Delayed Viral Clearance Accompanied by Early Impaired Humoral and Virus-Specific T-Cell Response in Patients with Coronavirus Disease 2019 and Interstitial Lung Disease
Abstract
1. Introduction
2. Methods
2.1. Study Cohort
2.2. Measurement of SARS-CoV-2 Viral Load on Nasopharyngeal Swabs
2.3. Enzyme-Linked Immunosorbent Assay
2.4. iFlash SARS-CoV-2 IgG Assay
2.5. SARS-CoV-2 Conventional Virus Neutralization Test
2.6. Peptide Pool Design and Preparation
2.7. Peripheral Blood Mononuclear Cell Isolation and Ex Vivo Stimulation
2.8. Flow Cytometry
2.9. Cytokine Assays
2.10. Statistical Analysis
3. Results
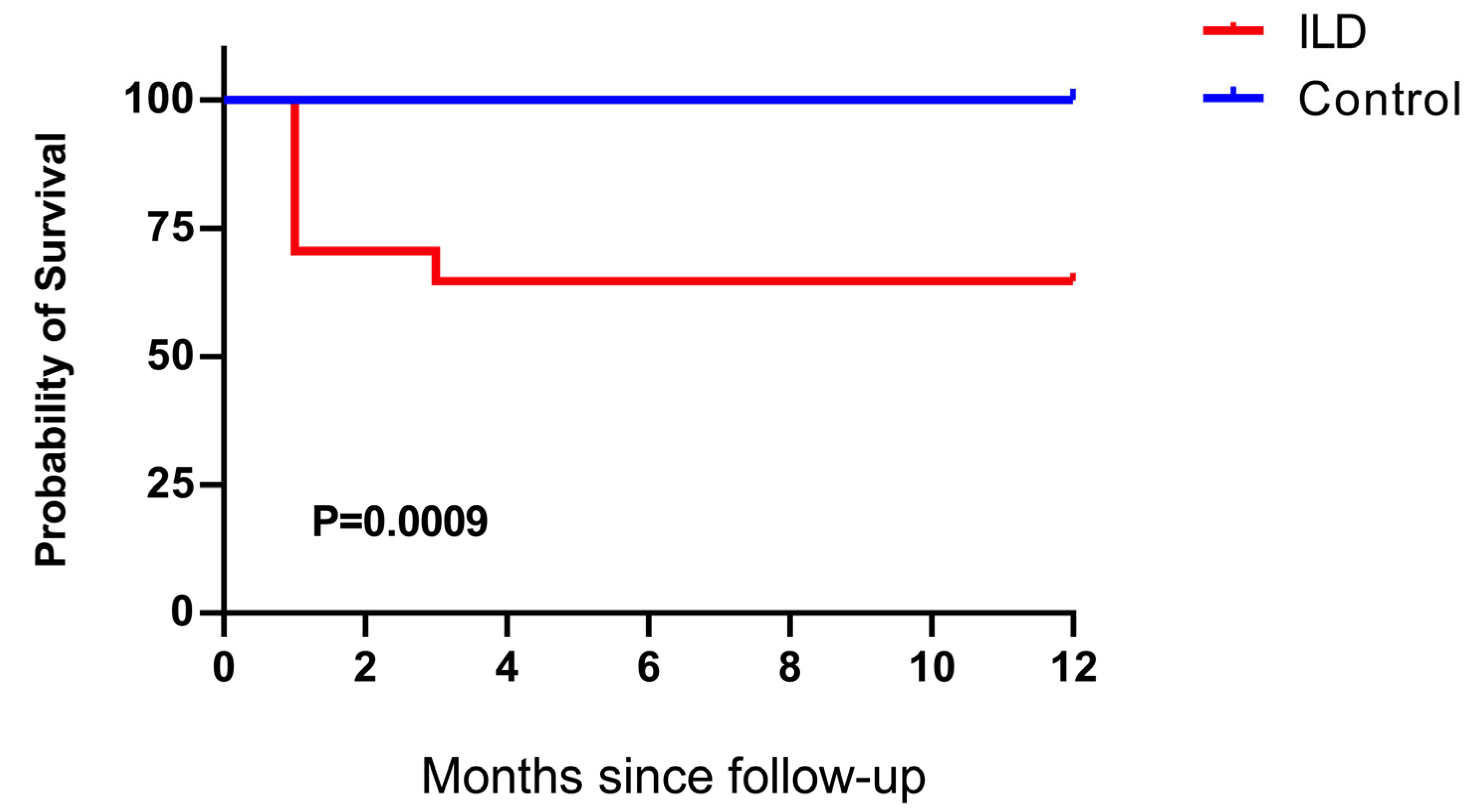
3.1. General Condition of the Study Cohort
3.2. Delayed Virus Clearance of SARS-CoV-2 Was Observed in Patients with ILD
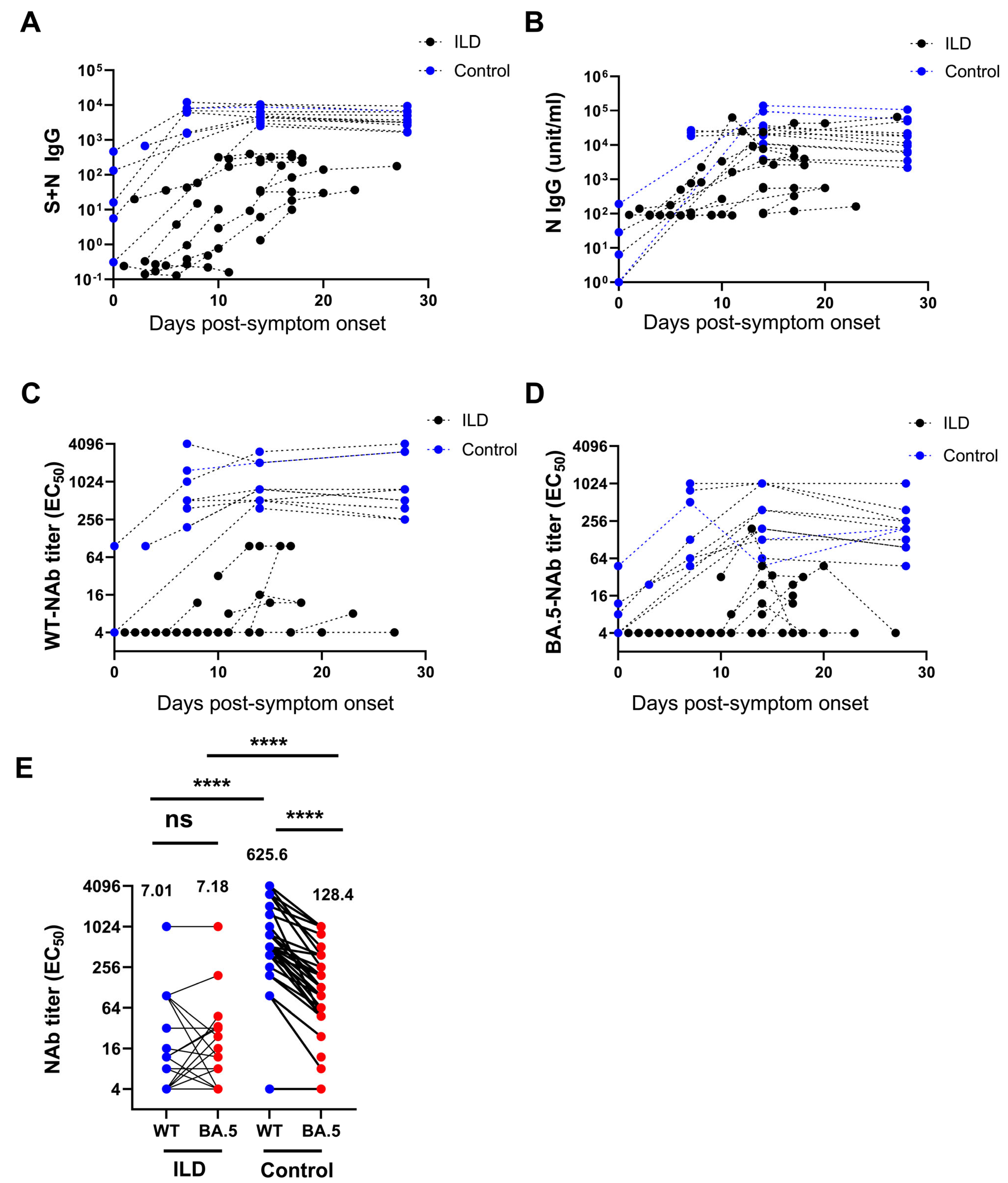
3.3. Decreased Level of Neutralizing Antibodies Against SARS-CoV-2 in Patients with ILD
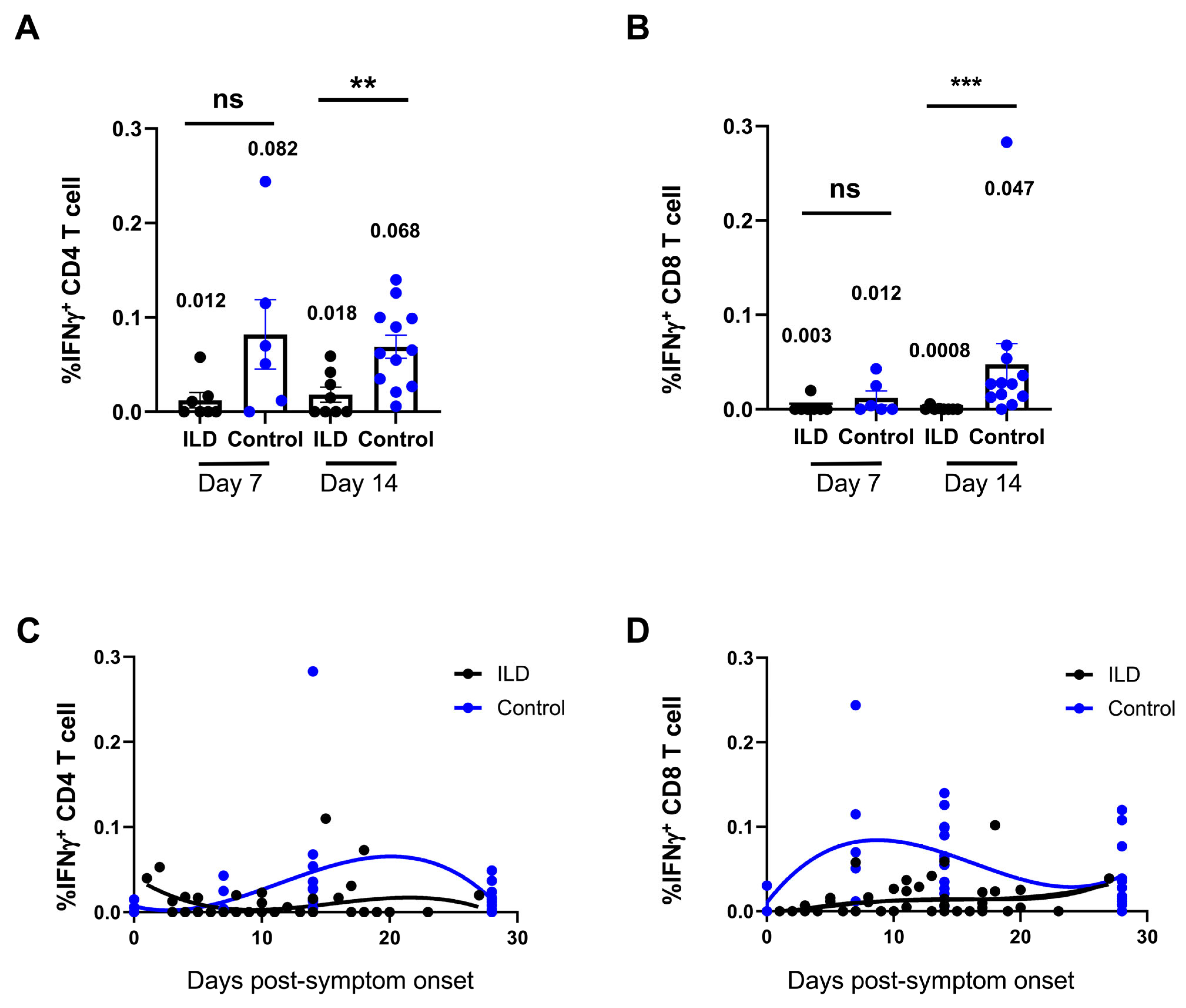
3.4. Suppression of Virus-Specific T Cells Against SARS-CoV-2 in Patients with ILD
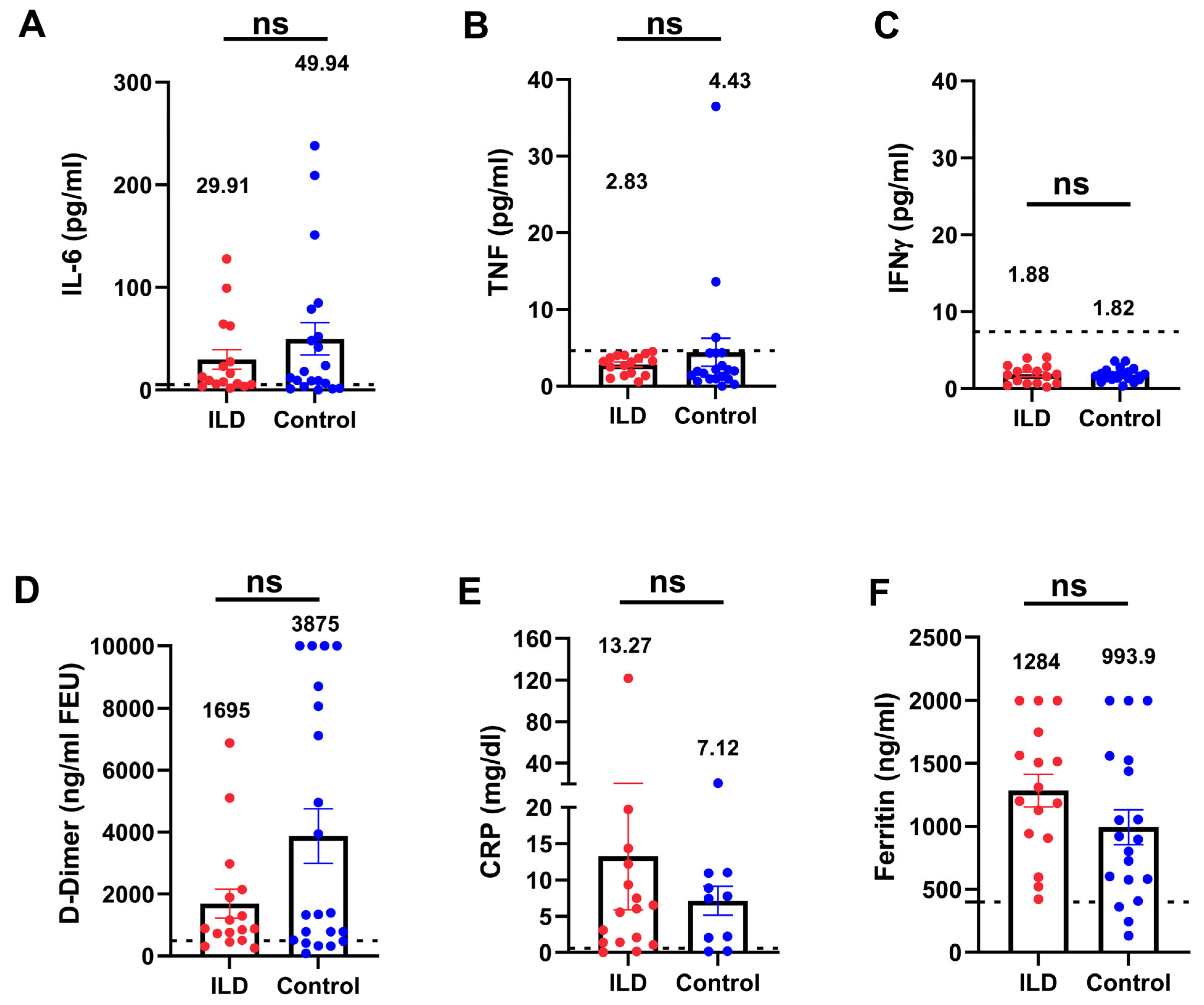
3.5. Inflammatory Parameters in Patients with ILD Compared with Those in the Control Group
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.J.; Dong, X.; Liu, G.H.; Gao, Y.D. Risk and Protective Factors for COVID-19 Morbidity, Severity, and Mortality. Clin. Rev. Allergy Immunol. 2023, 64, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.F.; Lakhani, D.A.; Sohail, A.H.; Maurer, J.; Sofka, S.; Sarwari, A.; Hadi, Y.B. Patients with idiopathic pulmonary fibrosis have poor clinical outcomes with COVID-19 disease: A propensity matched multicentre research network analysis. BMJ Open Respir. Res. 2021, 8, e000969. [Google Scholar] [CrossRef] [PubMed]
- Drake, T.M.; Docherty, A.B.; Harrison, E.M.; Quint, J.K.; Adamali, H.; Agnew, S.; Babu, S.; Barber, C.M.; Barratt, S.; Bendstrup, E.; et al. Outcome of Hospitalization for COVID-19 in Patients with Interstitial Lung Disease. An International Multicenter Study. Am. J. Respir. Crit. Care Med. 2020, 202, 1656–1665. [Google Scholar] [CrossRef]
- Gallay, L.; Uzunhan, Y.; Borie, R.; Lazor, R.; Rigaud, P.; Marchand-Adam, S.; Hirschi, S.; Israel-Biet, D.; Valentin, V.; Cottin, V. Risk Factors for Mortality after COVID-19 in Patients with Preexisting Interstitial Lung Disease. Am. J. Respir. Crit. Care Med. 2021, 203, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Pertzov, B.; Shmueli, E.; Ben Zvi, H.; Massarweh, A.; Barkan, T.; Ness, A.; Shostak, Y.; Freidkin, L.; Shtraichman, O.; Kramer, M.R. Humoral response among patients with interstitial lung disease vaccinated with the BNT162b2 SARS-Cov-2 vaccine: A prospective cohort study. Respir. Res. 2022, 23, 226. [Google Scholar] [CrossRef]
- Tan, A.T.; Linster, M.; Tan, C.W.; Le Bert, N.; Chia, W.N.; Kunasegaran, K.; Zhuang, Y.; Tham, C.Y.L.; Chia, A.; Smith, G.J.D.; et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021, 34, 108728. [Google Scholar] [CrossRef]
- Lyudovyk, O.; Kim, J.Y.; Qualls, D.; Hwee, M.A.; Lin, Y.H.; Boutemine, S.R.; Elhanati, Y.; Solovyov, A.; Douglas, M.; Chen, E.; et al. Impaired humoral immunity is associated with prolonged COVID-19 despite robust CD8 T cell responses. Cancer Cell 2022, 40, 738–753.e5. [Google Scholar] [CrossRef]
- Rodriguez, Y.; Rojas, M.; Beltran, S.; Polo, F.; Camacho-Dominguez, L.; Morales, S.D.; Gershwin, M.E.; Anaya, J.M. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J. Autoimmun. 2022, 132, 102898. [Google Scholar] [CrossRef]
- Safary, A.; Esalatmanesh, K.; Eftekharsadat, A.T.; Jafari Nakjavani, M.R.; Khabbazi, A. Autoimmune inflammatory rheumatic diseases post-COVID-19 vaccination. Int. Immunopharmacol. 2022, 110, 109061. [Google Scholar] [CrossRef]
- Li, X.; Tong, X.; Yeung, W.W.Y.; Kuan, P.; Yum, S.H.H.; Chui, C.S.L.; Lai, F.T.T.; Wan, E.Y.F.; Wong, C.K.H.; Chan, E.W.Y.; et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann. Rheum. Dis. 2022, 81, 564–568. [Google Scholar] [CrossRef]
- National Health Commission of the People’s Republic of China. Guidelines for the Diagnosis and Treatment of Corona Virus Disease-2019 Infection by the National Health Commission (Revision of 5th Trial Version). Available online: https://www.nhc.gov.cn/wjw/c100175/202002/323442ae04c24b3ba097d1c21eb9b9d1.shtml (accessed on 22 March 2020). (In Chinese)
- Andreano, E.; Piccini, G.; Licastro, D.; Casalino, L.; Johnson, N.V.; Paciello, I.; Dal Monego, S.; Pantano, E.; Manganaro, N.; Manenti, A.; et al. SARS-CoV-2 escape from a highly neutralizing COVID-19 convalescent plasma. Proc. Natl. Acad. Sci. USA 2021, 118, e2103154118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, X.; Zhong, J.; Zhou, Y.; Tang, Z.; Zhou, H.; He, J.; Mei, X.; Tang, Y.; Lin, B.; et al. Exposure to SARS-CoV-2 generates T-cell memory in the absence of a detectable viral infection. Nat. Commun. 2021, 12, 1724. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Mei, X.; Cao, J.; Zhong, J.; Huang, P.; Luo, Q.; Li, G.; Wei, R.; Zhong, N.; et al. SARS-CoV-2 vaccination-infection pattern imprints and diversifies T cell differentiation and neutralizing response against Omicron subvariants. Cell Discov. 2022, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Zhang, N.; Feng, M.; Liang, Z.; Zhao, X.; Gao, C.; Qin, Y.; Wu, Y.; Liu, G.; et al. Reduced activated regulatory T cells and imbalance of Th17/activated Treg cells marks renal involvement in ANCA-associated vasculitis. Mol. Immunol. 2020, 118, 19–29. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Y.; Tang, X. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China. Glob. Health Med. 2020, 2, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, H.; Yang, B.; Lee, S.K.; Park, T.S.; Park, D.W.; Moon, J.Y.; Kim, T.H.; Sohn, J.W.; Yoon, H.J.; et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur. Respir. J. 2021, 58, 2004125. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Mo, G.; Yuan, X.; Tao, Y.; Peng, X.; Wang, F.S.; Xie, L.; Sharma, L.; Dela Cruz, C.S.; Qin, E. Time Kinetics of Viral Clearance and Resolution of Symptoms in Novel Coronavirus Infection. Am. J. Respir. Crit. Care Med. 2020, 201, 1150–1152. [Google Scholar] [CrossRef]
- Qi, H.; Liu, B.; Wang, X.; Zhang, L. The humoral response and antibodies against SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 1008–1020. [Google Scholar] [CrossRef]
- Pablos, J.L.; Galindo, M.; Carmona, L.; Lledo, A.; Retuerto, M.; Blanco, R.; Gonzalez-Gay, M.A.; Martinez-Lopez, D.; Castrejon, I.; Alvaro-Gracia, J.M.; et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: A multicentric matched cohort study. Ann. Rheum. Dis. 2020, 79, 1544–1549. [Google Scholar] [CrossRef]
- Marty, P.K.; Van Keulen, V.P.; Erskine, C.L.; Shah, M.; Hummel, A.; Stachowitz, M.; Fatis, S.; Granger, D.; Block, M.S.; Duarte-Garcia, A.; et al. Antigen Specific Humoral and Cellular Immunity Following SARS-CoV-2 Vaccination in ANCA-Associated Vasculitis Patients Receiving B-Cell Depleting Therapy. Front. Immunol. 2022, 13, 834981. [Google Scholar] [CrossRef]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2020, 370, eabd4250. [Google Scholar] [CrossRef] [PubMed]
- Prendecki, M.; Clarke, C.; Edwards, H.; McIntyre, S.; Mortimer, P.; Gleeson, S.; Martin, P.; Thomson, T.; Randell, P.; Shah, A.; et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Dis. 2021, 80, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Mrak, D.; Tobudic, S.; Koblischke, M.; Graninger, M.; Radner, H.; Sieghart, D.; Hofer, P.; Perkmann, T.; Haslacher, H.; Thalhammer, R.; et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann. Rheum. Dis. 2021, 80, 1345–1350. [Google Scholar] [CrossRef]
- Sattler, A.; Schrezenmeier, E.; Weber, U.A.; Potekhin, A.; Bachmann, F.; Straub-Hohenbleicher, H.; Budde, K.; Storz, E.; Pross, V.; Bergmann, Y.; et al. Impaired humoral and cellular immunity after SARS-CoV-2 BNT162b2 (tozinameran) prime-boost vaccination in kidney transplant recipients. J. Clin. Investig. 2021, 131, e150175. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J.; Hlh Across Speciality Collaboration, U.K. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Group, R.C.; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar]
- D’Antiga, L. Coronaviruses and Immunosuppressed Patients: The Facts During the Third Epidemic. Liver Transpl. 2020, 26, 832–834. [Google Scholar] [CrossRef]
- Cheng, G.S.; Evans, S.E. The paradox of immunosuppressants and COVID-19. Eur. Respir. J. 2022, 59, 2102828. [Google Scholar] [CrossRef]
- Ward, D.; Gortz, S.; Thomson Ernst, M.; Andersen, N.N.; Kjaer, S.K.; Hallas, J.; Christensen, S.; Fynbo Christiansen, C.; Bastrup Israelsen, S.; Benfield, T.; et al. The effect of immunosuppressants on the prognosis of SARS-CoV-2 infection. Eur. Respir. J. 2022, 59, 2100769. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Qiu, S.; Yuan, Y.; Zong, Y.; Tuo, Z.; Li, J.; Liu, J. Clinical characteristics and treatment of patients infected with COVID-19 in Shishou, China. Lancet, 2020; preprint. [Google Scholar] [CrossRef]

| Case Number | Sex | Age | ILD Classification | Inactivated Vaccination History * | Severity of COVID-19 | SOFA on Admission | Lung Infiltrates (%) | Anti-Inflammatory | 3-Month Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute | Basic | MP (mg/day) | Immuno-Suppressant | ||||||||
| C1 | F | 67 | IPAF | No | Severe | 2 | 17 | 3 | 4 | CsA | Survive |
| C2 | F | 59 | CTD-ILD | No | Severe | 1 | 26 | 32 | 4 | CsA | Dead |
| C3 | M | 65 | CTD-ILD | No | Critically severe | 3 | 44 | 17 | 4 | CsA | Dead |
| C4 | M | 68 | IPAF | One dose | Severe | 0 | 6 | 7 | 4 | — | Survive |
| C5 | F | 55 | CTD-ILD | No | Moderate | 0 | 10 | 0 | 4 | Tac, HCQ | Survive |
| C6 | F | 65 | CTD-ILD | No | Severe | 0 | 18 | 12 | 8 | Tac | Survive |
| C7 | M | 63 | CTD-ILD | No | Severe | 2 | 2 | 9 | 16 | Tac, HCQ | Survive |
| C8 | M | 43 | IPAF | Three doses | Severe | 0 | 24 | 13 | 12 | MMF | Lung transplantation |
| C9 | F | 55 | IPAF | Three doses | Moderate | 0 | 12 | 2 | — | Tac | Survive |
| C10 | F | 67 | CTD-ILD | Three doses | Severe | 1 | 36 | 3 | 12 | — | Survive |
| C11 | M | 59 | CTD-ILD | Three doses | Moderate | 2 | 28 | 18 | — | MMF, Tac, HCQ | Survive |
| C12 | M | 72 | IPAF | Three doses | Severe | 0 | 25 | 34 | 8 | Tac | Survive |
| C13 | M | 62 | IPAF | Three doses | Critically severe | 3 | 32 | 40 | — | MMF | Dead |
| C14 | F | 59 | CTD-ILD | Three doses | Severe | 3 | 21 | 1 | 4 | MMF | Dead |
| C15 | M | 59 | CTD-ILD | Three doses | Severe | 1 | 17 | 15 | 8 | MMF | Survive |
| C16 | F | 83 | CTD-ILD | No | Critically severe | 4 | 58 | 0 | — | — | Dead |
| C17 | F | 70 | CTD-ILD | No | Moderate | 1 | — | — | 8 | Tac | Survive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Li, J.; Wei, R.; Guo, B.; Cui, T.; Huang, P.; Wang, Z.; Luo, Q.; Han, Q. Delayed Viral Clearance Accompanied by Early Impaired Humoral and Virus-Specific T-Cell Response in Patients with Coronavirus Disease 2019 and Interstitial Lung Disease. Vaccines 2025, 13, 655. https://doi.org/10.3390/vaccines13060655
Zhong J, Li J, Wei R, Guo B, Cui T, Huang P, Wang Z, Luo Q, Han Q. Delayed Viral Clearance Accompanied by Early Impaired Humoral and Virus-Specific T-Cell Response in Patients with Coronavirus Disease 2019 and Interstitial Lung Disease. Vaccines. 2025; 13(6):655. https://doi.org/10.3390/vaccines13060655
Chicago/Turabian StyleZhong, Jiaying, Juan Li, Rui Wei, Bingpeng Guo, Tingting Cui, Peiyu Huang, Zhongfang Wang, Qun Luo, and Qian Han. 2025. "Delayed Viral Clearance Accompanied by Early Impaired Humoral and Virus-Specific T-Cell Response in Patients with Coronavirus Disease 2019 and Interstitial Lung Disease" Vaccines 13, no. 6: 655. https://doi.org/10.3390/vaccines13060655
APA StyleZhong, J., Li, J., Wei, R., Guo, B., Cui, T., Huang, P., Wang, Z., Luo, Q., & Han, Q. (2025). Delayed Viral Clearance Accompanied by Early Impaired Humoral and Virus-Specific T-Cell Response in Patients with Coronavirus Disease 2019 and Interstitial Lung Disease. Vaccines, 13(6), 655. https://doi.org/10.3390/vaccines13060655






