Efficacy and Safety of a Tri-Valent Ready-to-Use Porcine Circovirus Type 2a, Mycoplasma hyopneumoniae and Lawsonia intracellularis Vaccine in Weaned Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccines
2.2. Efficacy Studies
2.2.1. PCV2a Challenge Infection
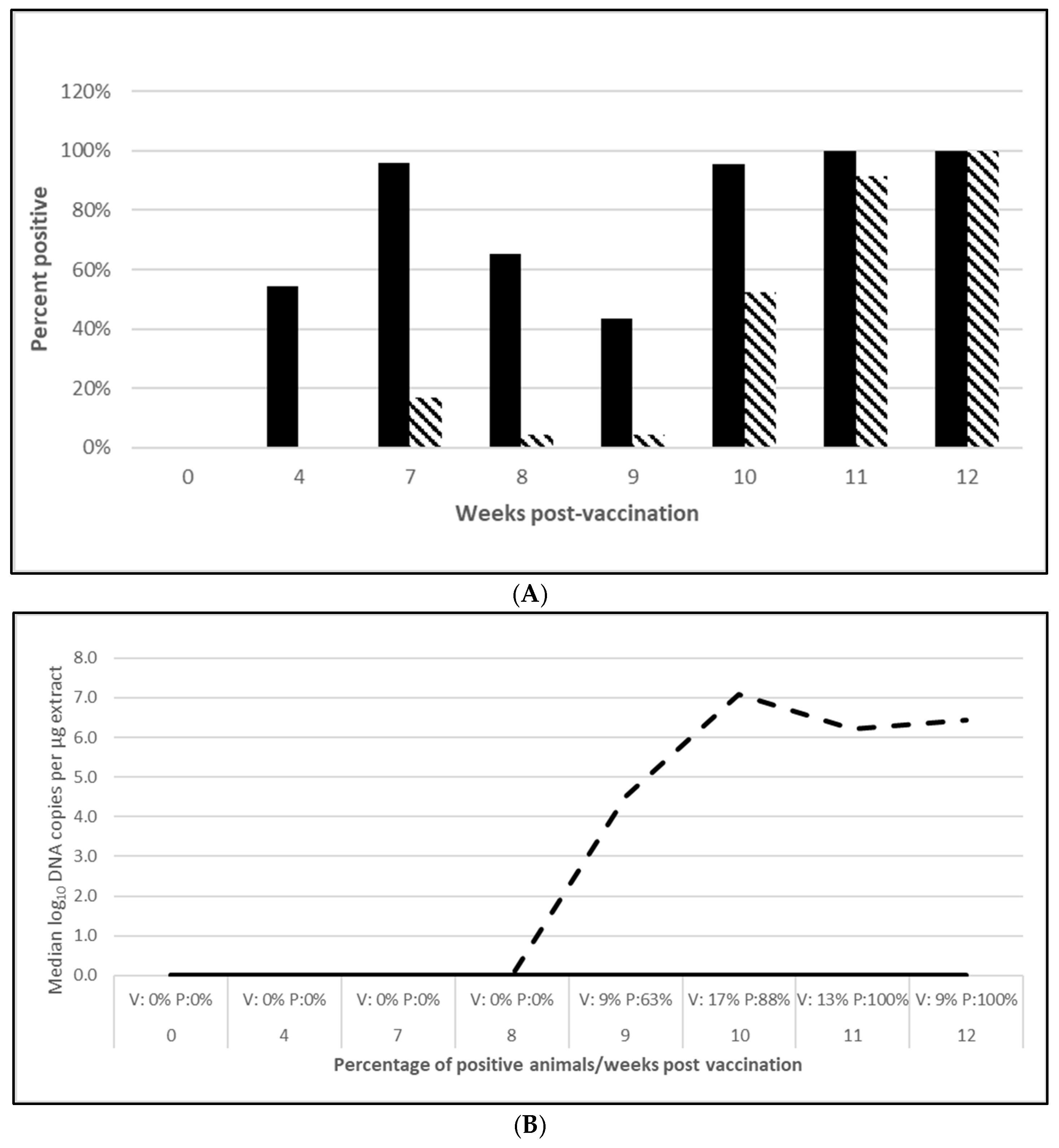
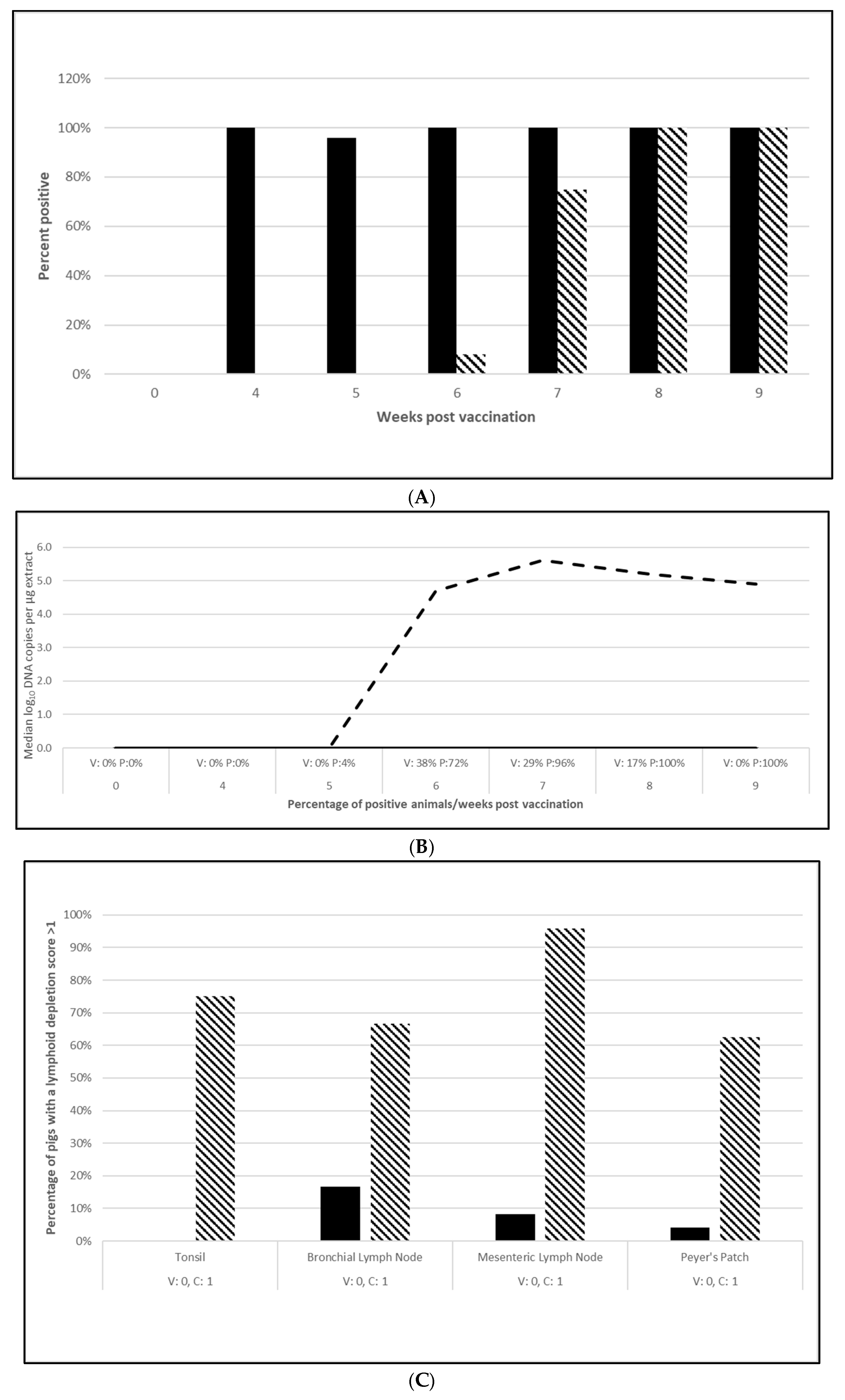
2.2.2. PCV2d Challenge Infections
2.2.3. M. Hyopneumoniae Challenge Infection
2.2.4. L. Intracellularis Challenge Infection
2.2.5. Serology
2.2.6. Quantification of PCV2 DNA
2.2.7. Quantification of Lawsonia DNA
2.2.8. Statistical Analysis
PCV2 Analysis
M. Hyopneumoniae Analysis
L. Intracellularis Analysis
3. Results
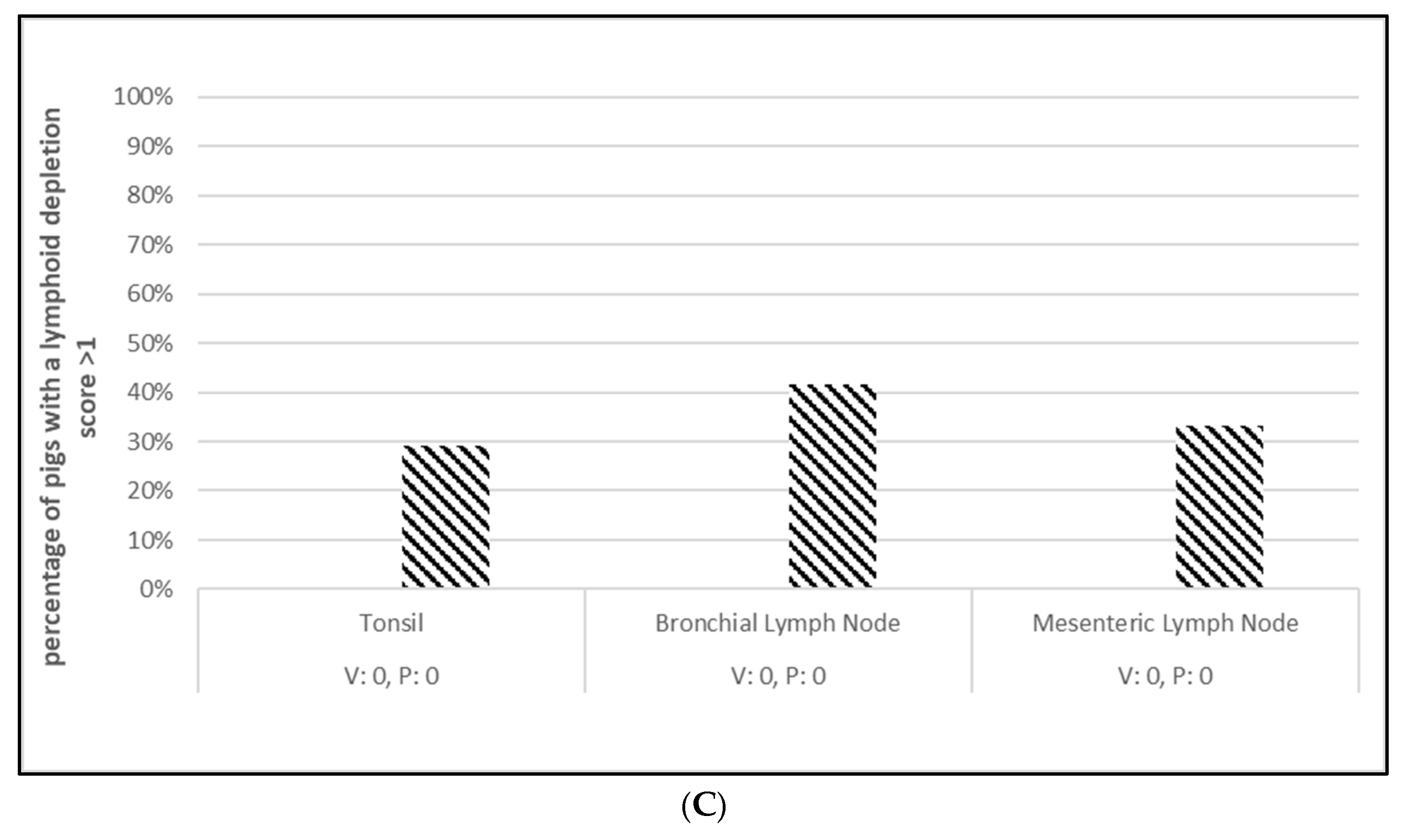
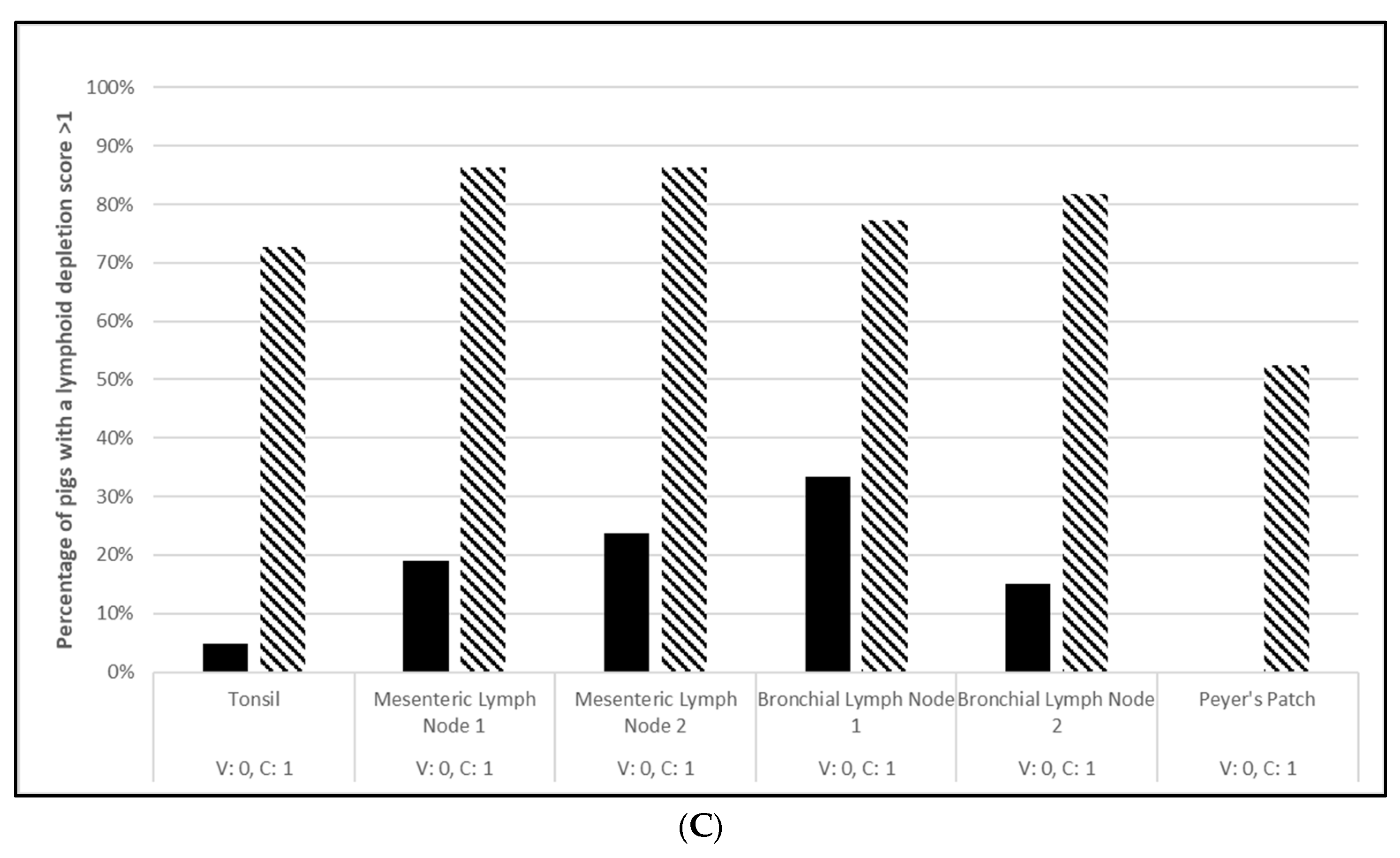
3.1. Safety
3.2. Efficacy
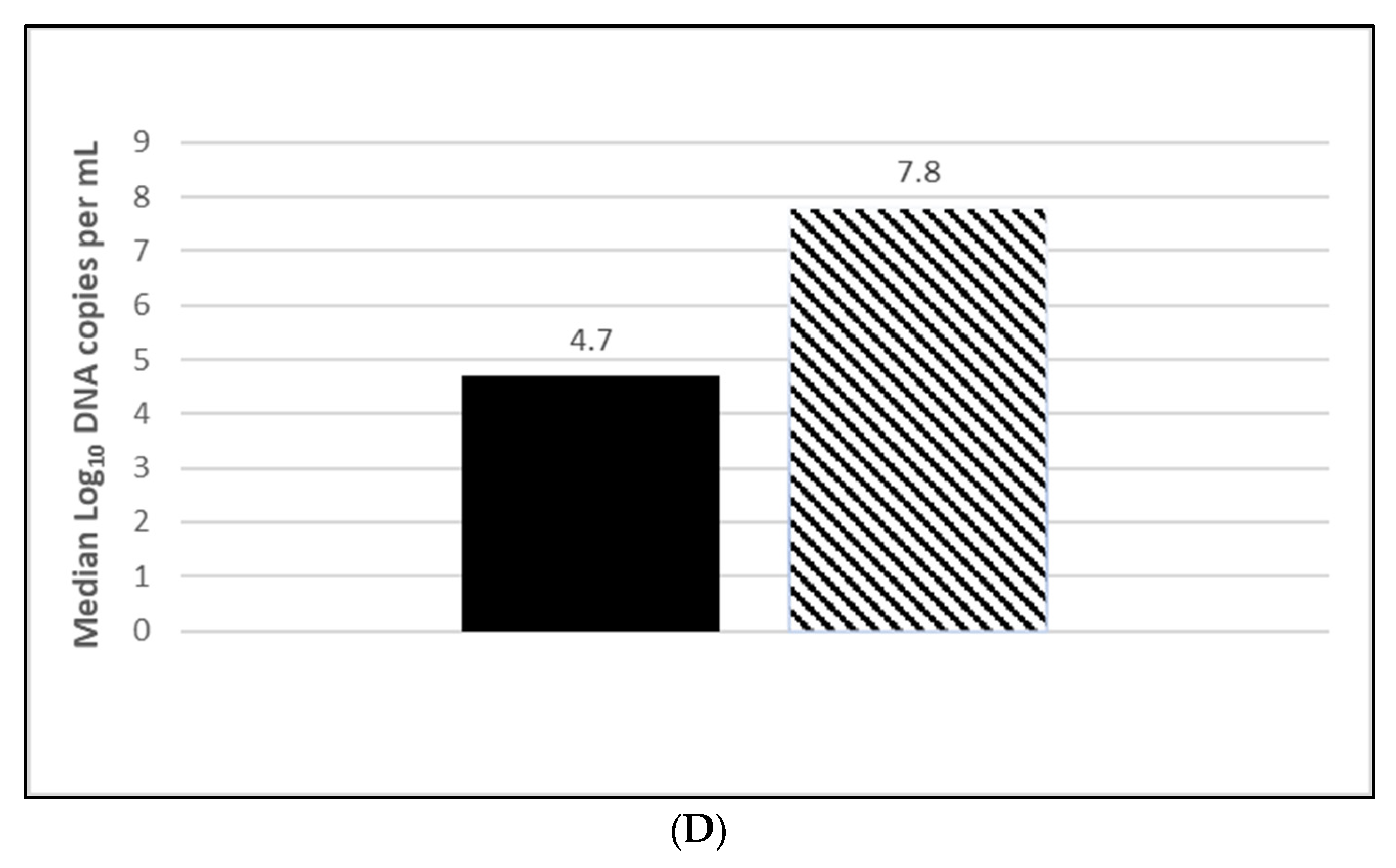
3.2.1. PCV2 Efficacy
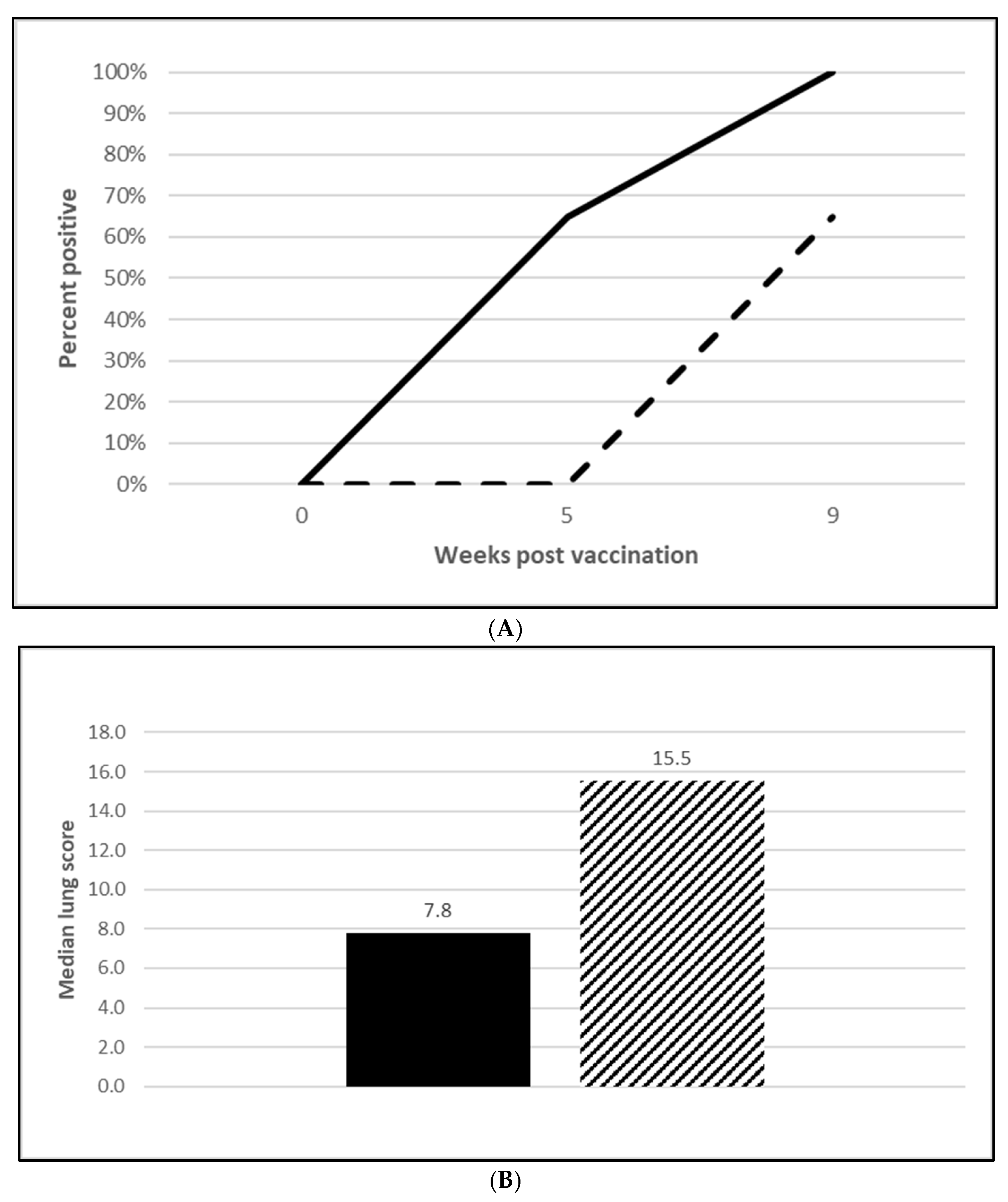
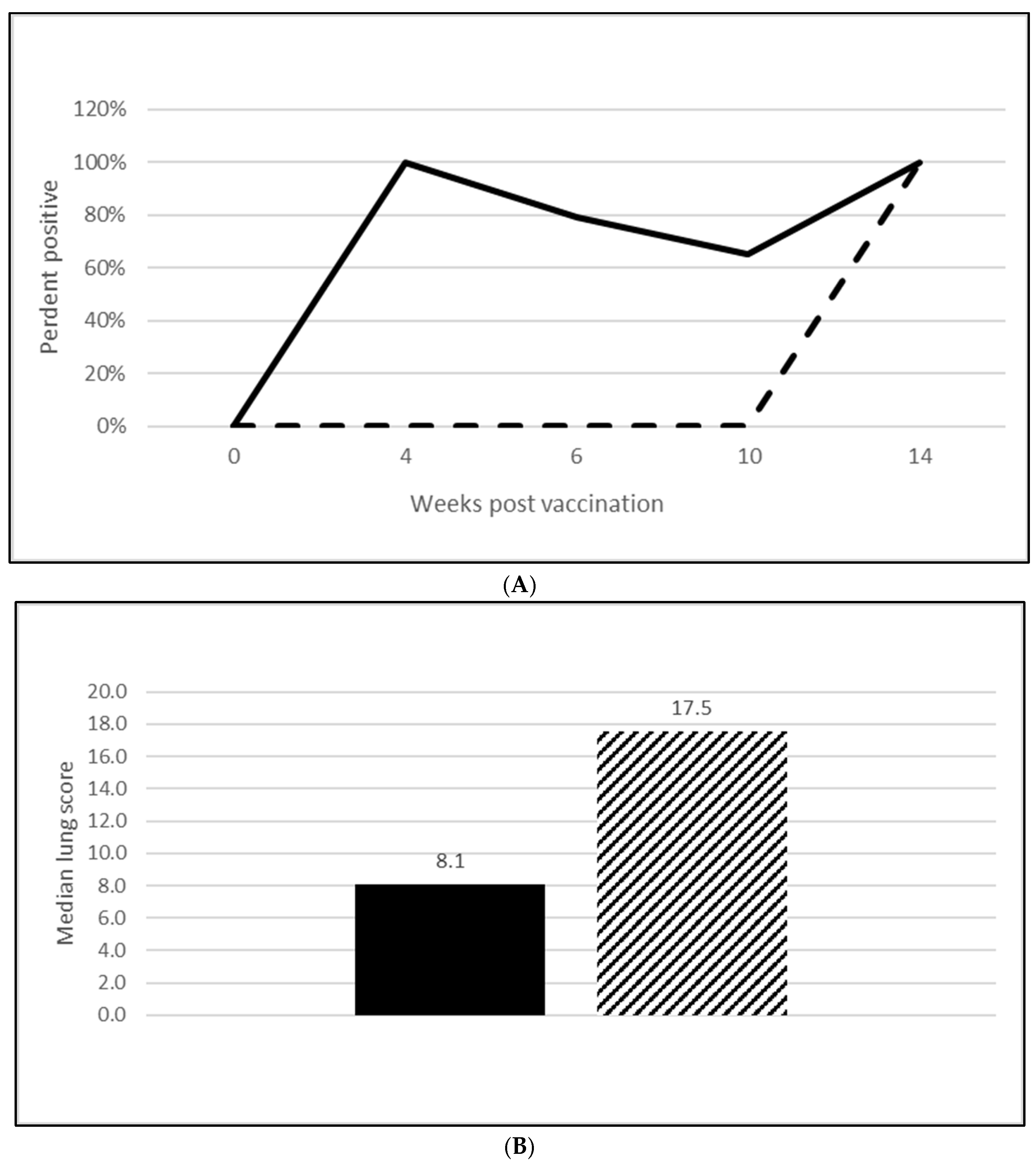
3.2.2. M. Hyopneumoniae Efficacy
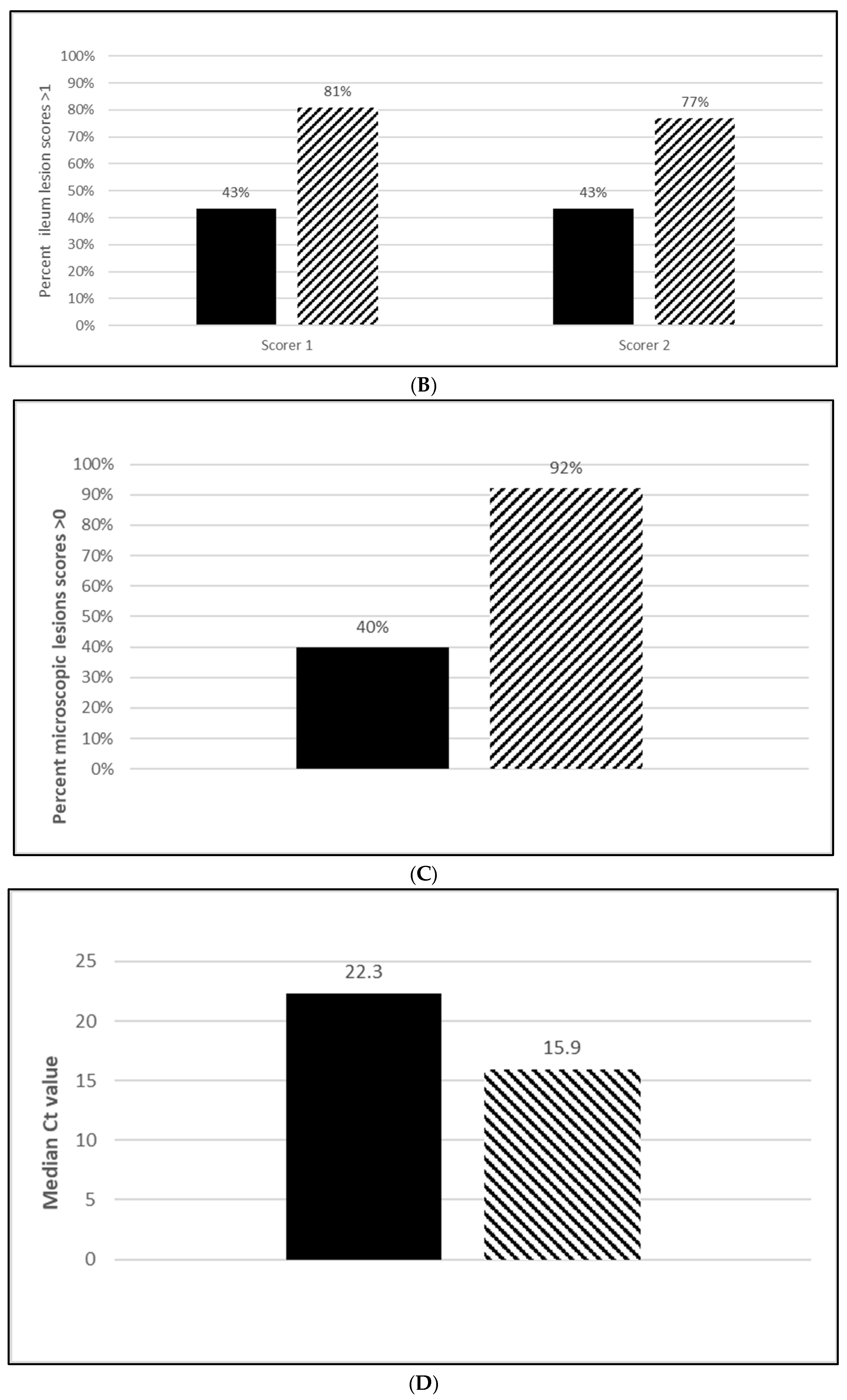
3.2.3. L. Intracellularis Efficacy
4. Discussion
5. Conclusions
- The trials successfully demonstrated the safety and efficacy of the first ready-to-use PCV2, M. hyopneumoniae, and L. intracellularis vaccine.
- The use of a tri-valent, ready to use vaccine, containing Porcine Circovirus Type 2a, M. hyopneumoniae, and L. intracellularis results in fewer injections, reducing animal stress.
- The use of the tri-valent vaccine supports a smaller carbon-footprint by reducing inventory and waste.
- The use of the tri-valent vaccine reduces labor needs during vaccination.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, C.; Seo, H.W.; Han, K.; Chae, C. Comparison of four commercial one-dose Porcine Circovirus Type 2 (PCV2) vaccines administered to pigs challenged with PCV2 and porcine reproductive and respiratory syndrome virus at 17 weeks postvaccination to control porcine respiratory disease complex under Korean field conditions. Clin. Vaccine Immunol. 2014, 21, 399–406. [Google Scholar] [PubMed]
- Witvliet, M.; Holtslag, H.; Nell, T.; Segers, R.; Fachinger, R.V. Efficacy and safety of a combined Porcine Circovirus and Mycoplasma hyopneumoniae vaccine in finishing pigs. Trials Vaccinol. 2014, 4, 43–49. [Google Scholar] [CrossRef][Green Version]
- Henning-Paula, I. Ongoing challenges posed by the infection dynamics of porcine circovirus 2. Vet. Rec. 2019, 184, 186–188. [Google Scholar] [CrossRef]
- Holst, S.; Yeske, P.; Pieters, M. Elimination of Mycoplasma hyopneumoniae from breed-to-wean- farms: A review of current protocols with emphasis on herd closure and medication. J. Swine Health Prod. 2015, 23, 321–330. [Google Scholar] [CrossRef]
- Sibila, M.; Pieters, M.; Molitor, T.; Maes, D.; Haesbrouck, F.; Segales, J. Current perspectives on the diagnosis and epidemiology of Mycoplasma hyopneumoniae infection. Vet. J. 2009, 181, 221–231. [Google Scholar] [CrossRef]
- Montesina, R.; Gutierrez, N.; Camacho, F.; Farnos, O.; Andrades, S.; Gonzalez, A.; Acosta, J.; Cortez-San Martin, M.; Sanchez, O.; Ruiz, A.; et al. Multi-antigenic recombinant subunit vaccine against Lawsonia intracellularis: The etiological agent of porcine proliferative enteropathy. Vaccine 2019, 37, 1340–1349. [Google Scholar] [CrossRef]
- Roerink, F.; Morgan, C.L.; Knetter, S.M.; Passat, M.-H.; Archibald, A.L.; Ait-Ali, T.; Strait, E.L. A novel inactivated vaccine against Lawsonia intracellularis induces rapid induction of humoral immunity, reduction of bacterial shedding and provides robust gut barrier function. Vaccine 2018, 36, 1500–1508. [Google Scholar] [CrossRef]
- Jacobson, M.; Fellstrom, C.; Jensen-Waern, C.M. Porcine proliferative enteropathy: An important disease with questions remaining to be solved. Vet. J. 2010, 184, 264–268. [Google Scholar] [CrossRef]
- Madson, D.M.; Patterson, A.R.; Ramamoorthy, S.; Pal, N.; Meng, X.J.; Opriessnig, T. Effect of a porcine circovirus type 2 (PCV2) vaccination of the dam on PCV2 replication in utero. Clin. Vaccine Immunol. 2009, 16, 830–834. [Google Scholar] [CrossRef]
- Guedes, R.M.C.; Gebhart, C.J.; Winkelman, N.L.; Mackie-Nuss, R.A. A comparative study of an indirect fluorescent antibody test and an immunoperoxidase monolayer assay for the diagnosis of porcine proliferative enteropathy. J. Vet. Diagn. Investig. 2002, 14, 420–423. [Google Scholar] [CrossRef]
- Pal, N.; Huang, Y.W.; Madson, D.M.; Kuster, C.; Meng, X.J.; Halbur, P.G.; Opriessnig, T. Development and validation of a duplex real-time PCR assay for the simultaneous detection and quantification of porcine circovirus type 2 and an internal control on porcine semen samples. J. Virol. Methods 2008, 149, 217–225. [Google Scholar] [CrossRef]
- Wattanaphansak, S.; Gebhart, C.J.; Anderson, J.M.; Singer, R.S. Development of a polymerase chain reaction assay for quantification of Lawsonia intracellularis. J. Vet. Diagn. Investig. 2010, 22, 598–602. [Google Scholar] [CrossRef]
- Wang, Y.; Noll, L.; Porter, E.; Stoy, C.; Zheng, W.; Liu, X.; Peddireddi, L.; Niederwerder, M.; Bai, J. Genetic diversity and prevalence of porcine circovirus type 3 (PCV3) and type 2 (PCV2) in the Midwest of the USA during 2016–2018. Transbound Emerg. Dis. 2020, 67, 1284–1294. [Google Scholar] [CrossRef]
- Kim, K.; Hahn, T.-W. Evaluation of novel recombinant porcine circovirus type 2d (PCV2d) vaccine in pigs naturally infected with PCV2d. Vaccine 2021, 39, 529–535. [Google Scholar] [CrossRef]
- Xiao, C.-T.; Harmon, K.M.; Halbur, P.G.; Opriessnig, T. PCV2d-2 is the predominant type of PCV2 DNA in pig samples collected in the U.S. during 2014–2016. Vet. Microbiol. 2016, 197, 72–77. [Google Scholar] [CrossRef]
- Kekarainen, T.; Segales, J. Porcine circovirus 2 immunology and viral evolution. Porc. Health Manag. 2015, 1, 17. [Google Scholar] [CrossRef]
- Maes, D.; Segales, J.; Meyns, T.; Sibila, M.; Pieters, M.; Haesebrouck, F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet. Microbiol. 2008, 126, 297–309. [Google Scholar] [CrossRef]
- Chae, C. Porcine respiratory disease complex: Interaction of vaccination and porcine circovirus type 2, porcine reproductive and respiratory syndrome virus, and Mycoplasma hyopneumoniae. Vet. J. 2016, 212, 1–6. [Google Scholar] [CrossRef]
- Nathues, H.; Doehring, S.; Woeste, H.; Farion, A.S.; Doherr, M.G.; Beilage, E.G. Individual risk factors for Mycoplasma hyopneumoniae infections in suckling pigs at the age of weaning. Acta Vet. Scand. 2015, 55, 44. [Google Scholar] [CrossRef]
- Baro, J.; Martinez, J. Porcine circovirus type 2 (PCV2) enteric disease: An independent condition or part of the systemic disease? Vet. Microbiol. 2015, 176, 83–87. [Google Scholar] [CrossRef]
- Obradovic, M.R.; Wilson, H.I. Immune response and protection against Lawsonia intracellularis infections in pigs. Vet. Immunol. Immunopathol. 2020, 219, 109959. [Google Scholar] [CrossRef] [PubMed]
- Helm, E.T.; Outhouse, A.C.; Schwartz, K.J.; Dekkers, J.C.M.; Lonergan, S.M.; Rauw, W.M.; Gabler, N.K. Impact of Mycoplasma hyopneumoniae and Lawsonia intracellularis on the performance of pigs divergently selected for feed efficiency. J. Anim. Sci. 2018, 96, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Helm, E.T.; Outhouse, A.C.; Schwartz, K.J.; Lonergan, S.M.; Curry, S.M.; Dekkers, J.C.M.; Gabler, N.K. Metabolic adaptation of pigs to a Mycoplasma hyopneumoniae and Lawsonia intracellularis dual challenge. J. Anim. Sci. 2018, 96, 3196–3207. [Google Scholar]



| Type of Study | Group | No. of Piglets | Challenge (Weeks Post-Vaccination) | Challenge Organism |
|---|---|---|---|---|
| Onset of Immunity | Circumvent CML | 25 | 7 | PCV2a/PRRSv * |
| Placebo | 25 | |||
| Onset of Immunity | Circumvent CML | 25 | 4 | PCV2d † |
| Placebo | 25 | |||
| Duration of Immunity | Circumvent CML | 25 | 16 | PCV2d |
| Placebo | 25 | |||
| Onset of Immunity | Circumvent CML | 35 | 5 | Mycoplasma hyopneumoniae |
| Placebo | 35 | |||
| Duration of Immunity | Circumvent CML | 70 | 10 | Mycoplasma hyopneumoniae |
| Placebo | 70 | |||
| Onset of Immunity | Circumvent CML | 40 | 5 | Lawsonia intracellularis |
| Placebo | 40 | |||
| Duration of Immunity | Circumvent CML | 50 | 20 | Lawsonia intracellularis |
| Placebo | 50 |
| Weeks | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SD * 0 | SD 3 | SD 4 | SD 5 | SD 6 | SD 7 | SD 8 | SD 9 | SD 10 | SD 12 | SD 13 | SD 14 | SD 16 | SD 17 | SD 18 | SD 19 | SD 20 | SD 23 | SD 27 | |
| PCV2a OOI ** | Vaccination with Circumvent CML or Placebo, Blood Sample | Blood Sample | PCV2a/PRRSV Challenge Blood Sample | Blood Sample (Weekly) | Collect Lymph Nodes, Blood Sample | ||||||||||||||
| PCV2d OOI | PCV2d Challenge Blood Sample | Blood Sample (Weekly) | Collect Lymph Nodes, Blood Sample | ||||||||||||||||
| PCV2d DOI *** | Blood Sample | Blood Sample | Blood Sample | Blood Sample | PCV2d Challenge Blood Sample | Blood Sample (Weekly) | Collect Lymph Nodes Peyer’s Patches Blood Sample | ||||||||||||
| M. hyopneumoniae OOI | M. hyopneumoniae Challenge, Blood Sample | Score Lung Lesions Blood Sample | |||||||||||||||||
| M. hyopneumoniae DOI | Blood Sample | Blood Sample | M. hyopneumoniae Challenge, Blood Sample | Score Lung Lesions Blood Sample | |||||||||||||||
| L. intracellularis OOI | Blood Sample | L. intracellularis Challenge, Blood Sample | Collect/Score Ileum Blood Sample | Blood Sample | |||||||||||||||
| L. intracellularis DOI | Blood Sample | Blood Sample | Blood Sample | Blood Sample | L. intracellularis Challenge, Blood Sample | Collect/Score Ileum Blood Sample | Blood Sample | ||||||||||||
| Number of Pigs | Breed | ||
|---|---|---|---|
| Male | Female | ||
| PCV2a OOI * | 24 | 25 | Large White/Duroc |
| PCV2d OOI | 33 | 17 | Yorkshire/Landrace |
| PCV2d DOI ** | 32 | 18 | Large White/Yorkshire |
| M. hyopneumoniae OOI | 32 | 38 | Yorkshire/Landrace |
| M. hyopneumoniae DOI *** | 36 | 32 | Yorkshire/Landrace |
| 39 | 31 | Large White/Landrace | |
| L. intracellularis OOI | 44 | 36 | Yorkshire/Landrace/Duroc |
| L. intracellularis DOI | 48 | 52 | Yorkshire/Landrace/Duroc |
| Study | Details | Vaccine | Placebo |
|---|---|---|---|
| PCV2a OOI | Number of pigs (n) | 25 | 24 |
| Pigs with local reactions (%) | 88 | 88 | |
| Pigs with a systemic reaction (%) | 0 | 0 | |
| PCV2d OOI | Number of pigs (n) | 25 | 25 |
| Pigs with local reactions (%) | 48 | 52 | |
| Pigs with a systemic reaction (%) | 0 | 0 | |
| PCV2d DOI | Number of pigs (n) | 25 | 25 |
| Pigs with local reactions (%) | 0 | 0 | |
| Pigs with a systemic reaction (%) | 0 | 0 | |
| M. hyopneumoniae OOI | Number of pigs (n) | 35 | 35 |
| Pigs with local reactions (%) | 3 | 9 | |
| Pigs with a systemic reaction (%) | 0 | 0 | |
| M. hyopneumoniae DOI | Number of pigs (n) | 70 | 80 |
| Pigs with local reactions (%) | 44 | 54 | |
| Pigs with a systemic reaction (%) | 0 | 0 | |
| L. intracellularis OOI | Number of pigs (n) | 40 | 44 |
| Pigs with local reactions (%) | 55 | 36 | |
| Pigs with a systemic reaction (%) | 0 | 0 | |
| L. intracellularis DOI | Number of pigs (n) | 50 | 50 |
| Pigs with local reactions (%) | 68 | 42 | |
| Pigs with a systemic reaction (%) | 0 | 0 | |
| All studies | Number of pigs (n) | 270 | 283 |
| Pigs with local reactions (%) | 44 | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, M.; Roerink, F.; Crowley, A.; Knetter, S.; Morgan, C.; Wei, H.; Segers, R. Efficacy and Safety of a Tri-Valent Ready-to-Use Porcine Circovirus Type 2a, Mycoplasma hyopneumoniae and Lawsonia intracellularis Vaccine in Weaned Pigs. Vaccines 2025, 13, 681. https://doi.org/10.3390/vaccines13070681
Allen M, Roerink F, Crowley A, Knetter S, Morgan C, Wei H, Segers R. Efficacy and Safety of a Tri-Valent Ready-to-Use Porcine Circovirus Type 2a, Mycoplasma hyopneumoniae and Lawsonia intracellularis Vaccine in Weaned Pigs. Vaccines. 2025; 13(7):681. https://doi.org/10.3390/vaccines13070681
Chicago/Turabian StyleAllen, Michelle, Frank Roerink, Abigail Crowley, Susan Knetter, Chandra Morgan, Huiling Wei, and Ruud Segers. 2025. "Efficacy and Safety of a Tri-Valent Ready-to-Use Porcine Circovirus Type 2a, Mycoplasma hyopneumoniae and Lawsonia intracellularis Vaccine in Weaned Pigs" Vaccines 13, no. 7: 681. https://doi.org/10.3390/vaccines13070681
APA StyleAllen, M., Roerink, F., Crowley, A., Knetter, S., Morgan, C., Wei, H., & Segers, R. (2025). Efficacy and Safety of a Tri-Valent Ready-to-Use Porcine Circovirus Type 2a, Mycoplasma hyopneumoniae and Lawsonia intracellularis Vaccine in Weaned Pigs. Vaccines, 13(7), 681. https://doi.org/10.3390/vaccines13070681






