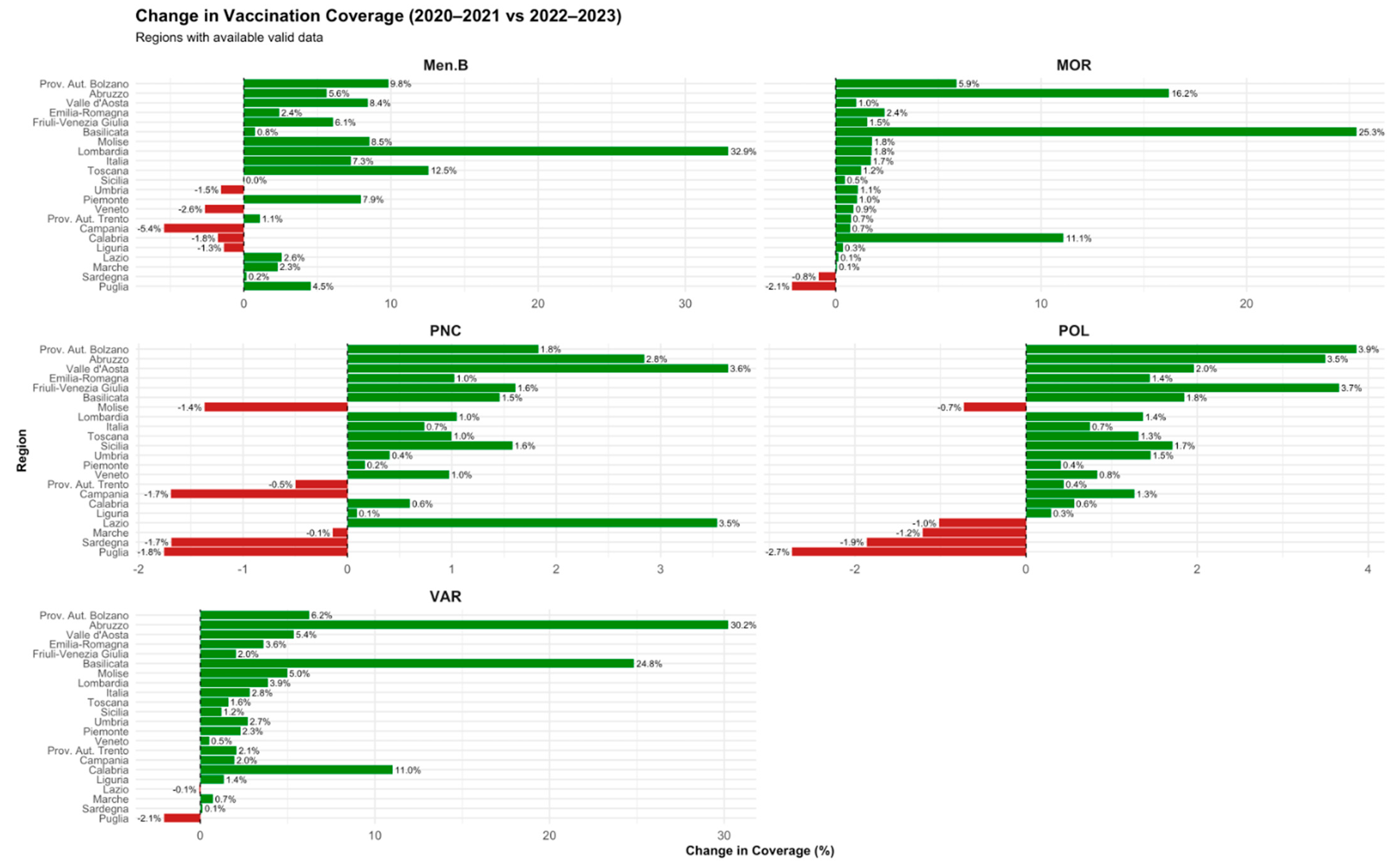

| Vaccine | Post-mandate (2015–2016 vs. 2017–2019) | Pandemic (2017–2019 vs. 2020–2021) | Post-pandemic (2020–2021 vs. 2022–2023) |
|---|---|---|---|
| Poliomyelitis | +0.24% to +3.53% (↑ in all regions) | −0.03% to −5.24% (↓ esp. in South) | +0.29% to +3.50% (↑ in most regions) |
| Measles | +2.41% to +11.05% (↑ broadly) | −0.08% to −24.94% (↓ variable) | +0.06% to +25.34% (↑, esp. in Center) |
| Varicella | +6.58% to +93.24% (↑, largest increase) | −1.51% to −21.92% (↓ in most) | +0.13% to +30.22% (↑ in most regions) |
| MenB | +18.48% to +79.32% (↑) | −1.82% to −3.62% (mostly ↓) | −0.5% to +5.63% (mixed) |
| Pneumococcal | +0.01% to +9.11% (↑) | −0.07% to −4.99% (↓) | −0.2% to +2.84% (slight recovery) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pennisi, F.; Silenzi, A.; Mammone, A.; Siddu, A.; Odone, A.; Sabbatucci, M.; Orioli, R.; D’Amelio, A.C.; Maraglino, F.; Rezza, G.; et al. Childhood Immunization Coverage Before, During and After the COVID-19 Pandemic in Italy. Vaccines 2025, 13, 683. https://doi.org/10.3390/vaccines13070683
Pennisi F, Silenzi A, Mammone A, Siddu A, Odone A, Sabbatucci M, Orioli R, D’Amelio AC, Maraglino F, Rezza G, et al. Childhood Immunization Coverage Before, During and After the COVID-19 Pandemic in Italy. Vaccines. 2025; 13(7):683. https://doi.org/10.3390/vaccines13070683
Chicago/Turabian StylePennisi, Flavia, Andrea Silenzi, Alessia Mammone, Andrea Siddu, Anna Odone, Michela Sabbatucci, Riccardo Orioli, Anna Carole D’Amelio, Francesco Maraglino, Giovanni Rezza, and et al. 2025. "Childhood Immunization Coverage Before, During and After the COVID-19 Pandemic in Italy" Vaccines 13, no. 7: 683. https://doi.org/10.3390/vaccines13070683
APA StylePennisi, F., Silenzi, A., Mammone, A., Siddu, A., Odone, A., Sabbatucci, M., Orioli, R., D’Amelio, A. C., Maraglino, F., Rezza, G., & Signorelli, C. (2025). Childhood Immunization Coverage Before, During and After the COVID-19 Pandemic in Italy. Vaccines, 13(7), 683. https://doi.org/10.3390/vaccines13070683







