Melanoma Vaccines: Comparing Novel Adjuvant Treatments in High-Risk Patients
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CPIs | Checkpoint inhibitors |
| TL | Tumor lysate, particle only |
| YCWPs | Yeast cell wall particles |
| DFS | Disease-free survival |
| HR | Hazard ratio |
| RFS | Recurrence-free survival |
| TLPLDC | Tumor lysate, particle-loaded dendritic cell |
| TLPO | Tumor lysate, particle only |
| ECOG | Eastern Cooperative Oncology Group |
| G-CSF | Granulocyte-colony stimulating factor |
| TLPLDC + G | Tumor lysate, particle-loaded dendritic cell + G-CSF |
| AEs | Adverse events |
| NCI | National Cancer Institute |
| CTCAE | Common Terminology Criteria for Adverse Events |
| TVEC | Talimogene laherparepvec |
| TME | Tumor microenvironment |
| ICIs | Immune checkpoint inhibitors |
References
- Agarwala, S.S. Current systemic therapy for metastatic melanoma. Expert Rev. Anticancer Ther. 2009, 9, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Jing, M.; Cai, Y.; Shi, J.; Zhang, X.; Zhu, B.; Yuan, F.; Zhang, J.; Xiao, M.; Chen, M. Frontiers|Adjuvant Treatments of Adult Melanoma: A Systematic Review and Network Meta-Analysis. Front. Oncol. 2022, 12, 926242. [Google Scholar] [CrossRef] [PubMed]
- Steininger, J.; Gellrich, F.F.; Schulz, A.; Westphal, D.; Beissert, S.; Meier, F. Systemic Therapy of Metastatic Melanoma: On the Road to Cure. Cancers 2021, 13, 1430. [Google Scholar] [CrossRef]
- Tsao, H.; Atkins, M.B.; Sober, A.J. Management of Cutaneous Melanoma. N. Engl. J. Med. 2004, 351, 998–1012. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Giacomo, A.M.D.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Nivolumab Monotherapy in Patients With Previously Untreated BRAF Wild-Type Advanced Melanoma. JAMA Oncol. 2019, 5, 187–194. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Robert, C.; Grob, J.J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Sileni, V.C.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Baraibar, I.; Melero, I.; Ponz-Sarvise, M.; Castanon, E.; Baraibar, I.; Melero, I.; Ponz-Sarvise, M.; Castanon, E. Safety and Tolerability of Immune Checkpoint Inhibitors (PD-1 and PD-L1) in Cancer. Drug Saf. 2019, 42, 281–294. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Vecchio, M.D.; Mandalá, M.; Gogas, H.; Arance, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.-J.; Chiarion-Sileni, V.; et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB–C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1465–1477. [Google Scholar] [CrossRef]
- Dummer, R.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Kirkwood, J.M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; et al. Five-Year Analysis of Adjuvant Dabrafenib plus Trametinib in Stage III Melanoma. N. Engl. J. Med. 2020, 383, 1139–1148. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; Wiel, B.A.v.d.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.M.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Demaria, P.J.B. Marijo, Cancer Vaccines. Hematol./Oncol. Clin. N. Am. 2019, 33, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Jou, J.; Harrington, K.J.; Zocca, M.-B.; Ehrnrooth, E.; Cohen, E.E.W. The Changing Landscape of Therapeutic Cancer Vaccines—Novel Platforms and Neoantigen Identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Kleponis, J.; Skelton, R.; Zheng, L. Fueling the engine and releasing the break: Combinational therapy of cancer vaccines and immune checkpoint inhibitors—PubMed. Cancer Biol. Med. 2015, 12, 201–208. [Google Scholar]
- Carpenter, E.L.; Van Decar, S.; Adams, A.M.; O’Shea, A.E.; McCarthy, P.; Chick, R.C.; Clifton, G.T.; Vreeland, T.; Valdera, F.A.; Tiwari, A.; et al. Prospective, randomized, double-blind phase 2B trial of the TLPO and TLPLDC vaccines to prevent recurrence of resected stage III/IV melanoma: A prespecified 36-month analysis. J. Immunother. Cancer 2023, 11, e006665. [Google Scholar] [CrossRef]
- Herbert, G.S.; Vreeland, T.J.; Clifton, G.T.; Greene, J.M.; Jackson, D.O.; Hardin, M.O.; Hale, D.F.; Berry, J.S.; Nichol, P.; Yin, S.; et al. Initial phase I/IIa trial results of an autologous tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine in patients with solid tumors. Vaccine 2018, 36, 3247–3253. [Google Scholar] [CrossRef]
- Vreeland, T.J.; Clifton, G.T.; Hale, D.F.; Chick, R.C.; Hickerson, A.T.; Cindass, J.L.; Adams, A.M.; Bohan, P.M.K.; Andtbacka, R.H.I.; Berger, A.C.; et al. A Phase IIb Randomized Controlled Trial of the TLPLDC Vaccine as Adjuvant Therapy After Surgical Resection of Stage III/IV Melanoma: A Primary Analysis. Ann. Surg. Oncol. 2021, 28, 6126–6137. [Google Scholar] [CrossRef]
- Burris, H.A., III; Patel, M.R.; Cho, D.C.; Clarke, J.M.; Gutierrez, M.; Zaks, T.Z.; Frederick, J.; Hopson, K.; Mody, K.; Binanti-Berube, A.; et al. A phase 1, open-label, multicenter study to assess the safety, tolerability, and immunogenicity of mRNA-4157 alone in subjects with resected solid tumors and in combination with pembrolizumab in subjects with unresectable solid tumors. J. Glob. Oncol. 2019, 5, 93. [Google Scholar] [CrossRef]
- Gainor, J.F.; Patel, M.R.; Weber, J.S.; Gutierrez, M.; Bauman, J.E.; Clarke, J.M.; Julian, R.; Scott, A.J.; Geiger, J.L.; Kirtane, K.; et al. T-cell Responses to Individualized Neoantigen Therapy mRNA-4157 (V940) Alone or in Combination with Pembrolizumab in the Phase 1 KEYNOTE-603 Study—PubMed. Cancer Discov. 2024, 14, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.M.; Carpenter, E.L.; Clifton, G.T.; Vreeland, T.J.; Chick, R.C.; O’Shea, A.E.; McCarthy, P.M.; Kemp Bohan, P.M.; Hickerson, A.T.; Valdera, F.A.; et al. Divergent clinical outcomes in a phase 2B trial of the TLPLDC vaccine in preventing melanoma recurrence and the impact of dendritic cell collection methodology: A randomized clinical trial. Cancer Immunol. Immunother. 2022, 72, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef]
- Sylvester, R.J.; Meijden, A.P.M.v.d.; Lamm, D.L. Intravesical Bacillus Calmette-Guerin Reduces the Risk of Progression in Patients with Superficial Bladder Cancer: A Meta-analysis of the Published Results of Randomized Clinical Trials. J. Urol. 2002, 168, 1964–1970. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Welty, N.E.; Gill, S.I. Cancer Immunotherapy Beyond Checkpoint Blockade: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2022, 4, 563–578. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Ibrahim, J.G.; Sosman, J.A.; Sondak, V.K.; Agarwala, S.S.; Ernstoff, M.S.; Rao, U. High-Dose Interferon Alfa-2b Significantly Prolongs Relapse-Free and Overall Survival Compared With the GM2-KLH/QS-21 Vaccine in Patients With Resected Stage IIB-III Melanoma: Results of Intergroup Trial E1694/S9512/C509801. J. Clin. Oncol. 2001, 19, 2370–2380. [Google Scholar] [CrossRef]
- Dreno, B.; Thompson, J.F.; Smithers, B.M.; Santinami, M.; Jouary, T.; Gutzmer, R.; Levchenko, E.; Rutkowski, P.; Grob, J.-J.; Korovin, S.; et al. MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018, 19, 916–929. [Google Scholar] [CrossRef]
- Bol, K.F.; Schreibelt, G.; Bloemendal, M.; van Willigen, W.W.; Hins-de Bree, S.; de Goede, A.L.; de Boer, A.J.; Bos, K.J.H.; Duiveman-de Boer, T.; Olde Nordkamp, M.A.M.; et al. Adjuvant dendritic cell therapy in stage IIIB/C melanoma: The MIND-DC randomized phase III trial. Nat. Commun. 2024, 15, 1–10. [Google Scholar] [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef] [PubMed]
- Janes, L.A.; Haykal, T.; Angeles, C.V. Vaccines in Melanoma: Past, Present, and Future—PubMed. Surg. Oncol. Clin. N. Am. 2025, 34, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Giobbie-Hurder, A.; Adu-Berchie, K.; Ranasinghe, S.; Lako, A.; Severgnini, M.; Thrash, E.M.; Weirather, J.L.; Baginska, J.; Manos, M.P.; et al. First-in-Human Clinical Trial of Vaccination with WDVAX, a Dendritic Cell Activating Scaffold Incorporating Autologous Tumor Cell Lysate, in Metastatic Melanoma Patients. Cancer Immunol. Res. 2025. [Google Scholar] [CrossRef]
| Trial Information | |||
|---|---|---|---|
| Moderna—mRNA * | Elios—TL ** | ||
| Technology | Vaccine Mechanism | mRNA encodes highly selective neoantigens that are translated in vivo and incorporated into antigen processing pathways, producing a tumor-specific immune response | Tumor lysate contains the entire tumor antigen repertoire loaded in YCWPs introduced into the cytoplasm of autologous dendritic cells (DCs) via phagocytosis |
| Samples Required | Tumor DNA and RNA, patient DNA and HLA | Tumor sample only for TLPO Tumor sample and DC for TLPLDC | |
| Antigen Isolation | Next-generation sequencing of samples is input into an internal mRNA vaccine bioinformatics system, which identifies the ideal neoantigens for mRNA manufacturing | Tumor lysate is created through freeze/thaw cycles of tumor sample | |
| Antigenic Repertoire | Up to 34 neoantigens | Total repertoire to include all neoantigens | |
| Time | 9 weeks | 1–3 days | |
| Trial Design | Phase | 2b | 2b |
| Prospective | Yes | Yes | |
| Randomized | Yes | Yes | |
| Blinding | Open label | Double blind | |
| Placebo Controlled | No | Yes | |
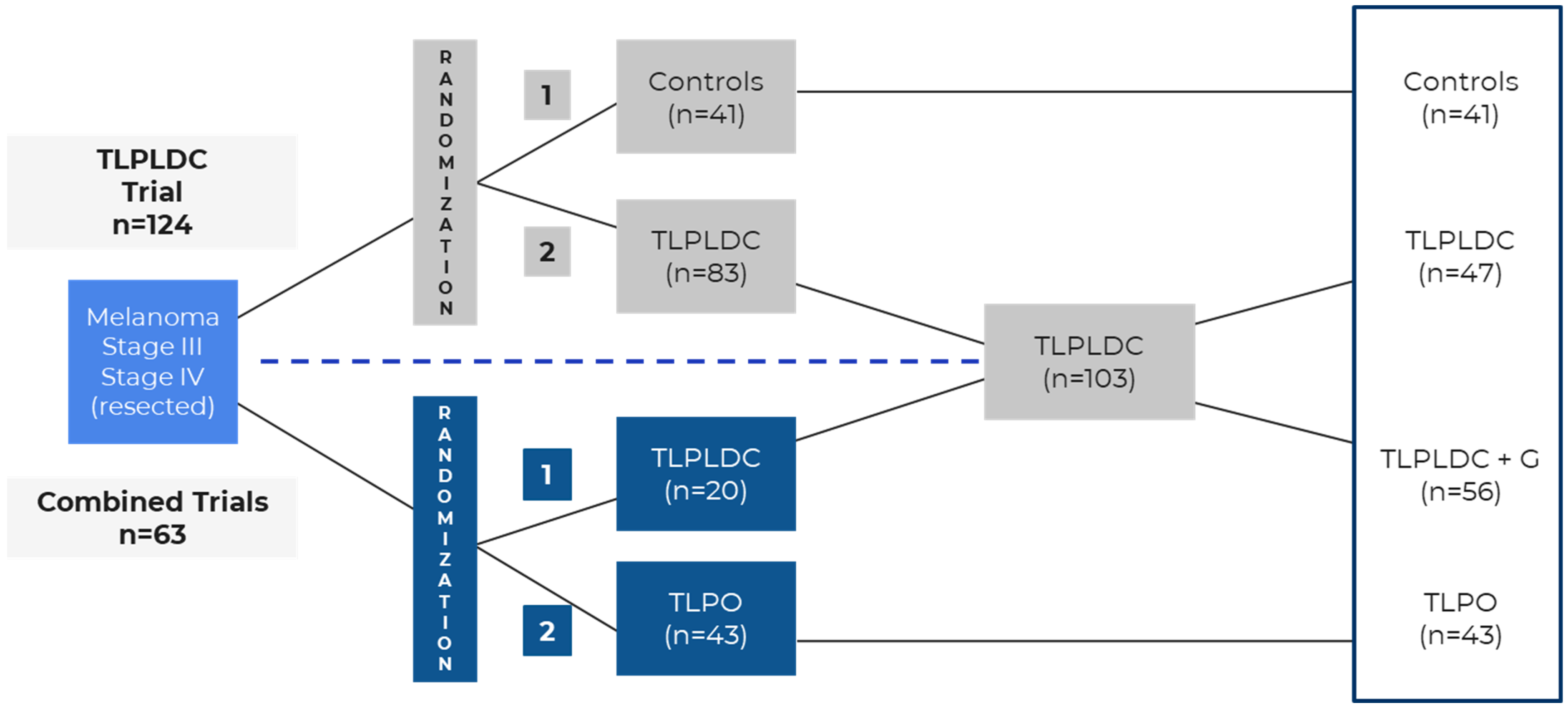
| Statistical Powering | Required 40 recurrence-free survival events, yielding a sample size of 150 to provide approximately 80% power to detect an HR of 0.5 with one-sided a = 0.10 | Assumed 60% recurrence, yielding a sample size of 120 for 80% power to detect an HR of 0.5 with two-sided a = 0.05 Trial was expanded to perform head-to-head comparison of TLPLDC vs. TLPO | |
| Protocol Amendments | None | Yes—to permit concurrent CPI therapy once FDA approved for use in the adjuvant setting | |
| Patients Enrolled | Melanoma Stage | IIIC or IIID—86% | III—74% |
| IV—14% | IV—26% | ||
| ECOG Performance Status | 0–1 | 0–1 | |
| Complete Resection, No Evidence of Disease | Yes, no more than 13 weeks prior to trial enrollment | Yes, no time restriction | |
| Results | Vaccine | mRNA | TLPLDC or TLPO |
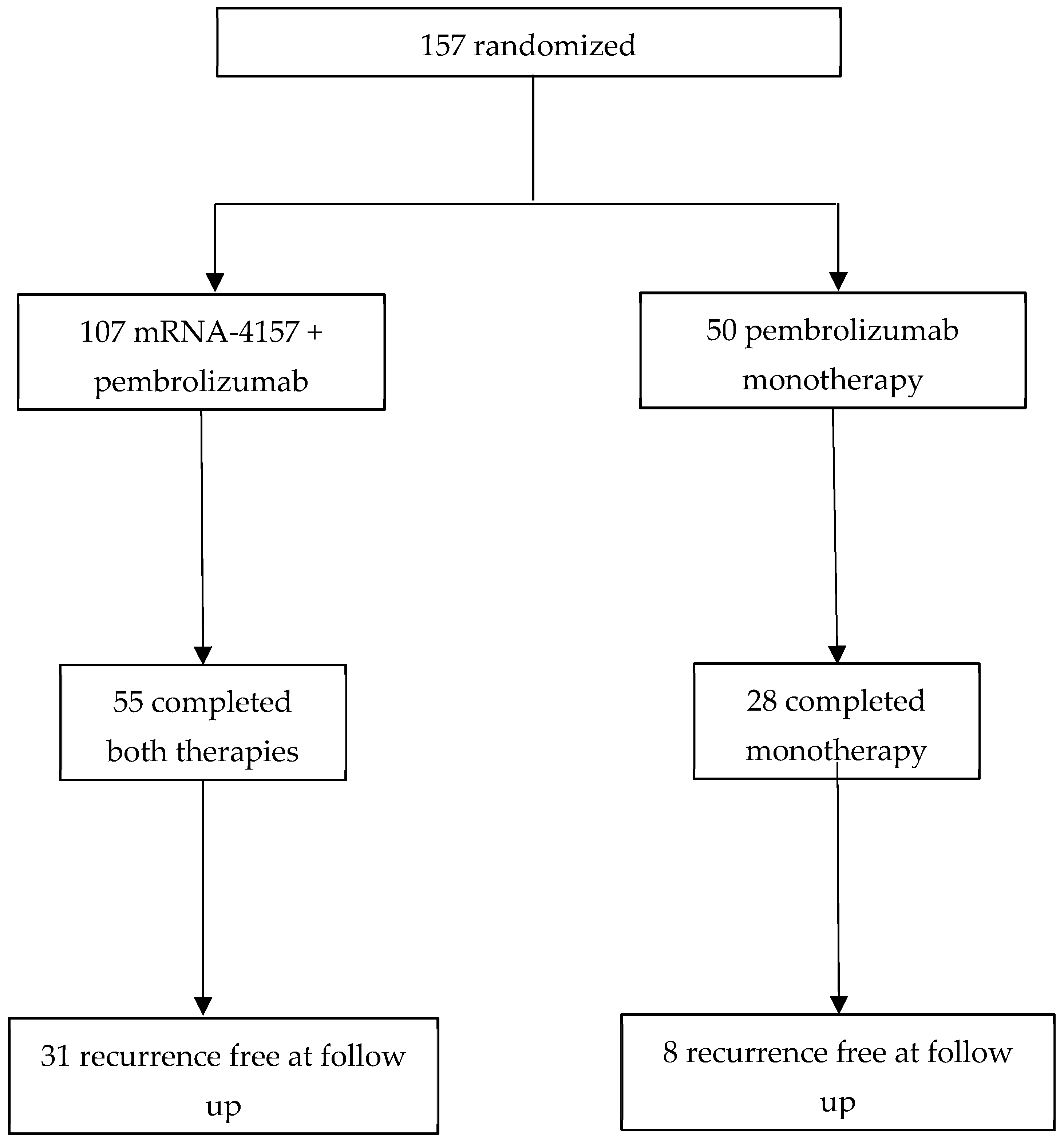
| Treatment Arms | n = 157
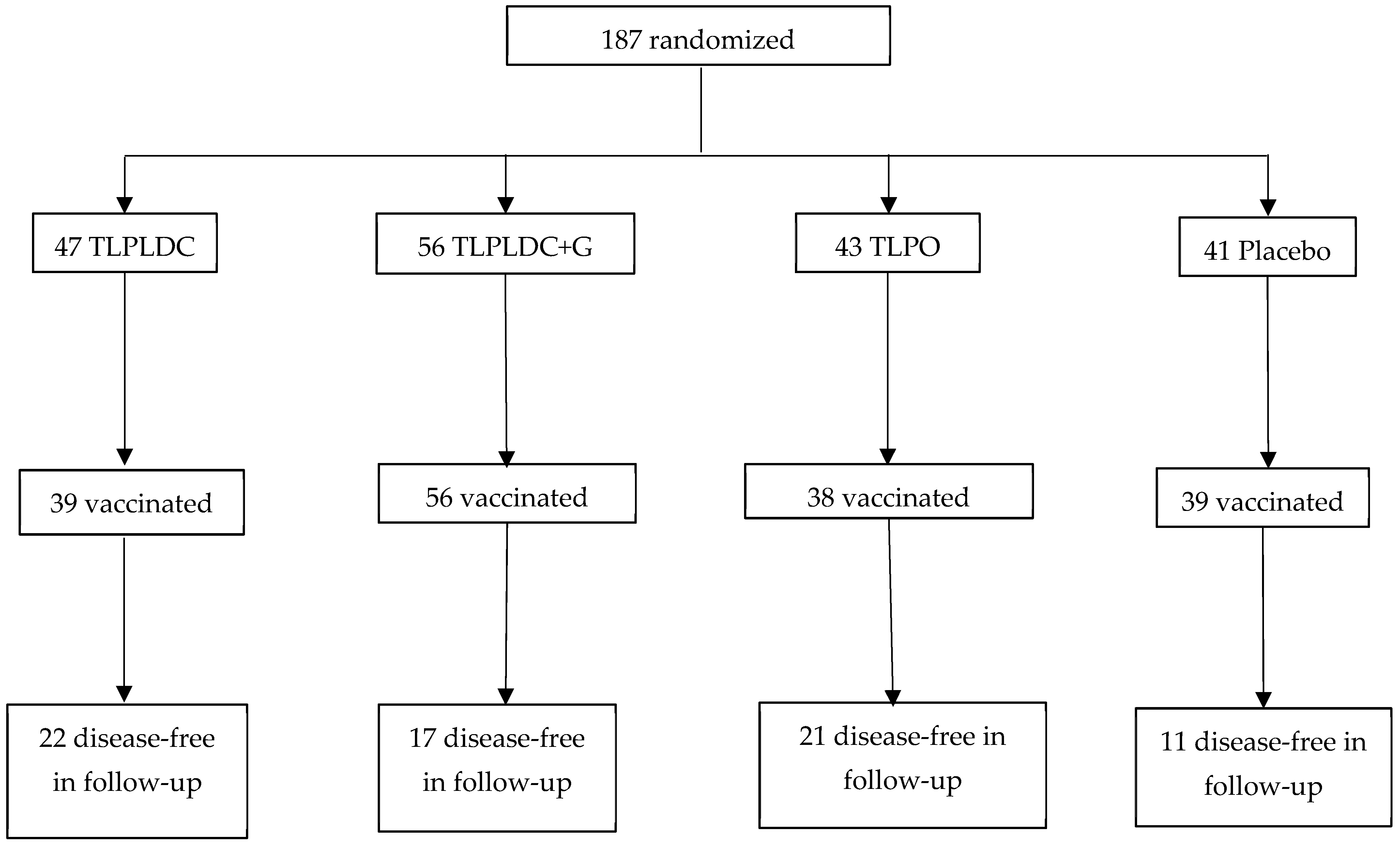
| n = 187
| |
| Median Follow-Up | ~24 months | ~36 months | |
| % of Patients on CPI | 100% concurrently (pembrolizumab) | 42–35% sequentially, 7% concurrently (pembrolizumab, nivolumab, and/or ipilimumab) | |
| Recurrence or Disease-Free Survival | RFS: HR = 0.56; p = 0.053 over 18 months | DFS: HR = 0.52; p < 0.01 over 36 months | |
| Overall Survival | Not reported | HR = 0.17; p = 0.011 | |
| Grade 3 Related AEs (No Grade 4 or 5 Related AEs Reported) | 12% of patients | <1% of patients (1 in TLPLDC + G) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broderick, J.C.; Adams, A.M.; Barbera, E.L.; Van Decar, S.; Clifton, G.T.; Peoples, G.E. Melanoma Vaccines: Comparing Novel Adjuvant Treatments in High-Risk Patients. Vaccines 2025, 13, 656. https://doi.org/10.3390/vaccines13060656
Broderick JC, Adams AM, Barbera EL, Van Decar S, Clifton GT, Peoples GE. Melanoma Vaccines: Comparing Novel Adjuvant Treatments in High-Risk Patients. Vaccines. 2025; 13(6):656. https://doi.org/10.3390/vaccines13060656
Chicago/Turabian StyleBroderick, Joseph C., Alexandra M. Adams, Elizabeth L. Barbera, Spencer Van Decar, Guy T. Clifton, and George E. Peoples. 2025. "Melanoma Vaccines: Comparing Novel Adjuvant Treatments in High-Risk Patients" Vaccines 13, no. 6: 656. https://doi.org/10.3390/vaccines13060656
APA StyleBroderick, J. C., Adams, A. M., Barbera, E. L., Van Decar, S., Clifton, G. T., & Peoples, G. E. (2025). Melanoma Vaccines: Comparing Novel Adjuvant Treatments in High-Risk Patients. Vaccines, 13(6), 656. https://doi.org/10.3390/vaccines13060656






