Safety and Efficacy of Simultaneous Vaccination with Polysaccharide Conjugate Vaccines Against Pneumococcal (13-Valent Vaccine) and Haemophilus Type B Infections in Children with Systemic Juvenile Idiopathic Arthritis: Prospective Cohort Study
Abstract
1. Introduction
2. Methods
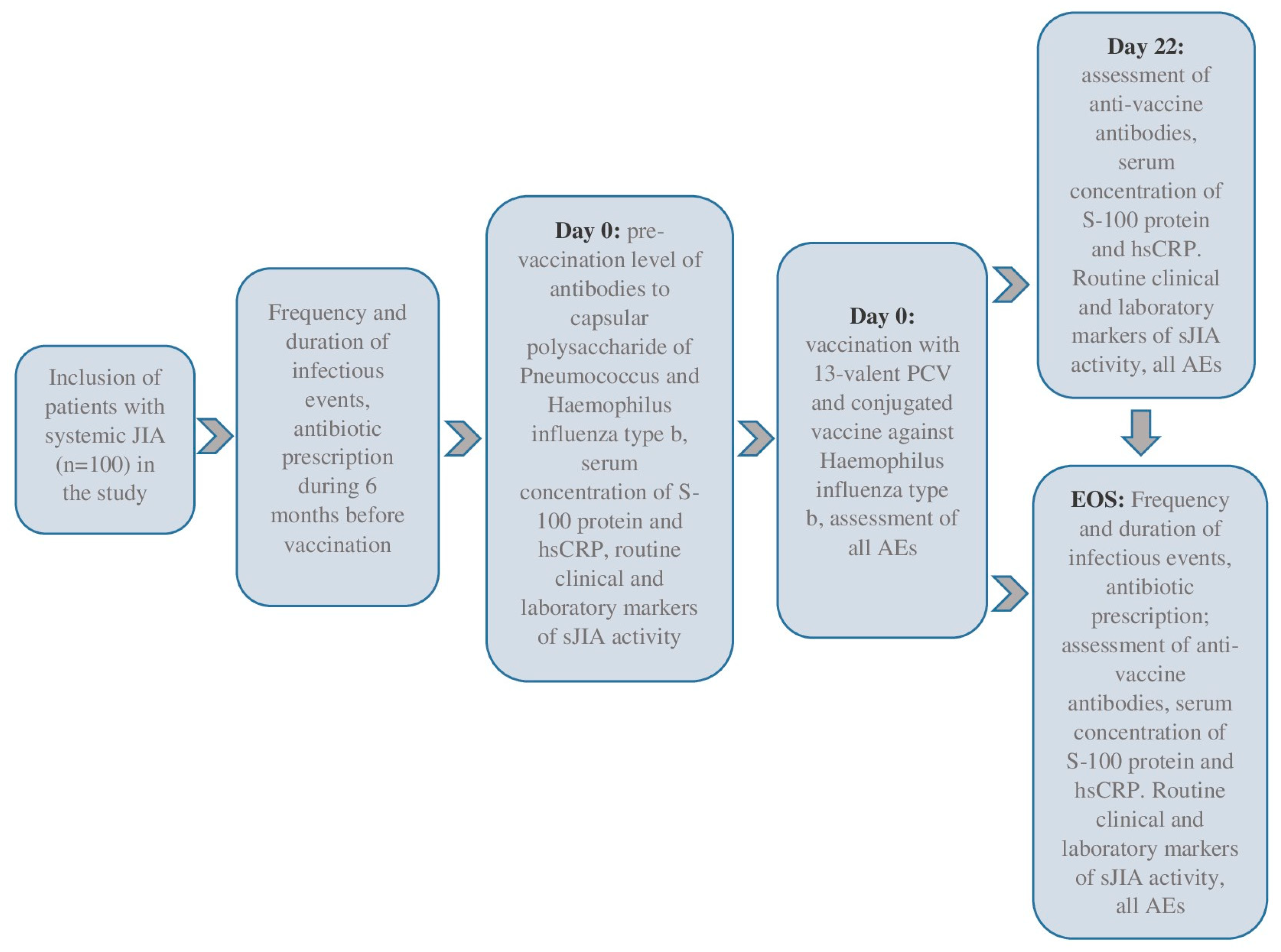
2.1. Study Design and Patient Selection
2.1.1. Inclusion Criteria
- -
- The age of patients included in this study is 2–18 years;
- -
- Diagnosis of sJIA according to the ILAR (International League of Associations for Rheumatology) criteria;
- -
- Treatment with either non-biologic or biologic disease-modifying antirheumatic drugs: tocilizumab, canakinumab, adalimumab, etanercept with or without disease-modifying antirheumatic drugs (DMARDs);
- -
- No previous vaccination against pneumococcal and Hemophilus influenza type b infections, except scheduled vaccination against these infections in the first year of life according to the national vaccine schedule. The national vaccine schedule included vaccination against pneumococcal and Hib infections at 3, 4.5, and 6 months, with revaccination after 15 months.
2.1.2. Exclusion Criteria
- -
- Intolerance to vaccine components in the past;
- -
- Signs of acute ENT and/or respiratory tract infection;
- -
- Mild respiratory, intestinal, or other infections with fever.
2.2. Vaccination
2.3. The Main Assessments and Outcomes of the Study
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Laboratory Signs of sJIA Activity
2.6. Clinical and Laboratory Assessment of sJIA Flare
2.7. The Following Changes Were Additionally Investigated:
- (a)
- Incidence of acute respiratory infection (ARI);
- (b)
- Frequency and duration of ARI antibacterial therapy.
2.8. Vaccination Safety
2.9. Statistical Analysis
2.10. Ethics
3. Results
3.1. Patient Characteristics
3.2. Analysis of the Influence of Treatment Regimen on the Vaccination Outcome
3.3. The Frequency of Acute Infectious Events and Antibiotic Use
3.4. Evaluation of Adverse Events After Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pardeo, M.; Bracaglia, C.; De Benedetti, F. Systemic juvenile idiopathic arthritis: New insights into pathogenesis and cytokine directed therapies. Best Pract. Res. Clin. Rheumatol. 2017, 31, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Hinze, C.H.; Foell, D.; Kessel, C. Treatment of systemic juvenile idiopathic arthritis. Nat. Rev. Rheumatol. 2023, 19, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Zhao, Z.; Ping, J.; Zhou, L.; Wang, Y.; Zhang, Y. Comparative efficacy and safety of different drugs in patients with systemic juvenile idiopathic arthritis: A systematic review and network meta-analysis. Medicine 2024, 103, e38002. [Google Scholar] [CrossRef]
- Morel, J.; Constantin, A.; Baron, G.; Dernis, E.; Flipo, R.M.; Rist, S.; Combe, B.; Gottenberg, J.E.; Schaeverbeke, T.; Soubrier, M.; et al. Risk factors of serious infections in patients with rheumatoid arthritis treated with tocilizumab in the French Registry REGATE. Rheumatology 2017, 56, 1746–1754. [Google Scholar] [CrossRef]
- Horneff, G.; Schulz, A.C.; Klotsche, J.; Hospach, A.; Minden, K.; Foeldvari, I.; Trauzeddel, R.; Ganser, G.; Weller-Heinemann, F.; Haas, J.P. Experience with etanercept, tocilizumab and interleukin-1 inhibitors in systemic onset juvenile idiopathic arthritis patients from the BIKER registry. Arthritis Res. Ther. 2017, 19, 256. [Google Scholar] [CrossRef] [PubMed]
- Yasin, S.; Schulert, G.S. Systemic juvenile idiopathic arthritis and macrophage activation syndrome: Update on pathogenesis and treatment. Curr. Opin. Rheumatol. 2018, 30, 514–520. [Google Scholar] [CrossRef]
- Klein, A.; Klotsche, J.; Hügle, B.; Minden, K.; Hospach, A.; Weller-Heinemann, F.; Schwarz, T.; Dressler, F.; Trauzeddel, R.; Hufnagel, M.; et al. Long-term surveillance of biologic therapies in systemic-onset juvenile idiopathic arthritis: Data from the German BIKER registry. Rheumatology 2020, 59, 2287–2298. [Google Scholar] [CrossRef]
- Tikhomirova, A.; Kidd, S.P. Haemophilus influenzae and Streptococcus pneumoniae: Living together in a biofilm. Pathog. Dis. 2013, 69, 114–126. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/en/news-room/fact-sheets/detail/pneumonia (accessed on 10 June 2025).
- Shea, K.M.; Edelsberg, J.; Weycker, D.; Farkouh, R.A.; Strutton, D.R.; Pelton, S.I. Rates of Pneumococcal Disease in Adults with Chronic Medical Conditions. Open Forum Infect. Dis. 2014, 1, ofu024. [Google Scholar] [CrossRef]
- Makarova, E.; Khabirova, A.; Volkova, N.; Gabrusskaya, T.; Ulanova, N.; Sakhno, L.; Revnova, M.; Kostik, M. Vaccination coverage in children with juvenile idiopathic arthritis, inflammatory bowel diseases, and healthy peers: Cross-sectional electronic survey data. World J. Clin. Pediatr. 2023, 12, 45–56. [Google Scholar] [CrossRef]
- Bass, A.R.; Chakravarty, E.; Akl, E.A.; Bingham, C.O.; Calabrese, L.; Cappelli, L.C.; Johnson, S.R.; Imundo, L.F.; Winthrop, K.L.; Arasaratnam, R.J.; et al. 2022 American College of Rheumatology Guideline for Vaccinations in Patients with Rheumatic and Musculoskeletal Diseases. Arthritis Care Res. 2023, 75, 449–464. [Google Scholar] [CrossRef] [PubMed]
- Nikishina, I.; Ruperto, N.; Kuzmina, N.; Shelepina, T.; Illarionova, O.; Salougina, S.; Kaleda, M.; Borodacheva, O.; Paediatric Rheumatology International Trials Organisation. The Russian version of the Childhood Health Assessment Questionnaire (CHAQ) and the Child Health Questionnaire (CHQ). Clin. Exp. Rheumatol. 2001, 19, S131–S135. [Google Scholar] [PubMed]
- Tibaldi, J.; Pistorio, A.; Aldera, E.; Puzone, L.; El Miedany, Y.; Pal, P.; Giri, P.P.; De, H.; Khubchandani, R.; Chavan, P.P.; et al. Development and initial validation of a composite disease activity score for systemic juvenile idiopathic arthritis. Rheumatology 2020, 59, 3505–3514. [Google Scholar] [CrossRef] [PubMed]
- Wallace, C.A.; Giannini, E.H.; Huang, B.; Itert, L.; Ruperto, N.; Childhood Arthritis Rheumatology Research Alliance (CARRA); Pediatric Rheumatology Collaborative Study Group (PRCSG); Paediatric Rheumatology International Trials Organisation (PRINTO). American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res. 2011, 63, 929–936. [Google Scholar] [CrossRef]
- Gertosio, C.; Licari, A.; De Silvestri, A.; Rebuffi, C.; Chiappini, E.; Marseglia, G.L. Efficacy, immunogenicity, and safety of available vaccines in children on biologics: A systematic review and meta-analysis. Vaccine 2022, 40, 2679–2695. [Google Scholar] [CrossRef]
- Keller, M.; Pittet, L.F.; Zimmermann, P. Immunogenicity and safety of routine vaccines in children and adolescents with rheumatic diseases on immunosuppressive treatment—A systematic review. Eur. J. Pediatr. 2021, 181, 1329–1362. [Google Scholar] [CrossRef]
- Alexeeva, E.I.; Vankova, D.D.; Dvoryakovskaya, T.M.; Isaeva, K.B.; Denisova, R.V.; Mamutova, A.V.; Chomakhidze, A.M.; Radygina, T.V.; Zubkova, I.V.; Tkachenko, N.E.; et al. Efficacy of Pneumococcal Polysaccharide Conjugate Vaccine (13-valent, Adsorbed) in Patients with Systemic Juvenile Idiopathic Arthritis Treated with Genetically Engineered Biologic Drugs (Tocilizumab or Canakinumab): Prospective Cohort Study. Curr. Pediatr. 2020, 19, 190–199. [Google Scholar] [CrossRef]
- Wahl, B.; O’Brien, K.L.; Greenbaum, A.; Majumder, A.; Liu, L.; Chu, Y.; Lukšić, I.; Nair, H.; McAllister, D.A.; Campbell, H.; et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: Global, regional, and national estimates for 2000–15. Lancet Glob. Health 2018, 6, e744–e757. [Google Scholar] [CrossRef]
- Wakabayashi, A.; Ishiguro, T.; Takaku, Y.; Miyahara, Y.; Kagiyama, N.; Takayanagi, N. Clinical characteristics and prognostic factors of pneumonia in patients with and without rheumatoid arthritis. PLoS ONE 2018, 13, e0201799. [Google Scholar] [CrossRef]
- Murali, S.; Marks, A.; Heeger, A.; Dako, F.; Febbo, J. Pneumonia in the Immunocompromised Host. Semin. Roentgenol. 2022, 57, 90–104. [Google Scholar] [CrossRef]
- Dumaine, C.; Bekkar, S.; Belot, A.; Cabrera, N.; Malik, S.; Von Scheven, A.; Carbasse, A.; Woerner, A.; Wouters, C.; Bouayed, K.; et al. Infectious adverse events in children with Juvenile Idiopathic Arthritis treated with Biological Agents in a real-life setting: Data from the JIRcohorte. Joint Bone Spine 2020, 87, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bauwens, J.; De Lusignan, S.; Weldesselassie, Y.G.; Sherlock, J.; Künzli, N.; Bonhoeffer, J. Safety of routine childhood vaccine coadministration versus separate vaccination. BMJ Glob. Health 2022, 7, e008215. [Google Scholar] [CrossRef]
- Dolhain, J.; Janssens, W.; Dindore, V.; Mihalyi, A. Infant vaccine co-administration: Review of 18 years of experience with GSK’s hexavalent vaccine co-administered with routine childhood vaccines. Expert Rev. Vaccines 2020, 19, 419–443. [Google Scholar] [CrossRef]
- Wasserman, M.; Chapman, R.; Lapidot, R.; Sutton, K.; Dillon-Murphy, D.; Patel, S.; Chilson, E.; Snow, V.; Farkouh, R.; Pelton, S. Twenty-Year Public Health Impact of 7- and 13-Valent Pneumococcal Conjugate Vaccines in US Children. Emerg. Infect. Dis. 2021, 27, 1627–1636. [Google Scholar] [CrossRef]
- Alexeeva, E.; Dvoryakovskaya, T.; Fetisova, A.; Kriulin, I.; Krekhova, E.; Kabanova, A.; Labinov, V.; Labinova, E.; Kostik, M. The Efficacy and Safety of Simultaneous Vaccination with Polysaccharide Conjugate Vaccines Against Pneumococcal (13-Valent Vaccine) and Haemophilus influenzae Type b Infections in Children with Juvenile Idiopathic Arthritis Without Systemic Manifestations: A Prospective Cohort Study. Vaccines 2025, 13, 177. [Google Scholar] [CrossRef]
- Rondaan, C.; Furer, V.; Heijstek, M.W.; Agmon-Levin, N.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; Van Laar, J.M.; et al. Efficacy, immunogenicity and safety of vaccination in adult patients with autoimmune inflammatory rheumatic diseases: A systematic literature review for the 2019 update of EULAR recommendations. RMD Open 2019, 5, e001035. [Google Scholar] [CrossRef]
- Camacho-Lovillo, M.S.; Bulnes-Ramos, A.; Goycochea-Valdivia, W.; Fernández-Silveira, L.; Núñez-Cuadros, E.; Neth, O.; Pérez-Romero, P. Immunogenicity and safety of influenza vaccination in patients with juvenile idiopathic arthritis on biological therapy using the microneutralization assay. Pediatr. Rheumatol. 2017, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Lakota, K.; Perdan-Pirkmajer, K.; Sodin-Šemrl, S.; Čučnik, S.; Šubelj, V.; Prosenc, K.; Mrak Poljšak, K.; Tomšič, M.; Ambrožič, A.; Praprotnik, S. The immunogenicity of seasonal and pandemic influenza vaccination in autoimmune inflammatory rheumatic patients—A 6-month follow-up prospective study. Clin. Rheumatol. 2019, 38, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Van Aalst, M.; Langedijk, A.C.; Spijker, R.; De Bree, G.J.; Grobusch, M.P.; Goorhuis, A. The effect of immunosuppressive agents on immunogenicity of pneumococcal vaccination: A systematic review and meta-analysis. Vaccine 2018, 36, 5832–5845. [Google Scholar] [CrossRef]
- Friedman, M.A.; Curtis, J.R.; Winthrop, K.L. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021, 80, 1255–1265. [Google Scholar] [CrossRef]
- Alexeeva, E.I.; Dvoryakovskaya, T.M.; Denisova, R.V.; Isaeva, K.B.; Soloshenko, M.A.; Mamutova, A.V.; Mayansky, N.A.; Tkachenko, N.E.; Zubkova, I.V.; Kaluzhnaya, T.A.; et al. Immunization With a Pneumococcal Polysaccharide Vaccine in Children with Juvenile Idiopathic Arthritis Without Systemic Manifestations: A Prospective Study. Curr. Pediatr. 2017, 16, 493–501. [Google Scholar] [CrossRef]
- Alfayadh, N.M.; Gowdie, P.J.; Akikusa, J.D.; Easton, M.L.; Buttery, J.P. Vaccinations Do Not Increase Arthritis Flares in Juvenile Idiopathic Arthritis: A Study of the Relationship between Routine Childhood Vaccinations on the Australian Immunisation Schedule and Arthritis Activity in Children with Juvenile Idiopathic Arthritis. Int. J. Rheumatol. 2020, 2020, 1078914. [Google Scholar] [CrossRef] [PubMed]
| sJIA Features | Results, n = 100 (%) |
|---|---|
| Demography | |
| Sex, female, n (%) | 54 (54) |
| Age at baseline *, years (SD) | 10.0 (4.5) |
| ENT diseases, n (%) | 38 (38) |
| Treatment at baseline | |
| NSAIDs, n (%) | 1 (1) |
| Glucocorticosteroids, n (%) | 30 (30) |
| Methotrexate, n (%) | 34 (34) |
| Cyclosporine A, n (%) | 15 (15) |
| Mofetyl mycophenolate, n (%) | 1 (1) |
| Sulfasalazine, n (%) | 2 (2) |
| Colchicine, n (%) | 4 (4) |
| Biologic treatment, n (%) | 91 (91) |
| Tocilizumab monotherapy | 24 (24) |
| Canakinumab monotherapy | 10 (10) |
| Adalimumab monotherapy | 1 (1) |
| Etanercept monotherapy | 1 (1) |
| Tocilizumab + nbDMARD | 16 (16) |
| Canakinumab + nbDMARD | 9 (9) |
| Adalimumab + nbDMARD | 1 (1) |
| Etanercept + nbDMARD | 1 (1) |
| Tocilizumab + GCs | 7 (7) |
| Canakinumab + GCs | 4 (4) |
| Tocilizumab + nbDMARD + GCs | 9 (9) |
| Canakinumab + nbDMARD + GCs | 7 (7) |
| Adalimumab + nbDMARD + GCs | 1 (1) |
| Parameters (n = 100) n (%)/Me (IQR) [Min; Max] | D1 | D22 | EOS | p1 | p2 | p3 | p Total |
|---|---|---|---|---|---|---|---|
| Anti-SP IgG titer, U/mL Me (IQR) [min; max] | 48.3 (18.2; 76.5) [0.8–299] | 103.5 (47.3; 185.4) [5.8–285.2] | 105 (48.7; 171.8) [2.03–282.7] | 0.0000001 | 0.0000001 | 0.249 | 0.000001 |
| Patients with protective titer anti-SP IgG, n (%) | 90 (91) | 99 (100) | 98 (99) | 0.008 | 0.027 | 1.0 | Na |
| Patients with a two-fold-increased anti-SP titer, n (%) | - | 48 (48) | 60 (61) | 0.0000001 | 0.0000001 | ||
| Anti-Hib titer, U/mL, Me (IQR) [min; max] | 0.64 (0.3; 3.2) [0.07–101] | 4 (3.5; 4.2) [0.2–35] | 4 (3.8; 4) [0.2–3.5] | 0.0000001 | 0.0000001 | 0.423 | 0.000001 |
| Patients with a protective anti-HIb titer, n (%) | 37 (37) | 92 (93) | 91 (92) | 0.0001 | 0.0001 | 1 | Na |
| Patients with a two-fold-increased anti-HIb titer, n (%) | - | 61 (62) | 78 (79) | 0.0000001 | 0.0000001 | ||
| S-100 U/mL (n.v. > 2.9), Me (IQR) [min; max] | 3.1 (1.9; 6.9) [0.4–114] | 3.2 (2.0; 5.6) [0.4–108] | 0.83 (0.5; 1.6) [0.01–114] | 0.062 | 0.0000001 | 0.0000001 | 0.000001 |
| hs-CRP, U/mL (n.v. > 8.2), Me (IQR) [min; max] | 0.5 (0.2; 1.8) [0.0–40.4] | 0.5 (0.2; 1.8) [0.01–102] | 0.8 (0.3; 2.1) [0.04–102] | 0.291 | 0.660 | 0.369 | 0.087 |
| Patients with systemic features, n (%) | 12/99 (12.1) | 4/99 (4.0) | 2/99 (2.0) | 0.014 | 0.017 | 0.001 | na |
| Patients in remission, n (%) | 70/99 (70.7) | 79/99 (79.8) | 84/97 (86.6) | 0.039 | 0.239 | 0.008 | na |
| Active joints Me (IQR) [min; max] | 0.0 (0.0; 0.0) [0.0–13.0] | 0.0 (0.0; 0.0) [0.0–13.0] | 0.0 (0.0; 0.0) [0.0–8.0] | 0.179 | 0.026 | 0.066 | 0.029 |
| Joints with limited ROM Me (IQR) [min; max] | 0.0 (0.0; 0.0) [0.0–31.0] | 0.0 (0.0; 0.0) [0.0–31.0] | 0.0 (0.0; 0.0) [0.0–31.0] | - | 0.068 | 0.084 | 0.116 |
| Morning stiffness min Me (IQR) [min; max] | 0.0 (0.0; 0.0) [0.0–180.0] | 0.0 (0.0; 0.0) [0.0–180.0] | 0.0 (0.0; 0.0) [0.0–30.0] | 0.108 | 0.012 | 0.024 | 0.048 |
| MDVAS, mm Me (IQR) [min; max] | 0.0 (0.0; 0.0) [0.0–68.0] | 0.0 (0.0; 0.0) [0.0–68.0] | 0.0 (0.0; 0.0) [0.0–55.0] | 0.043 | 0.009 | 0.017 | 0.017 |
| Patient/parent VAS mm, Me (IQR) [min; max] | 0.0 (0.0; 0.0) [0.0–77.0] | 0.0 (0.0; 0.0) [0.0–77.0] | 0.0 (0.0; 0.0) [0.0–60.0] | 0.043 | 0.005 | 0.01 | 0.004 |
| CHAQ, points Me (IQR) [min; max] | 0.0 (0.0; 0.0) [0.0–1.25] | 0.0 (0.0; 0.0) [0.0–1.25] | 0.0 (0.0; 0.0) [0.0–2.0] | 0.109 | 0.075 | 0.234 | 0.035 |
| ESR, mm/hr (n.v = 0–20) Me (IQR) [min; max] | 2.0 (2.0; 6.5) [0.0–35.0] | 2.0 (2.0; 5.0) [0.0–32.0] | 2.0 (2.0; 5.0) [0.0–30.0] | 0.006 | 0.005 | 0.392 | 0.017 |
| CRP, mg/mL (n.v. < 5) Me (IQR) [min; max] | 1.0 (0.8; 1.1) [0.0–242.0] | 1.0 (0.5; 1.0) [0.11–287.4] | 1.0 (0.2; 1.2) [0.0–102.0] | 0.136 | 0.005 | 0.033 | 0.001 |
| JADAS71 points Me (IQR) [min; max] | 0.0 (0.0; 0.0) [0.0–30.0] | 0.0 (0.0; 0.0) [0.0–28.0] | 0.0 (0.0; 0.0) [0.0–16.0] | 0.008 | 0.007 | 0.026 | 0.003 |
| JADAS71-CRP points Me (IQR) [min; max] | 0.0 (0.0; 0.0) [0.0–38.0] | 0.0 (0.0; 0.0) [0.0–28.0] | 0.0 (0.0; 0.0) [0.0–16.0] | 0.414 | 0.012 | 0.010 | 0.016 |
| sJADAS71 | 0.00 (0.0; 0.0) [0.0–38.0] | 0.00 (0.0; 0.0) [0.0–30.0] | 0.0 (0.0; 0.0) [0.0–16.0] | 0.647 | 0.034 | 0.01 | 0.020 |
| Predictor | β | SE | p-Value |
|---|---|---|---|
| Protective titer anti-SP IgG titer on D22 | |||
| Combined treatment * vs. bDMARD monotherapy | 0.002 | 0.038 | 0.955 |
| Protective titer anti-SP IgG titer at the EOS | |||
| Combined treatment * vs. bDMARD monotherapy | −0.05 | 0.037 | 0.173 |
| Protective titer anti-HIb IgG titer on D22 | |||
| Combined treatment * vs. bDMARD monotherapy | 0.004 | 0.05 | 0.936 |
| Protective titer anti-HIb IgG titer at the EOS | |||
| Combined treatment * vs. bDMARD monotherapy | −0.07 | 0.07 | 0.286 |
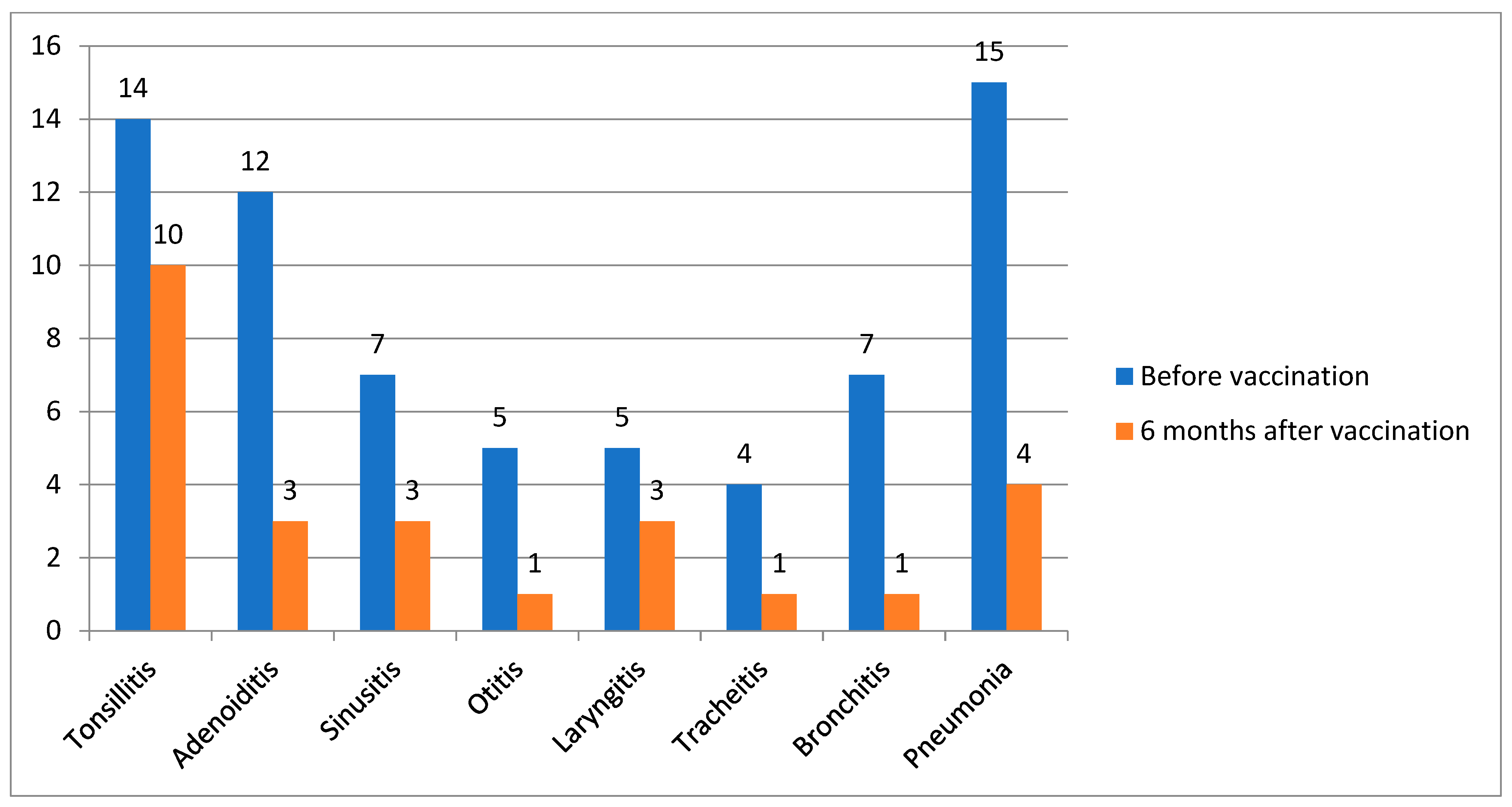
| Infection Burden Indicators, Me (IQR) [Min; Max] | 6 Months Prior to Vaccination | 6 Months After Vaccination | p-Value |
|---|---|---|---|
| Duration of ARI episode, days | 10.0 (7.5; 12.0) | 2.0 (1.0; 4.5) | 0.0000001 |
| The number of ARI episodes per patient | 4.0 (3.0; 5.0) | 2.0 (1.0; 2.0) | 0.0000001 |
| The number of courses of antibacterial drugs | 3.0 (2.0; 3.0) | 1.0 (0; 1.0) | 0.0000001 |
| Patients with ARI, n (%) | 99 (100) | 91 (92) | 0.014 |
| Patients require antibacterial drugs, n (%) | 99 (100) | 67 (68) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexeeva, E.; Dvoryakovskaya, T.; Kudlay, D.; Fetisova, A.; Kriulin, I.; Krekhova, E.; Kabanova, A.; Labinov, V.; Labinova, E.; Kostik, M. Safety and Efficacy of Simultaneous Vaccination with Polysaccharide Conjugate Vaccines Against Pneumococcal (13-Valent Vaccine) and Haemophilus Type B Infections in Children with Systemic Juvenile Idiopathic Arthritis: Prospective Cohort Study. Vaccines 2025, 13, 644. https://doi.org/10.3390/vaccines13060644
Alexeeva E, Dvoryakovskaya T, Kudlay D, Fetisova A, Kriulin I, Krekhova E, Kabanova A, Labinov V, Labinova E, Kostik M. Safety and Efficacy of Simultaneous Vaccination with Polysaccharide Conjugate Vaccines Against Pneumococcal (13-Valent Vaccine) and Haemophilus Type B Infections in Children with Systemic Juvenile Idiopathic Arthritis: Prospective Cohort Study. Vaccines. 2025; 13(6):644. https://doi.org/10.3390/vaccines13060644
Chicago/Turabian StyleAlexeeva, Ekaterina, Tatyana Dvoryakovskaya, Dmitry Kudlay, Anna Fetisova, Ivan Kriulin, Elizaveta Krekhova, Anna Kabanova, Vladimir Labinov, Elizaveta Labinova, and Mikhail Kostik. 2025. "Safety and Efficacy of Simultaneous Vaccination with Polysaccharide Conjugate Vaccines Against Pneumococcal (13-Valent Vaccine) and Haemophilus Type B Infections in Children with Systemic Juvenile Idiopathic Arthritis: Prospective Cohort Study" Vaccines 13, no. 6: 644. https://doi.org/10.3390/vaccines13060644
APA StyleAlexeeva, E., Dvoryakovskaya, T., Kudlay, D., Fetisova, A., Kriulin, I., Krekhova, E., Kabanova, A., Labinov, V., Labinova, E., & Kostik, M. (2025). Safety and Efficacy of Simultaneous Vaccination with Polysaccharide Conjugate Vaccines Against Pneumococcal (13-Valent Vaccine) and Haemophilus Type B Infections in Children with Systemic Juvenile Idiopathic Arthritis: Prospective Cohort Study. Vaccines, 13(6), 644. https://doi.org/10.3390/vaccines13060644







