A Promising Attenuated Rhabdovirus Vaccine Candidate Conferring Dual-Route Protection Against MSRV Disease in Largemouth Bass (Micropterus salmoides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish, Cells and Virus
2.2. Screening of the Attenuated Rhabdovirus Vaccine Candidate
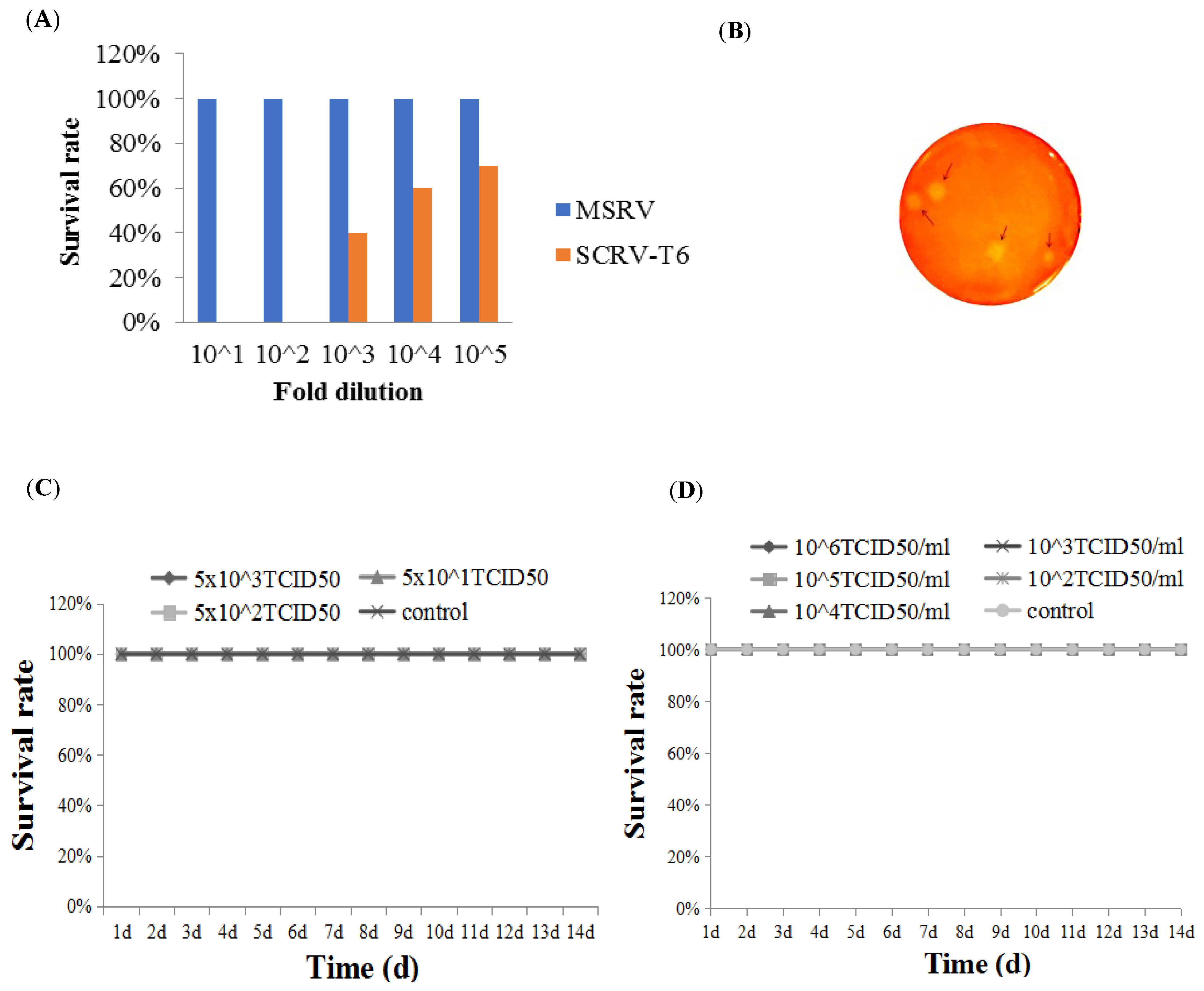
2.2.1. The Attenuated Strain Determination
2.2.2. Purification of Attenuated Rhabdovirus Isolate by Plaque Assay
2.2.3. Virulence Assessment of Clonal Strain by IP Injection and Immersion
2.3. Biological Characteristics of the Clonal MSRV-0509 Strain
2.3.1. Optimization of Inoculation Dose
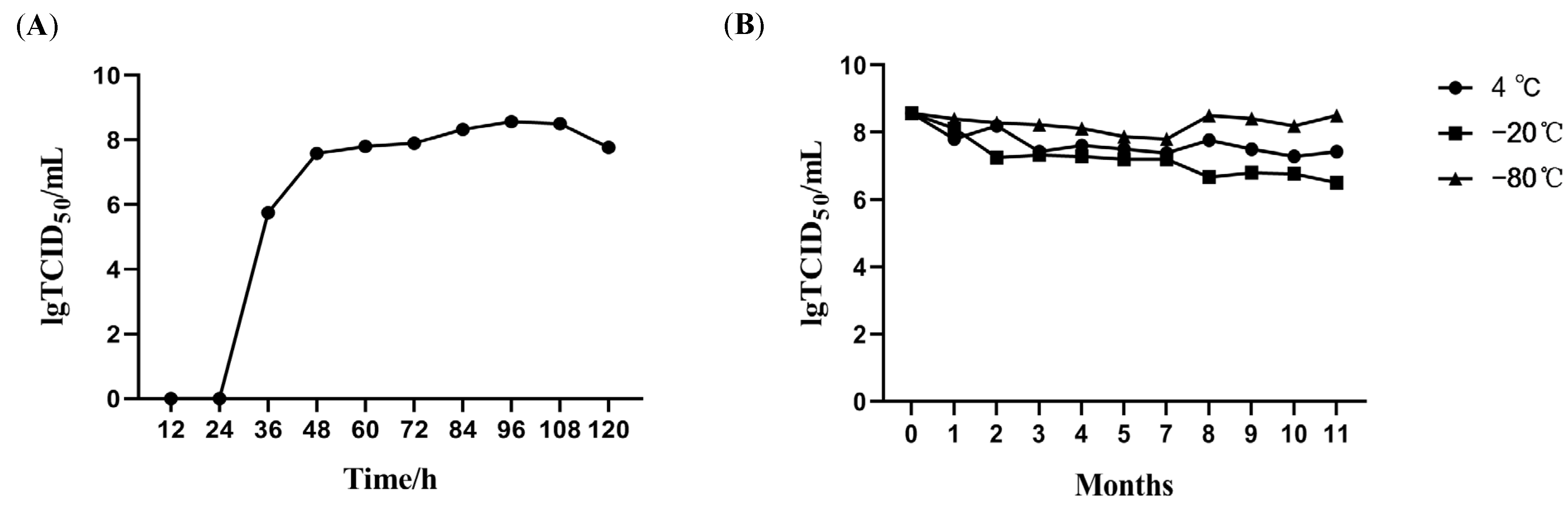
2.3.2. Growth Kinetics Analysis in CPB Cells
2.3.3. Physicochemical Characterization
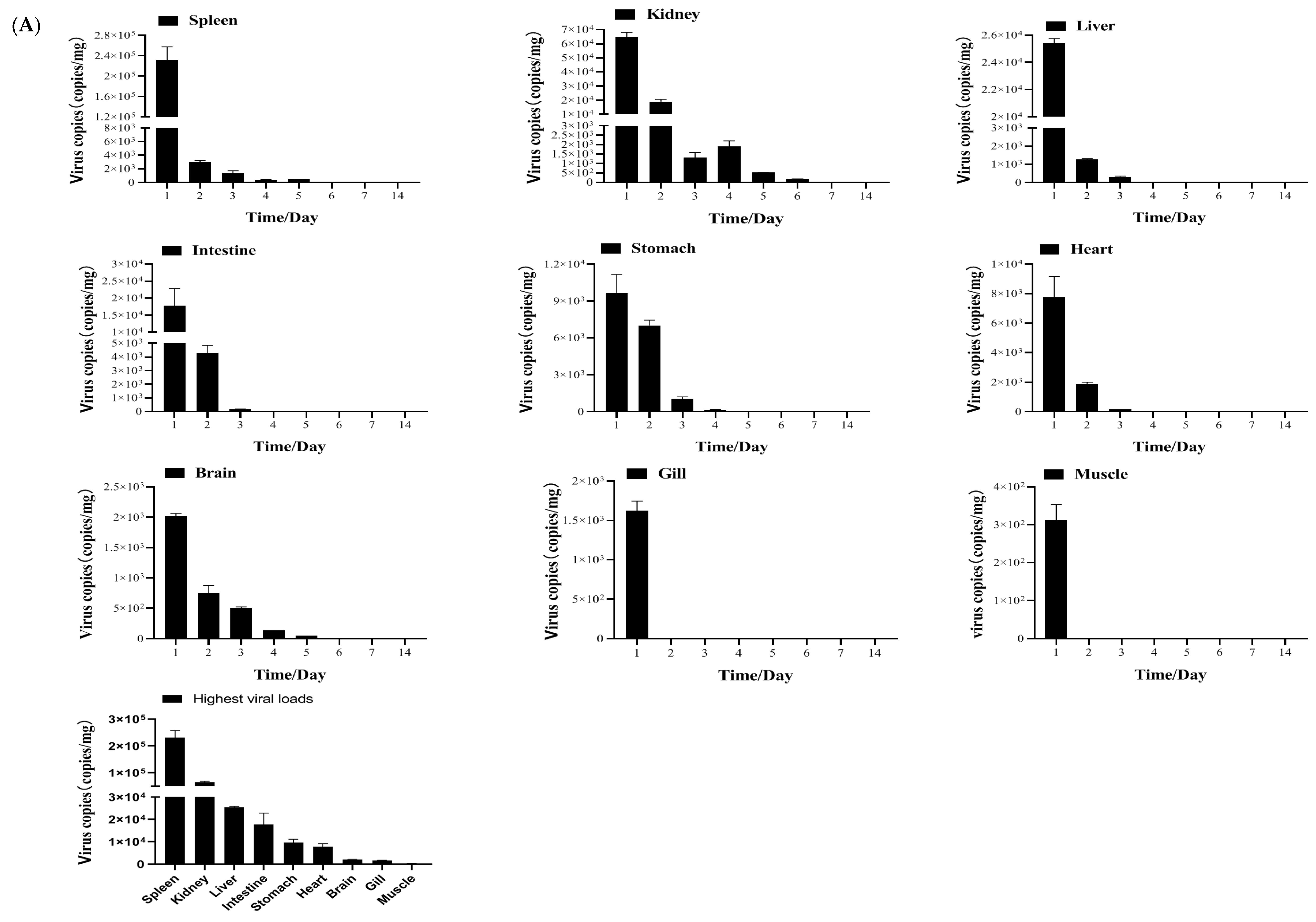
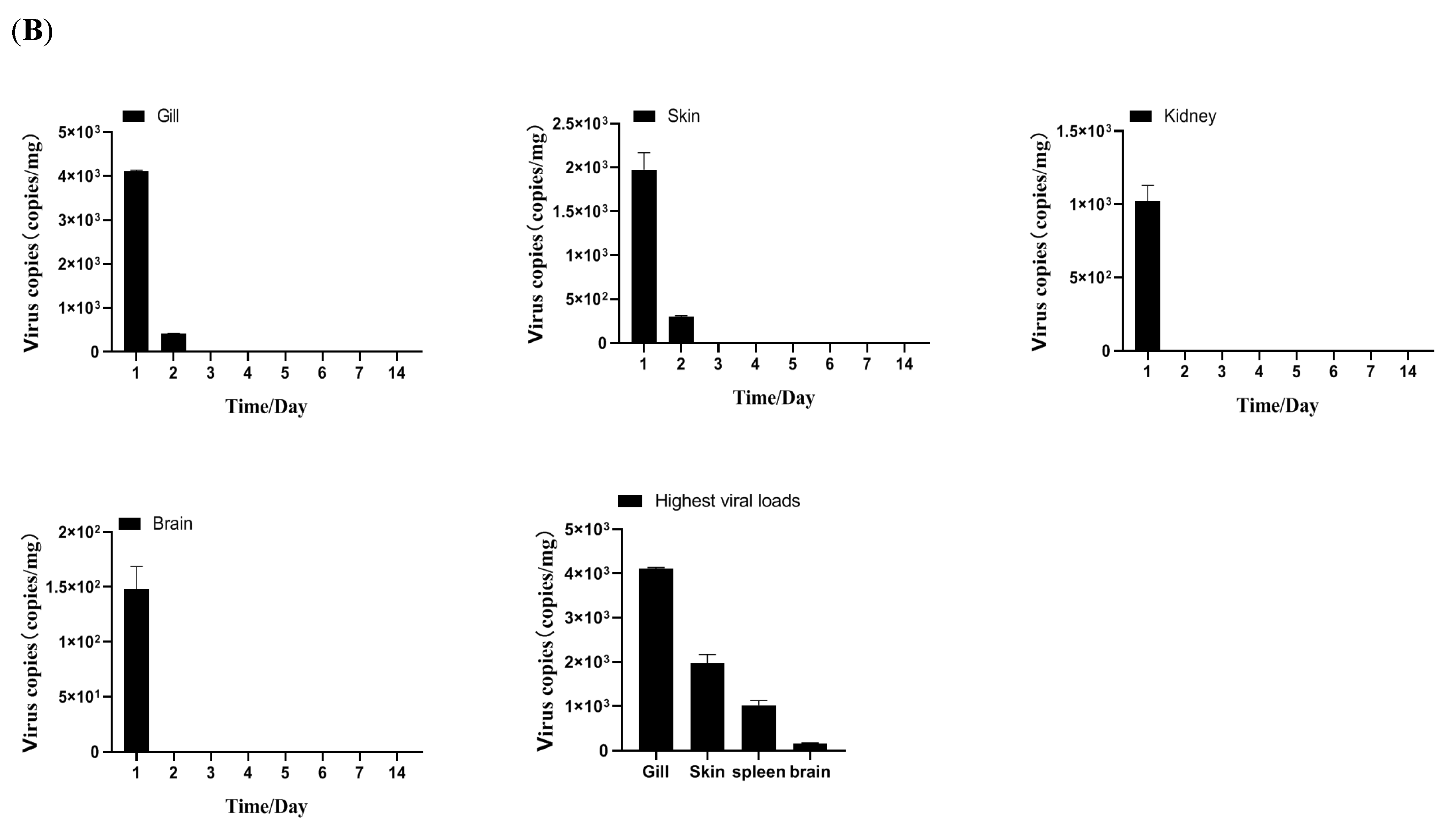
2.3.4. Tissue Distribution and Kinetics of MSRV-0509 in Largemouth Bass via Intraperitoneal (IP) Injection and Immersion
2.4. Evaluation of Protective Efficacy of Attenuated Vaccine Candidate
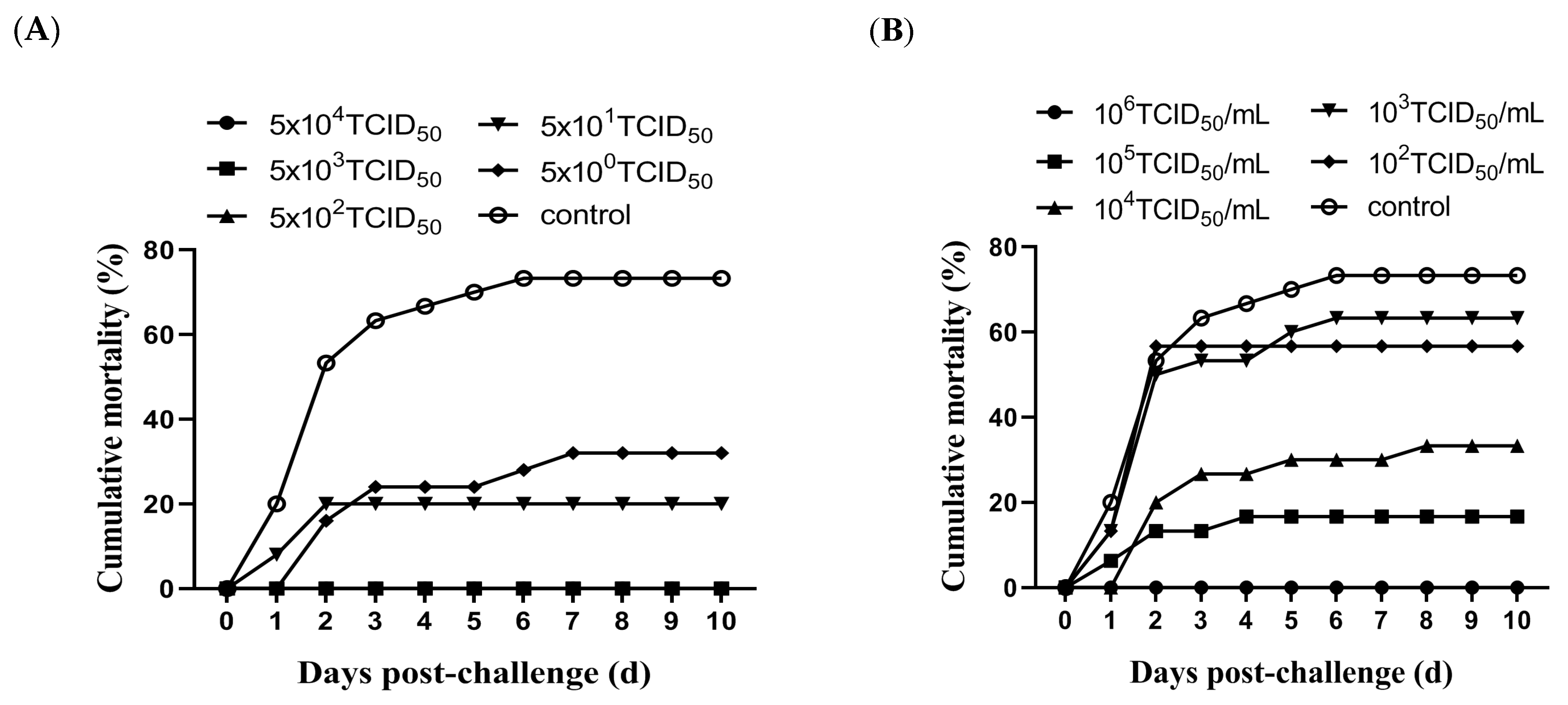
2.4.1. IP Injection Immunization with Different Doses
2.4.2. Immersion Immunization with Different Doses
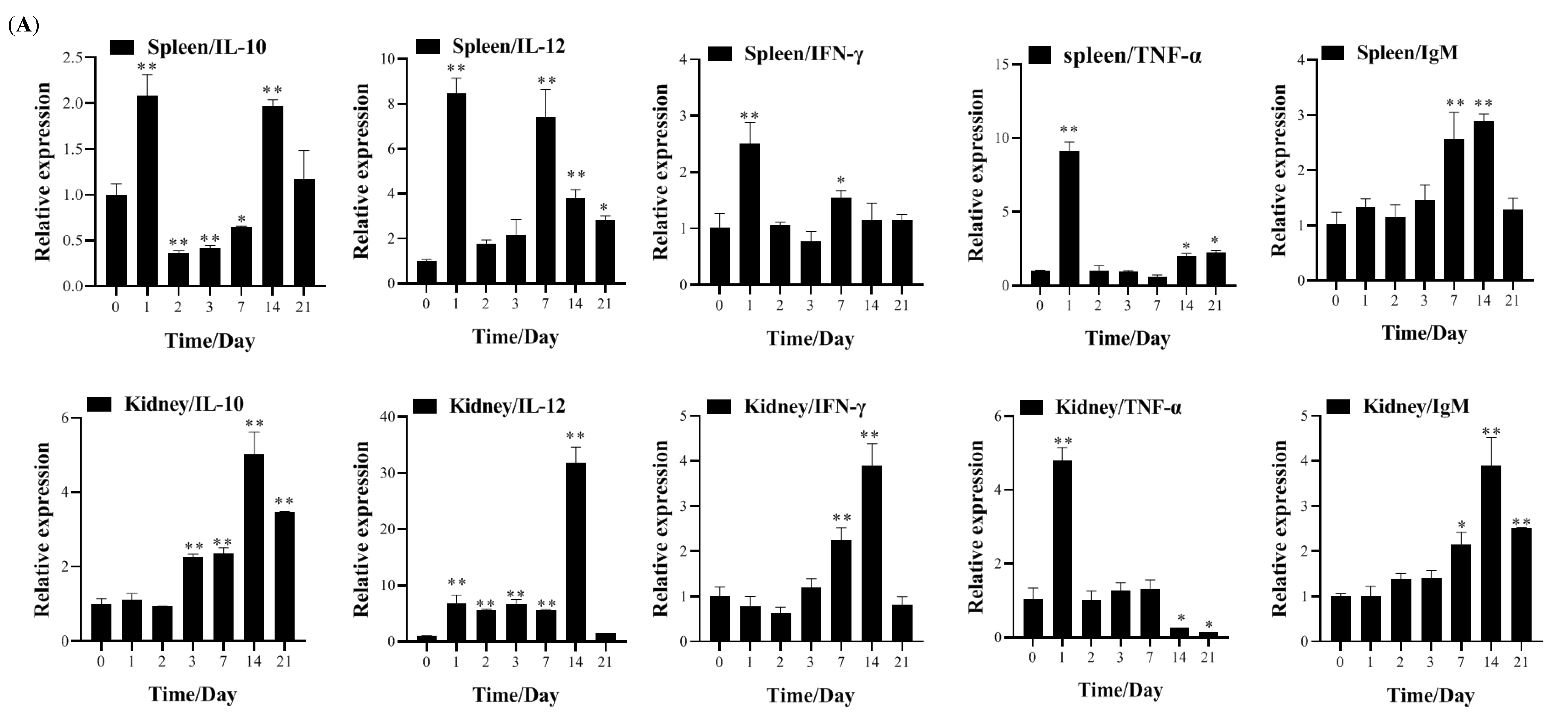
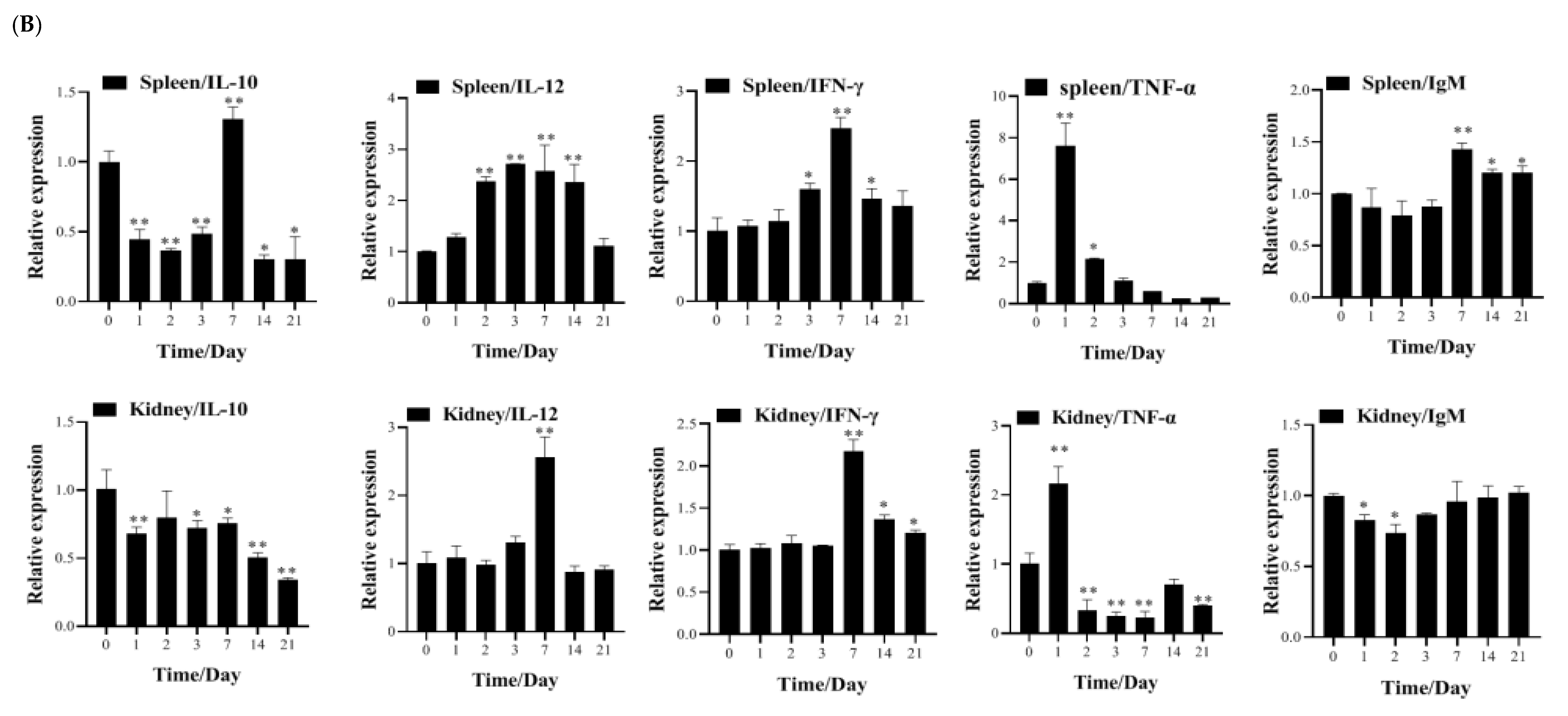
2.4.3. Transcriptional Analysis of Immune-Related Genes Post-Vaccination
2.4.4. Serum Neutralization Assay
2.5. Safety Test of the Attenuated Vaccine Candidate MSRV-0509
2.5.1. Single Overdose Immunization
2.5.2. Reversion to Virulence Test
2.6. Statistical Analysis
3. Results
3.1. Determination of the Attenuated Rhabdovirus Strain
3.2. Biological Characteristics of Clonal MSRV-0509 Strain
3.3. Evaluation of Protective Efficacy of Attenuated Vaccine Candidate MSRV-0509
3.4. Transcription of Immune-Related Genes and Serum Neutralization in Largemouth Bass Post-Vaccination
3.5. The Safety Evaluation of Single Overdose Vaccination and Virulence Reversion
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ministry of Agriculture and Rural Affairs of China. China Fishery Statistical Yearbook—2024; China Agriculture Press: Beijing, China, 2024.
- Fu, X.; Lin, Q.; Liang, H.; Liu, L.; Huang, Z.; Li, N.; Su, J. The biological features and genetic diversity of novel fish rhabdovirus isolates in China. Arch. Virol. 2017, 162, 2829–2834. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Li, S.; Xie, J.; Bai, J.; Chen, K.; Ma, D.; Jiang, X.; Lao, H.; Yu, L. Characterization of a ranavirus isolated from cultured largemouth bass (Micropterus salmoides) in China. Aquaculture 2011, 312, 198–204. [Google Scholar] [CrossRef]
- Fu, X.; Luo, M.; Zheng, G.; Liang, H.; Liu, L.; Lin, Q.; Niu, Y.; Luo, X.; Li, N. Determination and characterization of a novel birnavirus associated with massive mortality in largemouth bass. Microbiol. Spectr. 2022, 10, e0171621. [Google Scholar] [CrossRef]
- Saito, H.; Okamura, T.; Shibata, T.; Kato, G.; Sano, M. Development of a live attenuated vaccine candidate against herpesviral hematopoietic necrosis of goldfish. Aquaculture 2022, 552, 737974. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; An, N.; Li, D.C.; Huang, M.M.; Fei, H. Updates on infectious diseases of largemouth bass: A major review. Fish Shellfish Immunol. 2024, 154, 109976. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.J.; Freitas-Astua, J.; Bejerman, N.; Blasdell, K.R.; Breyta, R.; Dietzgen, R.G.; Fooks, A.R.; Kondo, H.; Kurath, G.; Kuzmin, I.V.; et al. ICTV virus taxonomy profile: Rhabdoviridae 2022. J. Gen. Virol. 2022, 103, 001689. [Google Scholar] [CrossRef]
- Jose Priya, T.A.; Kappalli, S. Modern biotechnological strategies for vaccine development in aquaculture—Prospects and challenges. Vaccine 2022, 40, 5873–5881. [Google Scholar] [CrossRef]
- Mondal, H.; Thomas, J. A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquac. Int. 2022, 30, 1971–2000. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, W.; Xu, Z. Current use and development of fish vaccines in China. Fish Shellfish Immunol. 2020, 96, 223–234. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef]
- Su, H.; Su, J. Cyprinid viral diseases and vaccine development. Fish Shellfish Immunol. 2018, 83, 84–95. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Klesius, P.H.; Drennan, J.D.; Evans, J.J. Efficacy of a modified live Flavobacterium columnare vaccine in fish. Fish Shellfish Immunol. 2011, 30, 304–308. [Google Scholar] [CrossRef]
- Fu, X.; Li, N.; Lai, Y.; Luo, X.; Wang, Y.; Shi, C.; Huang, Z.; Wu, S.; Su, J. A novel fish cell line derived from the brain of Chinese perch Siniperca chuatsi: Development and characterization. J. Fish Biol. 2015, 86, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, N.; Lin, Q.; Liu, L.; Liang, H.; Huang, Z.; Fu, X. An avirulent Micropterus salmoides rhabdovirus vaccine candidate protects Chinese perch against rhabdovirus infection. Fish Shellfish Immunol. 2018, 77, 474–480. [Google Scholar]
- Gui, L.; Li, Z.; Zhang, Q. Isolation and charaterization of a rhabdovirus from diseased flounder Paralichthys olivaceus. Acta Hydrobiol. Sin. 2007, 31, 345–353. [Google Scholar] [CrossRef]
- Munang’Andu, H.M.; Evensen, O. Correlates of protective immunity for fish vaccines. Fish Shellfish Immunol. 2019, 85, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, J.; Teng, Z.; Ren, M.; Dong, H.; Zhang, Y.; Ru, J.; Du, P.; Sun, S.; Guo, H. Four simple biomimetic mineralization methods to improve the thermostability and immunogenicity of virus-like particles as a vaccine against foot-and-mouth disease. Vaccines 2021, 9, 891. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, Z.; Zhang, C.; Jia, Y.; Qiu, D.; Zhu, B.; Wang, G. Carbon nanotubes-loaded subunit vaccine can increase protective immunity against rhabdovirus infections of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2020, 99, 548–554. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Zhang, G.; Zhou, G.; Yang, F.; Wang, E.; Liu, T.; Wang, G. High immune efficiency of bacterial nanocellulose loaded MSRV g protein vaccine for bath immunization. Aquaculture 2022, 560, 738579. [Google Scholar] [CrossRef]
- Yang, M.; Liang, J.; Luo, S.; Zhang, S.; Zhou, Q.; Lu, J.; Chen, J. Oral vaccination with recombinant saccharomyces cerevisiae expressing Micropterus salmoides rhabdovirus g protein elicits protective immunity in largemouth bass. Fish Shellfish Immunol. 2024, 145, 109364. [Google Scholar] [CrossRef]
- Ma, R.; Chen, W.; Guo, Z.; Jia, Y.; Zhu, B.; Wang, E.; Wang, G. Screening the potential part of the g protein antigen is an achievable strategy to improve the immune effect of DNA vaccine against MSRV infection. Fish Shellfish Immunol. 2022, 131, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, L.; Sun, Y.; Li, W.; Wang, R.; Sun, X.; Zhu, G.; Ma, S. Effects of seasonal changes on T-helper 1/T-helper 2 immune balance and eczema onset in rats. J. Tradit. Chin. Med. Sci. 2023, 10, 64–72. [Google Scholar] [CrossRef]
- Cousins, D.J.; Lee, T.H.; Staynov, D.Z. Cytokine coexpression during human Th1/Th2 cell differentiation: Direct evidence for coordinated expression of Th2 cytokines. J. Immunol. 2002, 169, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, A.; Qiu, X.; Yang, K.; Zhou, H. The IL-12 family cytokines in fish: Molecular structure, expression profile and function. Dev. Comp. Immunol. 2023, 141, 104643. [Google Scholar] [CrossRef]
| Primer Name | Sequence (5′-3′) |
|---|---|
| TNF-α-F | ACTTCGTCTACAGCCAGGCA |
| TNF-α-R | AGTAACGCGAGACCCTGTGG |
| IFN-γ-F | CGCCTCCATCAGCACCGACA |
| IFN-γ-R | CGGCAGCTCCCACAATGCTT |
| IL-10-F | ACAACCAGTGCTGCCGTT |
| IL-10-R | GCAGCGCTGTGTCTAAGTCA |
| IL-12-F | TCTTCCATCCTTGTGGTCTTCC |
| IL-12-R | CAGTTCCAGGTCAAAGTGGTC |
| IgM-F | ATTGTCAGGTCCATCGGGC |
| IgM-R | TACCGAATCACCTCGAGAGGGA |
| 18S-F | CATTCGTATTGTGCCGCTAGA |
| 18S-R | CAAATGCTTTCGCTTTGGTC |
| Numbers | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Titer (TCID50/mL) | 108.52 | 108.50 | 108.39 | 108.50 | 108.59 | 108.57 | 108.36 | 108.20 | 108.20 | 108.00 |
| MOI | 1.0 | 0.5 | 0.1 | 0.01 | 0.001 | 0.0001 |
|---|---|---|---|---|---|---|
| lgTCID50/mL | 6.410 | 7.000 | 7.289 | 7.800 | 8.000 | 8.386 |
| Treatment Conditions | lgTCID50/mL | |
|---|---|---|
| Temperature (°C) | 28 | 8.667 |
| 34 | 8.410 | |
| 40 | 8.289 | |
| 46 | 7.750 | |
| 52 | 4.000 | |
| 56 | 3.574 | |
| 60 | 0 | |
| Chloroform | Chloroform | 0 |
| Control | 8.0 | |
| pH | 3.0 | 3.563 |
| 5.0 | 7.800 | |
| 9.0 | 8.289 | |
| Control | 8.500 |
| Group | Serum Dilution Ratio | Negative Control | Positive Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1:2 | 1:4 | 1:8 | 1:16 | 1:32 | 1:64 | 1:128 | 1:256 | 1:512 | 1:1024 | |||
| IP injection Immersion | − | − | − | − | − | − | + | + | + | + | − | + |
| − | − | − | + | + | + | + | + | + | + | − | + | |
| Group | Vaccination Route | Dose (TCID50) | Fish Number/Group | Number of Deaths | Mortality (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| First Batch | Second Batch | Third Batch | First Batch | Second Batch | Third Batch | ||||
| Vaccine | IP injection | 5 × 103/0.1 mL/fish | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| Control | PBS/0.1 mL/fish | 30 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vaccine | Immersion | 107, for 1 h | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| Control | PBS, for 1 h | 30 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Passage | Ct Value | Viral Load (Copies/mg) |
|---|---|---|
| 1 | 33.841 | 511.8259 |
| 2 | 34.007 | 459.273 |
| 3 | ND | 0 |
| 4 | ND | 0 |
| 5 | ND | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Li, W.; Kong, M.; Liang, H.; Lin, Q.; Niu, Y.; Luo, X.; Ma, B.; Zhou, J.; Li, N. A Promising Attenuated Rhabdovirus Vaccine Candidate Conferring Dual-Route Protection Against MSRV Disease in Largemouth Bass (Micropterus salmoides). Vaccines 2025, 13, 645. https://doi.org/10.3390/vaccines13060645
Fu X, Li W, Kong M, Liang H, Lin Q, Niu Y, Luo X, Ma B, Zhou J, Li N. A Promising Attenuated Rhabdovirus Vaccine Candidate Conferring Dual-Route Protection Against MSRV Disease in Largemouth Bass (Micropterus salmoides). Vaccines. 2025; 13(6):645. https://doi.org/10.3390/vaccines13060645
Chicago/Turabian StyleFu, Xiaozhe, Wenxian Li, Minghui Kong, Hongru Liang, Qiang Lin, Yinjie Niu, Xia Luo, Baofu Ma, Jin Zhou, and Ningqiu Li. 2025. "A Promising Attenuated Rhabdovirus Vaccine Candidate Conferring Dual-Route Protection Against MSRV Disease in Largemouth Bass (Micropterus salmoides)" Vaccines 13, no. 6: 645. https://doi.org/10.3390/vaccines13060645
APA StyleFu, X., Li, W., Kong, M., Liang, H., Lin, Q., Niu, Y., Luo, X., Ma, B., Zhou, J., & Li, N. (2025). A Promising Attenuated Rhabdovirus Vaccine Candidate Conferring Dual-Route Protection Against MSRV Disease in Largemouth Bass (Micropterus salmoides). Vaccines, 13(6), 645. https://doi.org/10.3390/vaccines13060645







