Evolving SARS-CoV-2 Vaccines: From Current Solutions to Broad-Spectrum Protection
Abstract
1. Introduction
2. Immunogenic Features of SARS-CoV-2 Structural Proteins
2.1. Full-Length S Protein
2.2. RBD
2.3. NTD
2.4. S2 Subunit
2.5. Other Proteins
3. Development of SARS-CoV-2 Vaccines
3.1. Inactivated Vaccines and Live-Attenuated Vaccines
| No. | Vaccine Platform | Vaccine Name | Detailed Information | Immunization Route | Developers | Article |
|---|---|---|---|---|---|---|
| 1 | Inactivated vaccines | CoronaVac | Chemically inactivated SARS-CoV-2 and aluminum hydroxide as an adjuvant | IM | Sinovac Biotech | [52] |
| 2 | BBIBP-CorV | Inactivated SARS-CoV-2 against pre-Omicron strains | IM | Sinopharm BIBP | [53] | |
| 3 | Covaxin | Whole-virion inactivated SARS-CoV-2 vaccine with TLR7/8 agonist adsorbed to alum | IM | Bharat Biotech-ICMR-NIV | [54] | |
| 4 | QazCovid-in | Kazakhstan SARS-CoV-2 isolated, inactivated with formaldehyde, adjuvanted with alum | IM | RIBSP | [55] | |
| 5 | VLA2001 | β-Propiolactone inactivates virus with CpG 1018 and aluminum hydroxide | IM | Valneva SE | [56] | |
| 6 | WIV04 | Chemically inactivated SARS-CoV-2 WIV04 with aluminum hydroxide adjuvant | IM | WIBP | [57] | |
| 7 | KCONVAC | 19nCoV-CDC-Tan-Strain, chemically inactivated, with alum adjuvant | IM | Kangtai & Minhai, China | [58] | |
| 8 | BIV1-CovIran | Chemically inactivated SARS-CoV-2 and aluminum hydroxide as an adjuvant | IM | Pasteur Institute of Iran | [59] | |
| 9 | Live-attenuated vaccine | COVI-VAC | Mimic infection, stimulate immunity, codon deoptimization, enhanced safety | IN | Codagenix | [60] |
| 10 | ∆3678 | Deleted ORF 3, 6, 7, 8; ∆3678 replicates 7500-fold lower than wild-type in airway cultures | IN | DBMB | [61] | |
| 11 | dCoV | WA/1 strain with sub-optimal codons and deleted furin sites | IN/IM | SIIPL | [62] | |
| 12 | QazCOVID-Live | Attenuated SARS-CoV-2 via Vero cell passages | IN | RIBSP | [63] | |
| 13 | Protein-based vaccine | SCTV01E | Tetravalent vaccine with Alpha, Beta, Delta, Omicron BA.1 S-ECD, plus SCT-VA02B | IM | Sinocelltech | [64] |
| 14 | NVSI-06-07 | Trimeric RBDs from SARS-CoV-2; NVSI-06-07 boosts nAb response | IM | National Vaccine &Serum Institute, NVSI | [65] | |
| 15 | NVSI-06-09 | Trimeric RBD integrates Omicron and variant mutations into a mosaic vaccine | ||||
| 16 | Nuvaxovid (NVX-CoV2373) | Recombinant nanoparticle vaccine with S protein and Matrix-M adjuvant | IM | Novavax | [66] | |
| 17 | EpiVacCorona | SARS-CoV-2 S protein conjugated to carrier protein, adsorbed on aluminum hydroxide | IM | FSRCVB | [67] | |
| 18 | Zifivax (ZF2001) | Dimeric RBD with alum adjuvant, 3-dose regimen, robust T cell responses | IM | CAS Microbiology | [68] | |
| 19 | MVC-COV1901 | Utilize CHO cells and contain CpG 1018 and aluminum hydroxide as adjuvants | IM | MVC | [69] | |
| 20 | Corbevax | Pichia pastoris encodes SARS-CoV-2 RBD, adjuvanted with alum and CpG 1018 | IM | Baylor Vaccine Center& Biological E. Limited | [70] | |
| 21 | CIGB-66 (Abdala) | Pichia pastoris yeast platform encodes SARS-CoV-2 RBD, adjuvanted with alum adjuvant | IM | CIGB | [71] | |
| 22 | VidPrevtyn Beta | Bivalent vaccine (D614, Beta B.1.351) with GSK AS03 adjuvant | IM | Sanofi &GSK | [72] | |
| 23 | R-CNP | Nanoparticles with cholera toxin B subunit displaying SARS-CoV-2 RBD, alveoli delivery | IN | Clover Biopharmaceuticals | [73] | |
| 24 | SCTV01E-2 | Recombinant S-ECD protein from Beta, Omicron BA.1, BQ.1.1, XBB.1 | IM | Sinocelltech | [74] | |
| 25 | HR1LS | Target HR1, CH, SH regions, neutralize multiple coronaviruses in vitro | IM | Wang X, et al. | [39] | |
| 26 | Virus-like particle vaccine | Covifenz | Used plant-derived VLPs, demonstrated efficacy against the Delta and Gamma | IM | Medicago | [75] |
| 27 | DVLP | DC-targeting VLP with engineered Sindbis glycoprotein, packaging SARS-CoV-2 Spike mRNA | IM | SJTU | [76] | |
| 28 | DNA-based vaccine | INO-4800 | SARS-CoV-2 S-protein delivered intradermally via CELLECTRA® EP system | ID | Inovio Pharmaceuticals | [77] |
| 29 | ZyCoV-D | First COVID-19 DNA vaccine (spike gene) induces immunity | ID | Cadila Healthcare | [78] | |
| 30 | GX-19 | Encodes SARS-CoV-2 S1 and S2, using vaccine vector pGX27 | IM | Genexine | [79] | |
| 31 | GX-19N | Induces broad T cell responses, potentially cross-reactive | IM | Genexine | [80] | |
| 32 | RNA-based vaccine | BNT162b2 | Encodes full-length spike (prefusion conformation), robust T cell responses | IM | Pfizer/BioNTech | [81] |
| 33 | BNT162b2 BA.1 bivalent booster | Bivalent mRNA encoding the original Wuhan-Hu-1 strain and BA.1 | IM | Pfizer/BioNTech | [82] | |
| 34 | BNT162b2 BA.4/5 bivalent booster | Bivalent mRNA encoding the original Wuhan-Hu-1 strain and BA.4/5 | IM | Pfizer/BioNTech | [83] | |
| 35 | BNT162b2 Monovalent XBB.1.5 | XBB.1.5-specific changes in S spike protein, based on original BNT162b2 | IM | Pfizer/BioNTech | [84] | |
| 36 | Spikevax (mRNA-1273) | LNP-encapsulated mRNA vaccine encoding prefusion-stabilized SARS-CoV-2 S protein | IM | Moderna and NIAID VRC | [85] | |
| 37 | mRNA-1273.214 | Encoding ancestral Wuhan-Hu-1 and Omicron BA.1 spike mRNAs | IM | Moderna | [86] | |
| 38 | mRNA-1273.222 | Encoding ancestral SARS-CoV-2 and BA.4/5 spike proteins | [87] | |||
| 39 | mRNA-1273.815 | Encoding ancestral SARS-CoV-2 and XBB.1.5 spike | ||||
| 40 | RQ3013 | Pseudouridine-modified mRNAs in LNP encode S protein with B.1.1.7/B.1.351 mutations | IM | Shanghai Lanque Fudan University | [88] | |
| 41 | RQ3033 | Targets XBB.1.5, designed to prevent COVID-19 caused by XBB and EG.5 | IM | [89] | ||
| 42 | CVnCoV | Sequence-engineered mRNA encoding SARS-CoV-2 S protein, protected by LNP delivery | IM | CureVac | [90] | |
| 43 | CV2CoV | 2nd vaccine with optimized non-coding regions, enhanced antigen expression | IM | [91] | ||
| 44 | SYS6006 | Encodes S protein with S-2P, induces nAbs against WT, Delta, et al. in mice/NHPs | IM | CSPC Group | [92] | |
| 45 | ARCT-154 | Self-amplifying mRNA with modified S-protein | IM | Arcturus Therapeutics | [93] | |
| 46 | VLPCOV-01 | LNP-encapsulated RNA vaccine expressing membrane-anchored SARS-CoV-2 RBD | IM | Akahata W, et al. | [94] | |
| 47 | Viral vector vaccine | Vaxzevria (AZD1222) | ChAdOx1 vector encoding full-length SARS-CoV-2 spike with S-2P mutation | IM | AstraZeneca-Oxford | [95] |
| 48 | Gam-COVID-Vac | RAd26 and rAd5 vectors carrying SARS-CoV-2 spike glycoprotein gene | IM | GRI | [96] | |
| 49 | Ad5-nCoV | Ad5 vector encoding full-length SARS-CoV-2 spike with S-2P mutation | IM | CanSino Biologics | [97] | |
| 50 | Ad26.COV2. S | Ad26 vector encodes pre-fusion stabilized full-length SARS-CoV-2 spike protein | IM | Johnson & Johnson | [98] | |
| 51 | dNS1-RBD | Cold-adapted H1N1 NS1-deleted strain with inserted SARS-CoV-2 RBD | IN | Beijing Wantai | [99] | |
| 52 | BBV154 (iNCOVACC) | ChAd36 vector encodes pre-fusion stabilized SARS-CoV-2 spike with S-2P | IN/IM | Bharat Biotech | [100] | |
| 53 | AdCOVID | Intranasal Ad5 vectored vaccine encoding SARS-CoV-2 RBD | IN | Altimmune | [101] | |
| 54 | CVXGA1 | Recombinant PIV5 with SARS-CoV-2 spike, cytoplasmic tail replaced by PIV5 F | IN | University of Georgia | [102] | |
| 55 | NDV-HXP-S | Modified spike with six prolines, swapped domains for NDV integration | IN | PATH | [103] | |
| 56 | Patria | Live NDV vector vaccine expressing SARS-CoV-2 spike with S-2P mutation | IN | Avimex | [104] | |
| 57 | MVA-SARS-2-S | MVA vector vaccine expressing full-length SARS-CoV-2 S protein | IM | DZIF | [105] | |
| 58 | MVA-SARS-2-ST | With modified, stabilized SARS-CoV-2 S antigen and inactivated S1/S2 cleavage site | IM | DZIF | [106] | |
| 59 | MV-014-212 | RSV OE4 with SARS-CoV-2 S and RSV F tail | IM | Meissa Vaccines Inc | [107] | |
| 60 | GBP510 (SKYCovione) | Targets RBD of S protein, uses AS03 adjuvant to boost reactogenicity | IM | SKB | [108] |
3.2. Protein Subunit Vaccine
3.3. Virus-like Particle Vaccine
3.4. Nucleic Acid Vaccines
3.5. Vector Vaccines
4. Neutralizing Antibodies Against SARS-CoV-2
4.1. RBD Targeting
4.2. NTD Targeting
4.3. S2 Domain Targeting
5. Potential Strategies to Optimize COVID-19 Vaccines
5.1. Broadly NAbs Drive the Development of Broad-Spectrum Vaccines
5.2. Modification of Vaccine Antigen Composition
5.3. Advancing Long-Lasting Coronavirus Vaccines
5.4. Establishing Mucosal Immunization Protection
5.5. Development of Combination Vaccines for Respiratory Infectious Diseases
5.6. Multivalent Vaccines Expressing Multiple Viral Proteins
5.7. Other Strategies to Optimize Neutralizing Antibodies
6. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.J.; Dong, X.; Cao, Y.Y.; Yuan, Y.D.; Yang, Y.B.; Yan, Y.Q.; Akdis, C.A.; Gao, Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020, 75, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Peng, Y.; Huang, B.; Ding, X.; Wang, X.; Niu, P.; Meng, J.; Zhu, Z.; Zhang, Z.; Wang, J.; et al. Genome Composition and Divergence of the Novel Coronavirus (2019-nCoV) Originating in China. Cell Host Microbe 2020, 27, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Shi, J.; Wang, Y.; Liu, H.; Hu, Y.F.; Hu, B.; Shuai, H.; Yuen, T.T.; Chai, Y.; et al. Lineage-specific pathogenicity, immune evasion, and virological features of SARS-CoV-2 BA.2.86/JN.1 and EG.5.1/HK.3. Nat. Commun. 2024, 15, 8728. [Google Scholar] [CrossRef]
- Yevsieieva, L.V.; Lohachova, K.O.; Kyrychenko, A.; Kovalenko, S.M.; Ivanov, V.V.; Kalugin, O.N. Main and papain-like proteases as prospective targets for pharmacological treatment of coronavirus SARS-CoV-2. RSC Adv. 2023, 13, 35500–35524. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Consortium, C.-G.U.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Wang, R.; Lan, C.; Benlagha, K.; Camara, N.O.S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H.; et al. The interaction of innate immune and adaptive immune system. MedComm 2024, 5, e714. [Google Scholar] [CrossRef]
- Lan, J.; Chen, P.; Liu, W.; Ren, W.; Zhang, L.; Ding, Q.; Zhang, Q.; Wang, X.; Ge, J. Structural insights into the binding of SARS-CoV-2, SARS-CoV, and hCoV-NL63 spike receptor-binding domain to horse ACE2. Structure 2022, 30, 1432–1442 e1434. [Google Scholar] [CrossRef]
- Ren, W.; Zhu, Y.; Lan, J.; Chen, H.; Wang, Y.; Shi, H.; Feng, F.; Chen, D.Y.; Close, B.; Zhao, X.; et al. Susceptibilities of Human ACE2 Genetic Variants in Coronavirus Infection. J. Virol. 2022, 96, e0149221. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Deng, W.; Huang, B.; Gao, H.; Liu, J.; Ren, L.; Wei, Q.; Yu, P.; Xu, Y.; Qi, F.; et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 2020, 583, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, C.; Liu, X.; Chiu, M.C.; Wang, D.; Wei, Y.; Chu, H.; Cai, J.P.; Hau-Yee Chan, I.; Kak-Yuen Wong, K.; et al. Human Intestinal Organoids Recapitulate Enteric Infections of Enterovirus and Coronavirus. Stem Cell Rep. 2021, 16, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chu, H.; Wong, B.H.; Chiu, M.C.; Wang, D.; Li, C.; Liu, X.; Yang, D.; Poon, V.K.; Cai, J.; et al. Activation of C-Type Lectin Receptor and (RIG)-I-Like Receptors Contributes to Proinflammatory Response in Middle East Respiratory Syndrome Coronavirus-Infected Macrophages. J. Infect. Dis. 2020, 221, 647–659. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Lineburg, K.E.; Grant, E.J.; Swaminathan, S.; Chatzileontiadou, D.S.M.; Szeto, C.; Sloane, H.; Panikkar, A.; Raju, J.; Crooks, P.; Rehan, S.; et al. CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity 2021, 54, 1055–1065.e5. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Y.; Jian, F.; Yang, S.; Song, W.; Wang, P.; Yu, L.; Shao, F.; Cao, Y. Enhanced immune evasion of SARS-CoV-2 variants KP.3.1.1 and XEC through N-terminal domain mutations. Lancet Infect. Dis. 2025, 25, e6–e7. [Google Scholar] [CrossRef]
- Wang, Q.; Iketani, S.; Li, Z.; Liu, L.; Guo, Y.; Huang, Y.; Bowen, A.D.; Liu, M.; Wang, M.; Yu, J.; et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell 2023, 186, 279–286.e8. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qiu, T.; Huang, X.; Mao, Q.; Wang, Y.; Qiao, R.; Li, J.; Mao, T.; Wang, Y.; Cun, Y.; et al. Potent and broadly neutralizing antibodies against sarbecoviruses induced by sequential COVID-19 vaccination. Cell Discov. 2024, 10, 14. [Google Scholar] [CrossRef]
- Yu, X.; Wei, D.; Xu, W.; Li, Y.; Li, X.; Zhang, X.; Qu, J.; Yang, Z.; Chen, E. Reduced sensitivity of SARS-CoV-2 Omicron variant to antibody neutralization elicited by booster vaccination. Cell Discov. 2022, 8, 4. [Google Scholar] [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E.; Saliba, C.; Zepeda, S.K.; Culap, K.; Pinto, D.; VanBlargan, L.A.; De Marco, A.; di Iulio, J.; et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef]
- Salvatori, G.; Luberto, L.; Maffei, M.; Aurisicchio, L.; Roscilli, G.; Palombo, F.; Marra, E. SARS-CoV-2 SPIKE PROTEIN: An optimal immunological target for vaccines. J. Transl. Med. 2020, 18, 222. [Google Scholar] [CrossRef]
- Pramanick, I.; Sengupta, N.; Mishra, S.; Pandey, S.; Girish, N.; Das, A.; Dutta, S. Conformational flexibility and structural variability of SARS-CoV2 S protein. Structure 2021, 29, 834–845 e835. [Google Scholar] [CrossRef]
- Walls, A.C.; Park, Y.J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Xia, S.; Lan, Q.; Su, S.; Wang, X.; Xu, W.; Liu, Z.; Zhu, Y.; Wang, Q.; Lu, L.; Jiang, S. The role of furin cleavage site in SARS-CoV-2 spike protein-mediated membrane fusion in the presence or absence of trypsin. Signal Transduct. Target. Ther. 2020, 5, 92. [Google Scholar] [CrossRef]
- Barnes, C.O.; Jette, C.A.; Abernathy, M.E.; Dam, K.A.; Esswein, S.R.; Gristick, H.B.; Malyutin, A.G.; Sharaf, N.G.; Huey-Tubman, K.E.; Lee, Y.E.; et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 2020, 588, 682–687. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Zhou, H.; Zhu, H.; Jiang, S.; Wang, P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 2023, 23, 189–199. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Xu, W.; Deng, W.; Wang, Y.; Wang, M.; Wang, Q.; Hsieh, M.; Dong, J.; Wang, X.; et al. A novel STING agonist-adjuvanted pan-sarbecovirus vaccine elicits potent and durable neutralizing antibody and T cell responses in mice, rabbits and NHPs. Cell Res. 2022, 32, 269–287. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Yang, H.; Tang, C.; Li, Q.; Huang, Q.; Wang, H.; Wang, J.; Zhou, Y.; Yang, Y.; Chen, H.; et al. A protein vaccine of RBD integrated with immune evasion mutation shows broad protection against SARS-CoV-2. Signal Transduct. Target. Ther. 2024, 9, 301. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, G.; Guo, Y.; Zhou, T.; Gorman, J.; Lee, M.; Rapp, M.; Reddem, E.R.; Yu, J.; Bahna, F.; Bimela, J.; et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe 2021, 29, 819–833.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Jennewein, M.F.; MacCamy, A.J.; Akins, N.R.; Feng, J.L.; Homad, L.J.; Hurlburt, N.K.; Seydoux, E.; Wan, Y.H.; Stuart, A.B.; Edara, V.V.; et al. Isolation and characterization of cross-neutralizing coronavirus antibodies from COVID-19+subjects. Cell Rep. 2021, 36, 109353. [Google Scholar] [CrossRef]
- Low, J.S.; Jerak, J.; Tortorici, M.A.; McCallum, M.; Pinto, D.; Cassotta, A.; Foglierini, M.; Mele, F.; Abdelnabi, R.; Weynand, B.; et al. ACE2-binding exposes the SARS-CoV-2 fusion peptide to broadly neutralizing coronavirus antibodies. Science 2022, 377, 735–742. [Google Scholar] [CrossRef]
- Sun, X.Y.; Yi, C.Y.; Zhu, Y.F.; Ding, L.F.; Xia, S.; Chen, X.C.; Liu, M.; Gu, C.J.; Lu, X.; Fu, Y.D.; et al. Neutralization mechanism of a human antibody with pan-coronavirus reactivity including SARS-CoV-2. Nat. Microbiol. 2022, 7, 1063–1074. [Google Scholar] [CrossRef]
- Dacon, C.; Tucker, C.; Peng, L.H.; Lee, C.C.D.; Lin, T.H.; Yuan, M.; Cong, Y.; Wang, L.S.; Purser, L.; Williams, J.K.; et al. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science 2022, 377, 728–735. [Google Scholar] [CrossRef]
- Wang, X.L.; Sun, L.J.; Liu, Z.Z.; Xing, L.X.; Zhu, Y.; Xu, W.; Xia, S.; Lu, L.; Jiang, S.B. An engineered recombinant protein containing three structural domains in SARS-CoV-2 S2 protein has potential to act as a pan-human coronavirus entry inhibitor or vaccine antigen. Emerg. Microbes Infect. 2023, 12, 2244084. [Google Scholar] [CrossRef]
- Lu, Y.; Shen, F.; He, W.; Li, A.; Li, M.; Feng, X.; Zheng, Y.; Pang, W. HR121 targeting HR2 domain in S2 subunit of spike protein can serve as a broad-spectrum SARS-CoV-2 inhibitor via intranasal administration. Acta Pharm. Sin. B 2023, 13, 3339–3351. [Google Scholar] [CrossRef]
- Bai, Z.H.; Cao, Y.; Liu, W.J.; Li, J. The SARS-CoV-2 Nucleocapsid Protein and Its Role in Viral Structure, Biological Functions, and a Potential Target for Drug or Vaccine Mitigation. Viruses 2021, 13, 1115. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Tang, L.L.; Yu, X.L.; Zhou, J.; Chang, Y.F.; Wu, X. Bioinformatics analysis of epitope-based vaccine design against the novel SARS-CoV-2. Infect. Dis. Poverty 2020, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Bournazos, S.; Ravetch, J.V. Fcgamma Receptor Function and the Design of Vaccination Strategies. Immunity 2017, 47, 224–233. [Google Scholar] [CrossRef]
- Kar, M.; Johnson, K.E.E.; Vanderheiden, A.; Elrod, E.J.; Floyd, K.; Geerling, E.; Stone, E.T.; Salinas, E.; Banakis, S.; Wang, W.; et al. CD4+ and CD8+ T cells are required to prevent SARS-CoV-2 persistence in the nasal compartment. Sci. Adv. 2024, 10, eadp2636. [Google Scholar] [CrossRef]
- Buchholz, U.J.; Bukreyev, A.; Yang, L.; Lamirande, E.W.; Murphy, B.R.; Subbarao, K.; Collins, P.L. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc. Natl. Acad. Sci. USA 2004, 101, 9804–9809. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Wang, S.Y.; Zheng, Z.Q.; Yi, H.; Li, W.W.; Xu, Z.S.; Wang, Y.Y. SARS-CoV-2 membrane glycoprotein M antagonizes the MAVS-mediated innate antiviral response. Cell. Mol. Immunol. 2021, 18, 613–620. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, K.; Hu, Y.; Ye, Z.W.; Luo, W.; Luo, C.; Cao, H.; Wang, R.; Yue, X.; Liu, D.; et al. M protein ectodomain-specific immunity restrains SARS-CoV-2 variants replication. Front. Immunol. 2024, 15, 1450114. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Hao, X.; Wang, Y.; Zhang, X.; Ge, L.; Wang, P.; Tian, B.; Zhang, M. Coronavirus envelope protein activates TMED10-mediated unconventional secretion of inflammatory factors. Nat. Commun. 2024, 15, 8708. [Google Scholar] [CrossRef]
- Creech, C.B.; Walker, S.C.; Samuels, R.J. SARS-CoV-2 Vaccines. JAMA 2021, 325, 1318–1320. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Al Kaabi, N.; Zhang, Y.; Xia, S.; Yang, Y.; Al Qahtani, M.M.; Abdulrazzaq, N.; Al Nusair, M.; Hassany, M.; Jawad, J.S.; Abdalla, J.; et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA 2021, 326, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Huang, B.; Deng, W.; Quan, Y.; Wang, W.; Xu, W.; Zhao, Y.; Li, N.; Zhang, J.; et al. Development of an Inactivated Vaccine Candidate, BBIBP-CorV, with Potent Protection against SARS-CoV-2. Cell 2020, 182, 713–721 e719. [Google Scholar] [CrossRef]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Zakarya, K.; Kutumbetov, L.; Orynbayev, M.; Abduraimov, Y.; Sultankulova, K.; Kassenov, M.; Sarsenbayeva, G.; Kulmagambetov, I.; Davlyatshin, T.; Sergeeva, M.; et al. Safety and immunogenicity of a QazCovid-in(R) inactivated whole-virion vaccine against COVID-19 in healthy adults: A single-centre, randomised, single-blind, placebo-controlled phase 1 and an open-label phase 2 clinical trials with a 6 months follow-up in Kazakhstan. eClinicalMedicine 2021, 39, 101078. [Google Scholar] [CrossRef]
- Lazarus, R.; Taucher, C.; Brown, C.; Corbic Ramljak, I.; Danon, L.; Dubischar, K.; Duncan, C.J.A.; Eder-Lingelbach, S.; Faust, S.N.; Green, C.; et al. Safety and immunogenicity of the inactivated whole-virus adjuvanted COVID-19 vaccine VLA2001: A randomized, dose escalation, double-blind phase 1/2 clinical trial in healthy adults. J. Infect. 2022, 85, 306–317. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Pan, H.X.; Liu, J.K.; Huang, B.Y.; Li, G.F.; Chang, X.Y.; Liu, Y.F.; Wang, W.L.; Chu, K.; Hu, J.L.; Li, J.X.; et al. Immunogenicity and safety of a severe acute respiratory syndrome coronavirus 2 inactivated vaccine in healthy adults: Randomized, double-blind, and placebo-controlled phase 1 and phase 2 clinical trials. Chin. Med. J. 2021, 134, 1289–1298. [Google Scholar] [CrossRef]
- Mohraz, M.; Vahdat, K.; Ghamari, S.H.; Abbasi-Kangevari, M.; Ghasemi, E.; Ghabdian, Y.; Rezaei, N.; Pouya, M.A.; Abdoli, A.; Malekpour, M.R.; et al. Efficacy and safety of an inactivated virus-particle vaccine for SARS-CoV-2, BIV1-CovIran: Randomised, placebo controlled, double blind, multicentre, phase 3 clinical trial. BMJ 2023, 382, e070464. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Song, Y.; Coleman, J.R.; Stawowczyk, M.; Tafrova, J.; Tasker, S.; Boltz, D.; Baker, R.; Garcia, L.; et al. Scalable live-attenuated SARS-CoV-2 vaccine candidate demonstrates preclinical safety and efficacy. Proc. Natl. Acad. Sci. USA 2021, 118, e2102775118. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Liu, J.; Xia, H.; Zou, J.; Muruato, A.E.; Periasamy, S.; Kurhade, C.; Plante, J.A.; Bopp, N.E.; et al. A live-attenuated SARS-CoV-2 vaccine candidate with accessory protein deletions. Nat. Commun. 2022, 13, 4337. [Google Scholar] [CrossRef] [PubMed]
- Mehla, R.; Kokate, P.; Bhosale, S.R.; Vaidya, V.; Narayanan, S.; Shandil, R.K.; Singh, M.; Rudramurthy, G.R.; Naveenkumar, C.N.; Bharathkumar, K.; et al. A Live Attenuated COVID-19 Candidate Vaccine for Children: Protection against SARS-CoV-2 Challenge in Hamsters. Vaccines 2023, 11, 255. [Google Scholar] [CrossRef] [PubMed]
- Kutumbetov, L.; Myrzakhmetova, B.; Tussipova, A.; Zhapparova, G.; Tlenchiyeva, T.; Bissenbayeva, K.; Zhapar, K.; Zhugunissov, K.; Nurabayev, S.; Kerimbayev, A. Safety and Immunogenicity of the Live Attenuated Vaccine QazCOVID-Live Against Coronavirus Infection COVID-19: Pre-Clinical Study Results. Vaccines 2024, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Hannawi, S.; Yan, L.; Saifeldin, L.; Abuquta, A.; Alamadi, A.; Mahmoud, S.A.; Hassan, A.; Zhang, M.; Gao, C.; Chen, Y.; et al. Safety and immunogenicity of multivalent SARS-CoV-2 protein vaccines: A randomized phase 3 trial. eClinicalMedicine 2023, 64, 102195. [Google Scholar] [CrossRef]
- Kaabi, N.A.; Yang, Y.K.; Liang, Y.; Xu, K.; Zhang, X.F.; Kang, Y.; Jin, Y.Q.; Hou, J.W.; Zhang, J.; Yang, T.; et al. Safety and immunogenicity of a mosaic vaccine booster against Omicron and other SARS-CoV-2 variants: A randomized phase 2 trial. Signal Transduct. Target. Ther. 2023, 8, 20. [Google Scholar] [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef]
- Ryzhikov, A.B.; Ryzhikov, E.A.; Bogryantseva, M.P.; Usova, S.V.; Nechaeva, E.A.; Danilenko, E.D.; Pyankov, S.A.; Gudymo, A.S.; Moiseeva, A.A.; Onkhonova, G.S.; et al. Assessment of Safety and Prophylactic Efficacy of the EpiVacCorona Peptide Vaccine for COVID-19 Prevention (Phase III). Vaccines 2023, 11, 998. [Google Scholar] [CrossRef]
- Dai, L.; Gao, L.; Tao, L.; Hadinegoro, S.R.; Erkin, M.; Ying, Z.; He, P.; Girsang, R.T.; Vergara, H.; Akram, J.; et al. Efficacy and Safety of the RBD-Dimer-Based COVID-19 Vaccine ZF2001 in Adults. N. Engl. J. Med. 2022, 386, 2097–2111. [Google Scholar] [CrossRef]
- Kuo, T.Y.; Lin, M.Y.; Coffman, R.L.; Campbell, J.D.; Traquina, P.; Lin, Y.J.; Liu, L.T.; Cheng, J.; Wu, Y.C.; Wu, C.C.; et al. Development of CpG-adjuvanted stable prefusion SARS-CoV-2 spike antigen as a subunit vaccine against COVID-19. Sci. Rep. 2020, 10, 20085. [Google Scholar] [CrossRef]
- Thuluva, S.; Paradkar, V.; Gunneri, S.R.; Yerroju, V.; Mogulla, R.; Turaga, K.; Kyasani, M.; Manoharan, S.K.; Medigeshi, G.; Singh, J.; et al. Evaluation of safety and immunogenicity of receptor-binding domain-based COVID-19 vaccine (Corbevax) to select the optimum formulation in open-label, multicentre, and randomised phase-1/2 and phase-2 clinical trials. eBioMedicine 2022, 83, 104217. [Google Scholar] [CrossRef]
- Mas-Bermejo, P.I.; Dickinson-Meneses, F.O.; Almenares-Rodriguez, K.; Sanchez-Valdes, L.; Guinovart-Diaz, R.; Vidal-Ledo, M.; Galban-Garcia, E.; Olivera-Nodarse, Y.; Morgado-Vega, I.; Duenas-Carrera, S.; et al. Cuban Abdala vaccine: Effectiveness in preventing severe disease and death from COVID-19 in Havana, Cuba; A cohort study. Lancet Reg. Health Am. 2022, 16, 100366. [Google Scholar] [CrossRef] [PubMed]
- Dayan, G.H.; Rouphael, N.; Walsh, S.R.; Chen, A.; Grunenberg, N.; Allen, M.; Antony, J.; Asante, K.P.; Bhate, A.S.; Beresnev, T.; et al. Efficacy of a bivalent (D614 + B.1.351) SARS-CoV-2 recombinant protein vaccine with AS03 adjuvant in adults: A phase 3, parallel, randomised, modified double-blind, placebo-controlled trial. Lancet Respir. Med. 2023, 11, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Jiao, Z.; Li, X.; He, Z.; Li, Y.; Yang, F.; Zhao, X.; Wang, Y.; Huang, W.; Qin, M.; et al. Inhaled SARS-CoV-2 vaccine for single-dose dry powder aerosol immunization. Nature 2023, 624, 630–638. [Google Scholar] [CrossRef]
- Tang, J.; Xu, Q.; Zhu, C.; Xuan, K.; Li, T.; Li, Q.; Pang, X.; Zha, Z.; Li, J.; Qiao, L.; et al. Immunogenicity of Tetravalent Protein Vaccine SCTV01E-2 against SARS-CoV-2 EG.5 Subvaraint: A Phase 2 Trial. Vaccines 2024, 12, 175. [Google Scholar] [CrossRef]
- Su, H.; van Eerde, A.; Rimstad, E.; Bock, R.; Branza-Nichita, N.; Yakovlev, I.A.; Clarke, J.L. Plant-made vaccines against viral diseases in humans and farm animals. Front. Plant Sci. 2023, 14, 1170815. [Google Scholar] [CrossRef]
- Yin, D.; Zhong, Y.; Ling, S.; Lu, S.; Wang, X.; Jiang, Z.; Wang, J.; Dai, Y.; Tian, X.; Huang, Q.; et al. Dendritic-cell-targeting virus-like particles as potent mRNA vaccine carriers. Nat. Biomed. Eng. 2025, 9, 185–200. [Google Scholar] [CrossRef]
- Tebas, P.; Yang, S.; Boyer, J.D.; Reuschel, E.L.; Patel, A.; Christensen-Quick, A.; Andrade, V.M.; Morrow, M.P.; Kraynyak, K.; Agnes, J.; et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: A preliminary report of an open-label, Phase 1 clinical trial. eClinicalMedicine 2021, 31, 100689. [Google Scholar] [CrossRef]
- Dey, A.; Chozhavel Rajanathan, T.M.; Chandra, H.; Pericherla, H.P.R.; Kumar, S.; Choonia, H.S.; Bajpai, M.; Singh, A.K.; Sinha, A.; Saini, G.; et al. Immunogenic potential of DNA vaccine candidate, ZyCoV-D against SARS-CoV-2 in animal models. Vaccine 2021, 39, 4108–4116. [Google Scholar] [CrossRef]
- Seo, Y.B.; Suh, Y.S.; Ryu, J.I.; Jang, H.; Oh, H.; Koo, B.S.; Seo, S.H.; Hong, J.J.; Song, M.; Kim, S.J.; et al. Soluble Spike DNA Vaccine Provides Long-Term Protective Immunity against SARS-CoV-2 in Mice and Nonhuman Primates. Vaccines 2021, 9, 307. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Lee, J.; Suh, Y.S.; Song, Y.G.; Choi, Y.J.; Lee, K.H.; Seo, S.H.; Song, M.; Oh, J.W.; Kim, M.; et al. Safety and immunogenicity of two recombinant DNA COVID-19 vaccines containing the coding regions of the spike or spike and nucleocapsid proteins: An interim analysis of two open-label, non-randomised, phase 1 trials in healthy adults. Lancet Microbe 2022, 3, e173–e183. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Winokur, P.; Gayed, J.; Fitz-Patrick, D.; Thomas, S.J.; Diya, O.; Lockhart, S.; Xu, X.; Zhang, Y.; Bangad, V.; Schwartz, H.I.; et al. Bivalent Omicron BA.1-Adapted BNT162b2 Booster in Adults Older than 55 Years. N. Engl. J. Med. 2023, 388, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Xie, F.; Takhar, H.; Ogun, O.A.; Simmons, S.; et al. Effectiveness of BNT162b2 BA.4/5 bivalent mRNA vaccine against a range of COVID-19 outcomes in a large health system in the USA: A test-negative case-control study. Lancet Respir. Med. 2023, 11, 1089–1100. [Google Scholar] [CrossRef]
- Modjarrad, K.; Che, Y.; Chen, W.; Wu, H.; Cadima, C.I.; Muik, A.; Maddur, M.S.; Tompkins, K.R.; Martinez, L.T.; Cai, H.; et al. Preclinical characterization of the Omicron XBB.1.5-adapted BNT162b2 COVID-19 vaccine. NPJ Vaccines 2024, 9, 229. [Google Scholar] [CrossRef]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schafer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Chalkias, S.; Harper, C.; Vrbicky, K.; Walsh, S.R.; Essink, B.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; Chang, Y.; et al. A Bivalent Omicron-Containing Booster Vaccine against COVID-19. N. Engl. J. Med. 2022, 387, 1279–1291. [Google Scholar] [CrossRef]
- Chalkias, S.; Whatley, J.L.; Eder, F.; Essink, B.; Khetan, S.; Bradley, P.; Brosz, A.; McGhee, N.; Tomassini, J.E.; Chen, X.; et al. Original SARS-CoV-2 monovalent and Omicron BA.4/BA.5 bivalent COVID-19 mRNA vaccines: Phase 2/3 trial interim results. Nat. Med. 2023, 29, 2325–2333. [Google Scholar] [CrossRef]
- Tan, S.; Zhao, J.; Hu, X.; Li, Y.; Wu, Z.; Lu, G.; Yu, Z.; Du, B.; Liu, Y.; Li, L.; et al. Preclinical evaluation of RQ3013, a broad-spectrum mRNA vaccine against SARS-CoV-2 variants. Sci. Bull. 2023, 68, 3192–3206. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Liang, Z.; Cui, T.; Chen, D.; Li, G.; Xu, H.; Liu, S.; Zhong, N.; Huang, W.; et al. Immune escape of BA.2.86 is comparable to XBB subvariants from the plasma of BA.5- and BA.5-XBB-convalescent subpopulations. J. Med. Virol. 2024, 96, e29417. [Google Scholar] [CrossRef]
- Kremsner, P.G.; Ahuad Guerrero, R.A.; Arana-Arri, E.; Aroca Martinez, G.J.; Bonten, M.; Chandler, R.; Corral, G.; De Block, E.J.L.; Ecker, L.; Gabor, J.J.; et al. Efficacy and safety of the CVnCoV SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): A randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 2022, 22, 329–340. [Google Scholar] [CrossRef]
- Gebre, M.S.; Rauch, S.; Roth, N.; Yu, J.; Chandrashekar, A.; Mercado, N.B.; He, X.; Liu, J.; McMahan, K.; Martinot, A.; et al. Optimization of non-coding regions for a non-modified mRNA COVID-19 vaccine. Nature 2022, 601, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lei, W.; Kang, B.; Yang, H.; Wang, Y.; Lu, Y.; Lv, L.; Sun, Y.; Zhang, J.; Wang, X.; et al. A novel mRNA vaccine, SYS6006, against SARS-CoV-2. Front. Immunol. 2022, 13, 1051576. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.T.; Hughes, S.G.; Ta, V.T.; Phan, L.T.; Do, Q.; Nguyen, T.V.; Pham, A.T.V.; Thi Ngoc Dang, M.; Nguyen, L.V.; Trinh, Q.V.; et al. Safety, immunogenicity and efficacy of the self-amplifying mRNA ARCT-154 COVID-19 vaccine: Pooled phase 1, 2, 3a and 3b randomized, controlled trials. Nat. Commun. 2024, 15, 4081. [Google Scholar] [CrossRef] [PubMed]
- Akahata, W.; Sekida, T.; Nogimori, T.; Ode, H.; Tamura, T.; Kono, K.; Kazami, Y.; Washizaki, A.; Masuta, Y.; Suzuki, R.; et al. Safety and immunogenicity of SARS-CoV-2 self-amplifying RNA vaccine expressing an anchored RBD: A randomized, observer-blind phase 1 study. Cell Rep. Med. 2023, 4, 101134. [Google Scholar] [CrossRef]
- Madhi, S.A.; Kwatra, G.; Richardson, S.I.; Koen, A.L.; Baillie, V.; Cutland, C.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; et al. Durability of ChAdOx1 nCoV-19 (AZD1222) vaccine and hybrid humoral immunity against variants including omicron BA.1 and BA.4 6 months after vaccination (COV005): A post-hoc analysis of a randomised, phase 1b-2a trial. Lancet Infect. Dis. 2023, 23, 295–306. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cardenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Yuan, L.; Zhang, L.; Zhang, L.; Zhao, H.; Chen, C.; Wang, X.; Han, J.; Chen, Y.; et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci. Bull. 2022, 67, 1372–1387. [Google Scholar] [CrossRef]
- Hassan, A.O.; Kafai, N.M.; Dmitriev, I.P.; Fox, J.M.; Smith, B.K.; Harvey, I.B.; Chen, R.E.; Winkler, E.S.; Wessel, A.W.; Case, J.B.; et al. A Single-Dose Intranasal ChAd Vaccine Protects Upper and Lower Respiratory Tracts against SARS-CoV-2. Cell 2020, 183, 169–184 e113. [Google Scholar] [CrossRef]
- King, R.G.; Silva-Sanchez, A.; Peel, J.N.; Botta, D.; Dickson, A.M.; Pinto, A.K.; Meza-Perez, S.; Allie, S.R.; Schultz, M.D.; Liu, M.; et al. Single-Dose Intranasal Administration of AdCOVID Elicits Systemic and Mucosal Immunity against SARS-CoV-2 and Fully Protects Mice from Lethal Challenge. Vaccines 2021, 9, 881. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Li, K.; Rowe, D.K.; Diaz, M.C.H.; Griffin, E.F.; Beavis, A.C.; Johnson, S.K.; Padykula, I.; Jones, C.A.; Briggs, K.; et al. Protection of K18-hACE2 mice and ferrets against SARS-CoV-2 challenge by a single-dose mucosal immunization with a parainfluenza virus 5-based COVID-19 vaccine. Sci. Adv. 2021, 7, eabi5246. [Google Scholar] [CrossRef]
- Tcheou, J.; Raskin, A.; Singh, G.; Kawabata, H.; Bielak, D.; Sun, W.; Gonzalez-Dominguez, I.; Sather, D.N.; Garcia-Sastre, A.; Palese, P.; et al. Safety and Immunogenicity Analysis of a Newcastle Disease Virus (NDV-HXP-S) Expressing the Spike Protein of SARS-CoV-2 in Sprague Dawley Rats. Front. Immunol. 2021, 12, 791764. [Google Scholar] [CrossRef]
- Lara-Puente, J.H.; Carreno, J.M.; Sun, W.; Suarez-Martinez, A.; Ramirez-Martinez, L.; Quezada-Monroy, F.; Paz-De la Rosa, G.; Vigueras-Moreno, R.; Singh, G.; Rojas-Martinez, O.; et al. Safety and Immunogenicity of a Newcastle Disease Virus Vector-Based SARS-CoV-2 Vaccine Candidate, AVX/COVID-12-HEXAPRO (Patria), in Pigs. mBio 2021, 12, e0190821. [Google Scholar] [CrossRef]
- Tscherne, A.; Schwarz, J.H.; Rohde, C.; Kupke, A.; Kalodimou, G.; Limpinsel, L.; Okba, N.M.A.; Bosnjak, B.; Sandrock, I.; Odak, I.; et al. Immunogenicity and efficacy of the COVID-19 candidate vector vaccine MVA-SARS-2-S in preclinical vaccination. Proc. Natl. Acad. Sci. USA 2021, 118, e2026207118. [Google Scholar] [CrossRef]
- Meyer Zu Natrup, C.; Tscherne, A.; Dahlke, C.; Ciurkiewicz, M.; Shin, D.L.; Fathi, A.; Rohde, C.; Kalodimou, G.; Halwe, S.; Limpinsel, L.; et al. Stabilized recombinant SARS-CoV-2 spike antigen enhances vaccine immunogenicity and protective capacity. J. Clin. Investig. 2022, 132, e159895. [Google Scholar] [CrossRef]
- Tioni, M.F.; Jordan, R.; Pena, A.S.; Garg, A.; Wu, D.; Phan, S.I.; Weiss, C.M.; Cheng, X.; Greenhouse, J.; Orekov, T.; et al. Mucosal administration of a live attenuated recombinant COVID-19 vaccine protects nonhuman primates from SARS-CoV-2. NPJ Vaccines 2022, 7, 85. [Google Scholar] [CrossRef]
- Walls, A.C.; Miranda, M.C.; Schafer, A.; Pham, M.N.; Greaney, A.; Arunachalam, P.S.; Navarro, M.J.; Tortorici, M.A.; Rogers, K.; O’Connor, M.A.; et al. Elicitation of broadly protective sarbecovirus immunity by receptor-binding domain nanoparticle vaccines. Cell 2021, 184, 5432–5447.e16. [Google Scholar] [CrossRef]
- Jin, L.; Li, Z.; Zhang, X.; Li, J.; Zhu, F. CoronaVac: A review of efficacy, safety, and immunogenicity of the inactivated vaccine against SARS-CoV-2. Hum. Vaccin Immunother. 2022, 18, 2096970. [Google Scholar] [CrossRef]
- Goh, Y.S.; Fong, S.W.; Rouers, A.; Chang, Z.W.; Tay, M.Z.; Chavatte, J.M.; Zhuo, N.Z.; Hor, P.X.; Loh, C.Y.; Huang, Y.; et al. Heterologous booster vaccination with CoronaVac following prime vaccination with mRNA vaccine. Clin. Transl. Immunology 2022, 11, e1403. [Google Scholar] [CrossRef]
- Soliman, R.M.; Nishioka, K.; Murakoshi, F.; Nakaya, T. Use of live attenuated recombinant Newcastle disease virus carrying avian paramyxovirus 2 HN and F protein genes to enhance immune responses against species A rotavirus VP6 protein. Vet. Res. 2024, 55, 16. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.H.; Jang, Y. Cold-Adapted Live Attenuated SARS-Cov-2 Vaccine Completely Protects Human ACE2 Transgenic Mice from SARS-Cov-2 Infection. Vaccines 2020, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zheng, T.; Xu, K.; Han, Y.; Xu, L.; Huang, E.; An, Y.; Cheng, Y.; Li, S.; Liu, M.; et al. A Universal Design of Betacoronavirus Vaccines against COVID-19, MERS, and SARS. Cell 2020, 182, 722–733 e711. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, Y.; Dai, L.; Wang, J.; He, P.; Li, C.; Fang, X.; Wang, C.; Zhao, X.; Huang, E.; et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: Two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect. Dis. 2021, 21, 1107–1119. [Google Scholar] [CrossRef]
- Hsieh, S.M.; Liu, W.D.; Huang, Y.S.; Lin, Y.J.; Hsieh, E.F.; Lian, W.C.; Chen, C.; Janssen, R.; Shih, S.R.; Huang, C.G.; et al. Safety and immunogenicity of a Recombinant Stabilized Prefusion SARS-CoV-2 Spike Protein Vaccine (MVC-COV1901) Adjuvanted with CpG 1018 and Aluminum Hydroxide in healthy adults: A Phase 1, dose-escalation study. eClinicalMedicine 2021, 38, 100989. [Google Scholar] [CrossRef]
- Chen, W.H.; Wei, J.; Kundu, R.T.; Adhikari, R.; Liu, Z.; Lee, J.; Versteeg, L.; Poveda, C.; Keegan, B.; Villar, M.J.; et al. Genetic modification to design a stable yeast-expressed recombinant SARS-CoV-2 receptor binding domain as a COVID-19 vaccine candidate. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129893. [Google Scholar] [CrossRef]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Yang, J.; He, X.; Shi, H.; He, C.; Lei, H.; He, H.; Yang, L.; Wang, W.; Shen, G.; Yang, J.; et al. Recombinant XBB.1.5 boosters induce robust neutralization against KP.2- and KP.3-included JN.1 sublineages. Signal Transduct. Target. Ther. 2025, 10, 47. [Google Scholar] [CrossRef]
- Janssen, Y.F.; Feitsma, E.A.; Boersma, H.H.; Alleva, D.G.; Lancaster, T.M.; Sathiyaseelan, T.; Murikipudi, S.; Delpero, A.R.; Scully, M.M.; Ragupathy, R.; et al. Phase I interim results of a phase I/II study of the IgG-Fc fusion COVID-19 subunit vaccine, AKS-452. Vaccine 2022, 40, 1253–1260. [Google Scholar] [CrossRef]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Miles, A.P.; McClellan, H.A.; Rausch, K.M.; Zhu, D.; Whitmore, M.D.; Singh, S.; Martin, L.B.; Wu, Y.; Giersing, B.K.; Stowers, A.W.; et al. Montanide ISA 720 vaccines: Quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine 2005, 23, 2530–2539. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Balke, I.; Zinkhan, S.; Zeltina, V.; Liu, X.; Chang, X.; Krenger, P.S.; Plattner, K.; Gharailoo, Z.; Vogt, A.S.; et al. A scalable and highly immunogenic virus-like particle-based vaccine against SARS-CoV-2. Allergy 2022, 77, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lai, H. Plant-derived virus-like particles as vaccines. Hum. Vaccines Immunother. 2013, 9, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Davies, H.M. Review article: Commercialization of whole-plant systems for biomanufacturing of protein products: Evolution and prospects. Plant Biotechnol. J. 2010, 8, 845–861. [Google Scholar] [CrossRef]
- Medicago and GSK Announce Positive Phase 3 Efficacy and Safety Results for Adjuvanted Plant-Based COVID-19 Vaccine Candidate. Available online: https://www.gsk.com/en-gb/media/press-releases/medicago-and-gsk-announce-positive-phase-3-efficacy-and-safety-results/ (accessed on 7 December 2021).
- Marsian, J.; Lomonossoff, G.P. Molecular pharming—VLPs made in plants. Curr. Opin. Biotechnol. 2016, 37, 201–206. [Google Scholar] [CrossRef]
- Joyce, M.G.; King, H.A.D.; Elakhal-Naouar, I.; Ahmed, A.; Peachman, K.K.; Macedo Cincotta, C.; Subra, C.; Chen, R.E.; Thomas, P.V.; Chen, W.H.; et al. A SARS-CoV-2 ferritin nanoparticle vaccine elicits protective immune responses in nonhuman primates. Sci. Transl. Med. 2022, 14, eabi5735. [Google Scholar] [CrossRef]
- Ma, X.; Zou, F.; Yu, F.; Li, R.; Yuan, Y.; Zhang, Y.; Zhang, X.; Deng, J.; Chen, T.; Song, Z.; et al. Nanoparticle Vaccines Based on the Receptor Binding Domain (RBD) and Heptad Repeat (HR) of SARS-CoV-2 Elicit Robust Protective Immune Responses. Immunity 2020, 53, 1315–1330.e9. [Google Scholar] [CrossRef]
- Swanson, K.A.; Rainho-Tomko, J.N.; Williams, Z.P.; Lanza, L.; Peredelchuk, M.; Kishko, M.; Pavot, V.; Alamares-Sapuay, J.; Adhikarla, H.; Gupta, S.; et al. A respiratory syncytial virus (RSV) F protein nanoparticle vaccine focuses antibody responses to a conserved neutralization domain. Sci. Immunol. 2020, 5, eaba6466. [Google Scholar] [CrossRef]
- Karch, C.P.; Bai, H.; Torres, O.B.; Tucker, C.A.; Michael, N.L.; Matyas, G.R.; Rolland, M.; Burkhard, P.; Beck, Z. Design and characterization of a self-assembling protein nanoparticle displaying HIV-1 Env V1V2 loop in a native-like trimeric conformation as vaccine antigen. Nanomedicine 2019, 16, 206–216. [Google Scholar] [CrossRef]
- Miranda, M.C.; Kepl, E.; Navarro, M.J.; Chen, C.; Johnson, M.; Sprouse, K.R.; Stewart, C.; Palser, A.; Valdez, A.; Pettie, D.; et al. Potent neutralization of SARS-CoV-2 variants by RBD nanoparticle and prefusion-stabilized spike immunogens. NPJ Vaccines 2024, 9, 184. [Google Scholar] [CrossRef]
- Leitner, W.W.; Ying, H.; Restifo, N.P. DNA and RNA-based vaccines: Principles, progress and prospects. Vaccine 1999, 18, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Frankland, T.B.; Ackerson, B.K.; Takhar, H.; Ogun, O.A.; Simmons, S.; Zamparo, J.M.; et al. BNT162b2 vaccine effectiveness against SARS-CoV-2 omicron BA.4 and BA.5. Lancet Infect. Dis. 2022, 22, 1663–1665. [Google Scholar] [CrossRef]
- Cheng, S.M.S.; Mok, C.K.P.; Li, J.K.C.; Chan, K.K.P.; Luk, K.S.; Lee, B.H.W.; Gu, H.; Chan, K.C.K.; Tsang, L.C.H.; Yiu, K.Y.S.; et al. Cross-neutralizing antibody against emerging Omicron subvariants of SARS-CoV-2 in infection-naive individuals with homologous BNT162b2 or BNT162b2(WT + BA.4/5) bivalent booster vaccination. Virol. J. 2024, 21, 70. [Google Scholar] [CrossRef]
- Usdan, L.; Patel, S.; Rodriguez, H.; Xu, X.; Lee, D.Y.; Finn, D.; Wyper, H.; Lowry, F.S.; Mensa, F.J.; Lu, C.; et al. A Bivalent Omicron-BA.4/BA.5-Adapted BNT162b2 Booster in >/=12-Year-Olds. Clin. Infect. Dis. 2024, 78, 1194–1203. [Google Scholar] [CrossRef]
- Khong, K.W.; Liu, D.; Leung, K.Y.; Lu, L.; Lam, H.Y.; Chen, L.; Chan, P.C.; Lam, H.M.; Xie, X.; Zhang, R.; et al. Antibody Response of Combination of BNT162b2 and CoronaVac Platforms of COVID-19 Vaccines against Omicron Variant. Vaccines 2022, 10, 160. [Google Scholar] [CrossRef]
- Scheaffer, S.M.; Lee, D.; Whitener, B.; Ying, B.; Wu, K.; Liang, C.Y.; Jani, H.; Martin, P.; Amato, N.J.; Avena, L.E.; et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice. Nat. Med. 2023, 29, 247–257. [Google Scholar] [CrossRef]
- Nham, E.; Song, J.Y.; Sohn, J.W.; Choi, W.S.; Wie, S.H.; Lee, J.; Lee, J.S.; Jeong, H.W.; Eom, J.S.; Choi, Y.J.; et al. Real-world effectiveness of COVID-19 XBB.1.5 monovalent mRNA vaccine: Analysis over nine months. Vaccine 2025, 59, 127275. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Ayoub, H.H.; Coyle, P.; Tang, P.; Hasan, M.R.; Yassine, H.M.; Al Thani, A.A.; Al-Kanaani, Z.; Al-Kuwari, E.; Jeremijenko, A.; et al. Differential protection against SARS-CoV-2 reinfection pre- and post-Omicron. Nature 2025, 639, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Fox, C.B. Development of thermostable vaccine adjuvants. Expert Rev. Vaccines 2021, 20, 497–517. [Google Scholar] [CrossRef]
- Arte, K.S.; Chen, M.; Patil, C.D.; Huang, Y.; Qu, L.; Zhou, Q. Recent advances in drying and development of solid formulations for stable mRNA and siRNA lipid nanoparticles. J. Pharm. Sci. 2025, 114, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Kisakov, D.N.; Belyakov, I.M.; Kisakova, L.A.; Yakovlev, V.A.; Tigeeva, E.V.; Karpenko, L.I. The use of electroporation to deliver DNA-based vaccines. Expert Rev. Vaccines 2024, 23, 102–123. [Google Scholar] [CrossRef]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef]
- Guo, X.; Guo, M.; Cai, R.; Hu, M.; Rao, L.; Su, W.; Liu, H.; Gao, F.; Zhang, X.; Liu, J.; et al. mRNA compartmentalization via multimodule DNA nanostructure assembly augments the immunogenicity and efficacy of cancer mRNA vaccine. Sci. Adv. 2024, 10, eadp3680. [Google Scholar] [CrossRef]
- Wang, S.; Liang, B.; Wang, W.; Li, L.; Feng, N.; Zhao, Y.; Wang, T.; Yan, F.; Yang, S.; Xia, X. Viral vectored vaccines: Design, development, preventive and therapeutic applications in human diseases. Signal Transduct. Target. Ther. 2023, 8, 149. [Google Scholar] [CrossRef]
- van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020, 586, 578–582. [Google Scholar] [CrossRef]
- Kirsebom, F.C.M.; Andrews, N.; Sachdeva, R.; Stowe, J.; Ramsay, M.; Lopez Bernal, J. Effectiveness of ChAdOx1-S COVID-19 booster vaccination against the Omicron and Delta variants in England. Nat. Commun. 2022, 13, 7688. [Google Scholar] [CrossRef]
- Kohmer, N.; Stein, S.; Schenk, B.; Grikscheit, K.; Metzler, M.; Rabenau, H.F.; Widera, M.; Herrmann, E.; Wicker, S.; Ciesek, S. Heterologous prime-boost immunization with ChAdOx1-S and BNT162b2: Reactogenicity and immunogenicity in a prospective cohort study. Int. J. Infect. Dis. 2023, 128, 166–175. [Google Scholar] [CrossRef]
- Gray, G.; Collie, S.; Goga, A.; Garrett, N.; Champion, J.; Seocharan, I.; Bamford, L.; Moultrie, H.; Bekker, L.G. Effectiveness of Ad26.COV2.S and BNT162b2 Vaccines against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Jeewandara, C.; Fernando, S.; Pushpakumara, P.D.; Ramu, S.T.; Kamaladasa, A.; Gunasekara, B.; Aberathna, I.S.; Kuruppu, H.; Ranasinghe, T.; Dayarathne, S.; et al. Immune responses following the first dose of the Sputnik V (Gam-COVID-Vac). Sci. Rep. 2022, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.F.; Guo, X.L.; Gou, J.B.; Hou, L.H.; Song, Z.Z.; Zhu, T.; Pan, H.X.; Zhu, J.H.; Shi, F.J.; Du, P.; et al. Immunogenicity and safety of heterologous immunisation with Ad5-nCOV in healthy adults aged 60 years and older primed with an inactivated SARS-CoV-2 vaccine (CoronaVac): A phase 4, randomised, observer-blind, non-inferiority trial. Lancet Reg. Health West. Pac. 2023, 38, 100829. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.M.S.; Mok, C.K.P.; Leung, Y.W.Y.; Ng, S.S.; Chan, K.C.K.; Ko, F.W.; Chen, C.; Yiu, K.; Lam, B.H.S.; Lau, E.H.Y.; et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat. Med. 2022, 28, 486–489. [Google Scholar] [CrossRef]
- Hocknell, P.K.; Wiley, R.D.; Wang, X.; Evans, T.G.; Bowers, W.J.; Hanke, T.; Federoff, H.J.; Dewhurst, S. Expression of human immunodeficiency virus type 1 gp120 from herpes simplex virus type 1-derived amplicons results in potent, specific, and durable cellular and humoral immune responses. J. Virol. 2002, 76, 5565–5580. [Google Scholar] [CrossRef]
- Pichla-Gollon, S.L.; Lin, S.W.; Hensley, S.E.; Lasaro, M.O.; Herkenhoff-Haut, L.; Drinker, M.; Tatsis, N.; Gao, G.P.; Wilson, J.M.; Ertl, H.C.; et al. Effect of preexisting immunity on an adenovirus vaccine vector: In vitro neutralization assays fail to predict inhibition by antiviral antibody in vivo. J. Virol. 2009, 83, 5567–5573. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Veness, B.; Berger, D.; Hamad, N.; Bari, N. Thrombosis with Thrombocytopenia Syndrome (TTS) following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination—A risk-benefit analysis for people < 60 years in Australia. Vaccine 2021, 39, 4784–4787. [Google Scholar] [CrossRef]
- Tica, J.; Rezelj, V.V.; Baron, B.; van Paassen, V.; Zaidman, J.; Fairlie, L.; Scheper, G.; Le Gars, M.; Struyf, F.; Douoguih, M.; et al. Safety and immunogenicity of Ad26.COV2.S in adolescents: Phase 2 randomized clinical trial. Hum. Vaccines Immunother. 2025, 21, 2450120. [Google Scholar] [CrossRef]
- Liu, C.; Hadiatullah, H.; Yuchi, Z. Identification of a potent SARS-CoV-2 neutralizing nanobody targeting the receptor-binding domain of the spike protein. Int. J. Biol. Macromol. 2024, 281, 136403. [Google Scholar] [CrossRef]
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 229–237. [Google Scholar] [CrossRef]
- Shi, R.; Shan, C.; Duan, X.; Chen, Z.; Liu, P.; Song, J.; Song, T.; Bi, X.; Han, C.; Wu, L.; et al. A human neutralizing antibody targets the receptor-binding site of SARS-CoV-2. Nature 2020, 584, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Baum, A.; Ajithdoss, D.; Copin, R.; Zhou, A.; Lanza, K.; Negron, N.; Ni, M.; Wei, Y.; Mohammadi, K.; Musser, B.; et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science 2020, 370, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Gilchuk, P.; Case, J.B.; Binshtein, E.; Chen, R.E.; Nkolola, J.P.; Schafer, A.; Reidy, J.X.; Trivette, A.; Nargi, R.S.; et al. Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature 2020, 584, 443–449. [Google Scholar] [CrossRef]
- Westendorf, K.; Zentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. Cell Rep. 2022, 39, 110812. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, D.K.; Lee, J.; Kim, Y.I.; Seo, J.M.; Kim, Y.G.; Jeong, J.H.; Kim, M.; Kim, J.I.; Kim, P.; et al. A therapeutic neutralizing antibody targeting receptor binding domain of SARS-CoV-2 spike protein. Nat. Commun. 2021, 12, 288. [Google Scholar] [CrossRef]
- Chen, Z.; Feng, L.; Wang, L.; Zhang, L.; Zheng, B.; Fu, H.; Li, F.; Liu, L.; Lv, Q.; Deng, R.; et al. A broadly neutralizing antibody against the SARS-CoV-2 Omicron sub-variants BA.1, BA.2, BA.2.12.1, BA.4, and BA.5. Signal Transduct. Target. Ther. 2025, 10, 14. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef]
- Swart, I.C.; Debski-Antoniak, O.J.; Zegar, A.; de Bouter, T.; Chatziandreou, M.; van den Berg, M.; Drulyte, I.; Pyrc, K.; de Haan, C.A.M.; Hurdiss, D.L.; et al. A bivalent spike-targeting nanobody with anti-sarbecovirus activity. J. Nanobiotechnol. 2025, 23, 196. [Google Scholar] [CrossRef]
- Liang, K.H.; Chiang, P.Y.; Ko, S.H.; Chou, Y.C.; Lu, R.M.; Lin, H.T.; Chen, W.Y.; Lin, Y.L.; Tao, M.H.; Jan, J.T.; et al. Antibody cocktail effective against variants of SARS-CoV-2. J. Biomed. Sci. 2021, 28, 80. [Google Scholar] [CrossRef]
- Rubio, A.A.; Baharani, V.A.; Dadonaite, B.; Parada, M.; Abernathy, M.E.; Wang, Z.; Lee, Y.E.; Eso, M.R.; Phung, J.; Ramos, I.; et al. Bispecific antibodies targeting the N-terminal and receptor binding domains potently neutralize SARS-CoV-2 variants of concern. Sci. Transl. Med. 2025, 17, eadq5720. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.J.; VanBlargan, L.A.; Liu, Z.; Gilchuk, P.; Zhao, H.; Chen, R.E.; Raju, S.; Chong, Z.; Whitener, B.M.; Shrihari, S.; et al. A broadly reactive antibody targeting the N-terminal domain of SARS-CoV-2 spike confers Fc-mediated protection. Cell Rep. Med. 2023, 4, 101305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Muecksch, F.; Cho, A.; Gaebler, C.; Hoffmann, H.H.; Ramos, V.; Zong, S.; Cipolla, M.; Johnson, B.; Schmidt, F.; et al. Analysis of memory B cells identifies conserved neutralizing epitopes on the N-terminal domain of variant SARS-Cov-2 spike proteins. Immunity 2022, 55, 998–1012.e8. [Google Scholar] [CrossRef]
- Niu, X.; Li, Z.; Wang, J.; Jian, F.; Yu, Y.; Song, W.; Yisimayi, A.; Du, S.; Zhang, Z.; Wang, Q.; et al. Omicron-specific ultra-potent SARS-CoV-2 neutralizing antibodies targeting the N1/N2 loop of Spike N-terminal domain. Emerg. Microbes Infect. 2024, 13, 2412990. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Zhang, Y.; Cheng, L.; Zhang, L.; Yan, Q.; Liu, X.; Chen, J.; Dai, J.; Guo, Y.; et al. Defining the features and structure of neutralizing antibody targeting the silent face of the SARS-CoV-2 spike N-terminal domain. MedComm 2024, 5, e70008. [Google Scholar] [CrossRef]
- Onodera, T.; Kita, S.; Adachi, Y.; Moriyama, S.; Sato, A.; Nomura, T.; Sakakibara, S.; Inoue, T.; Tadokoro, T.; Anraku, Y.; et al. A SARS-CoV-2 antibody broadly neutralizes SARS-related coronaviruses and variants by coordinated recognition of a virus-vulnerable site. Immunity 2021, 54, 2385–2398.e2310. [Google Scholar] [CrossRef]
- Shi, W.; Wang, L.; Zhou, T.; Sastry, M.; Yang, E.S.; Zhang, Y.; Chen, M.; Chen, X.; Choe, M.; Creanga, A.; et al. Vaccine-elicited murine antibody WS6 neutralizes diverse beta-coronaviruses by recognizing a helical stem supersite of vulnerability. Structure 2022, 30, 1233–1244.e7. [Google Scholar] [CrossRef]
- Zhou, P.; Yuan, M.; Song, G.; Beutler, N.; Shaabani, N.; Huang, D.; He, W.T.; Zhu, X.; Callaghan, S.; Yong, P.; et al. A human antibody reveals a conserved site on beta-coronavirus spike proteins and confers protection against SARS-CoV-2 infection. Sci. Transl. Med. 2022, 14, eabi9215. [Google Scholar] [CrossRef]
- Li, C.J.; Chao, T.L.; Chang, T.Y.; Hsiao, C.C.; Lu, D.C.; Chiang, Y.W.; Lai, G.C.; Tsai, Y.M.; Fang, J.T.; Ieong, S.; et al. Neutralizing Monoclonal Antibodies Inhibit SARS-CoV-2 Infection through Blocking Membrane Fusion. Microbiol. Spectr. 2022, 10, e0181421. [Google Scholar] [CrossRef]
- Fan, C.; Cohen, A.A.; Park, M.; Hung, A.F.; Keeffe, J.R.; Gnanapragasam, P.N.P.; Lee, Y.E.; Gao, H.; Kakutani, L.M.; Wu, Z.; et al. Neutralizing monoclonal antibodies elicited by mosaic RBD nanoparticles bind conserved sarbecovirus epitopes. Immunity 2022, 55, 2419–2435.e10. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Q.; Chang, F.; Yang, J.; Zhang, X.; Wang, Q.; Chen, J.; Teng, S.; Liu, Y.; Zheng, X.; et al. Broad cross neutralizing antibodies against sarbecoviruses generated by SARS-CoV-2 infection and vaccination in humans. NPJ Vaccines 2024, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Casner, R.G.; Nair, M.S.; Yu, J.; Guo, Y.; Wang, M.; Chan, J.F.; Cerutti, G.; Iketani, S.; Liu, L.; et al. A monoclonal antibody that neutralizes SARS-CoV-2 variants, SARS-CoV, and other sarbecoviruses. Emerg. Microbes Infect. 2022, 11, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Chia, W.N.; Tan, C.W.; Tan, A.W.K.; Young, B.; Starr, T.N.; Lopez, E.; Fibriansah, G.; Barr, J.; Cheng, S.; Yeoh, A.Y.; et al. Potent pan huACE2-dependent sarbecovirus neutralizing monoclonal antibodies isolated from a BNT162b2-vaccinated SARS survivor. Sci. Adv. 2023, 9, eade3470. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, W.; Liu, H.; Sang, Z.; Nambulli, S.; Tubiana, J.; Williams, K.L., Jr.; Duprex, W.P.; Schneidman-Duhovny, D.; Wilson, I.A.; et al. Superimmunity by pan-sarbecovirus nanobodies. Cell Rep. 2022, 39, 111004. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Liu, Y.; Xing, X.; Li, C.; Wang, X.; Shi, J.; Ma, W.; Li, J.; Chen, Y.; et al. Potent and broadly neutralizing antibodies against sarbecoviruses elicited by single ancestral SARS-CoV-2 infection. Commun. Biol. 2025, 8, 378. [Google Scholar] [CrossRef]
- Martinez, D.R.; Schafer, A.; Gobeil, S.; Li, D.; De la Cruz, G.; Parks, R.; Lu, X.; Barr, M.; Stalls, V.; Janowska, K.; et al. A broadly cross-reactive antibody neutralizes and protects against sarbecovirus challenge in mice. Sci. Transl. Med. 2022, 14, eabj7125. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Reddem, E.R.; Casner, R.G.; Nair, M.S.; Yu, J.; Chan, J.F.; Wang, M.; Cerutti, G.; et al. An antibody class with a common CDRH3 motif broadly neutralizes sarbecoviruses. Sci. Transl. Med. 2022, 14, eabn6859. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, R.; Chen, J.; Wei, J.; Wei, Z.; Yang, Z.; Zhu, K.; Yang, Y.; Yang, Q.; Liu, N.; et al. Super broad and protective nanobodies against Sarbecoviruses including SARS-CoV-1 and the divergent SARS-CoV-2 subvariant KP.3.1.1. PLoS Pathog. 2024, 20, e1012625. [Google Scholar] [CrossRef]
- Li, T.; Xue, W.; Zheng, Q.; Song, S.; Yang, C.; Xiong, H.; Zhang, S.; Hong, M.; Zhang, Y.; Yu, H.; et al. Cross-neutralizing antibodies bind a SARS-CoV-2 cryptic site and resist circulating variants. Nat. Commun. 2021, 12, 5652. [Google Scholar] [CrossRef]
- Starr, T.N.; Czudnochowski, N.; Liu, Z.; Zatta, F.; Park, Y.J.; Addetia, A.; Pinto, D.; Beltramello, M.; Hernandez, P.; Greaney, A.J.; et al. SARS-CoV-2 RBD antibodies that maximize breadth and resistance to escape. Nature 2021, 597, 97–102. [Google Scholar] [CrossRef]
- Hurtado, J.; Rogers, T.F.; Jaffe, D.B.; Adams, B.A.; Bangaru, S.; Garcia, E.; Capozzola, T.; Messmer, T.; Sharma, P.; Song, G.; et al. Deep repertoire mining uncovers ultra-broad coronavirus neutralizing antibodies targeting multiple spike epitopes. Cell Rep. 2024, 43, 114307. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.; De Marco, A.; Lempp, F.A.; Tortorici, M.A.; Pinto, D.; Walls, A.C.; Beltramello, M.; Chen, A.; Liu, Z.; Zatta, F.; et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell 2021, 184, 2332–2347.e16. [Google Scholar] [CrossRef] [PubMed]
- Hurlburt, N.K.; Homad, L.J.; Sinha, I.; Jennewein, M.F.; MacCamy, A.J.; Wan, Y.H.; Boonyaratanakornkit, J.; Sholukh, A.M.; Jackson, A.M.; Zhou, P.; et al. Structural definition of a pan-sarbecovirus neutralizing epitope on the spike S2 subunit. Commun. Biol. 2022, 5, 342. [Google Scholar] [CrossRef] [PubMed]
- Piepenbrink, M.S.; Park, J.G.; Deshpande, A.; Loos, A.; Ye, C.; Basu, M.; Sarkar, S.; Khalil, A.M.; Chauvin, D.; Woo, J.; et al. Potent universal beta-coronavirus therapeutic activity mediated by direct respiratory administration of a Spike S2 domain-specific human neutralizing monoclonal antibody. PLoS Pathog. 2022, 18, e1010691. [Google Scholar] [CrossRef]
- Sauer, M.M.; Tortorici, M.A.; Park, Y.J.; Walls, A.C.; Homad, L.; Acton, O.J.; Bowen, J.E.; Wang, C.; Xiong, X.; de van der Schueren, W.; et al. Structural basis for broad coronavirus neutralization. Nat. Struct. Mol. Biol. 2021, 28, 478–486. [Google Scholar] [CrossRef]
- Pinto, D.; Sauer, M.M.; Czudnochowski, N.; Low, J.S.; Tortorici, M.A.; Housley, M.P.; Noack, J.; Walls, A.C.; Bowen, J.E.; Guarino, B.; et al. Broad betacoronavirus neutralization by a stem helix-specific human antibody. Science 2021, 373, 1109–1116. [Google Scholar] [CrossRef]
- Wang, C.; van Haperen, R.; Gutierrez-Alvarez, J.; Li, W.; Okba, N.M.A.; Albulescu, I.; Widjaja, I.; van Dieren, B.; Fernandez-Delgado, R.; Sola, I.; et al. A conserved immunogenic and vulnerable site on the coronavirus spike protein delineated by cross-reactive monoclonal antibodies. Nat. Commun. 2021, 12, 1715. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, Z.; Li, Q.; Wang, B.; Yu, Y.; Wu, J.; Nie, J.; Ding, R.; Wang, H.; Zhang, Y.; et al. Ten emerging SARS-CoV-2 spike variants exhibit variable infectivity, animal tropism, and antibody neutralization. Commun. Biol. 2021, 4, 1196. [Google Scholar] [CrossRef]
- Jones, B.E.; Brown-Augsburger, P.L.; Corbett, K.S.; Westendorf, K.; Davies, J.; Cujec, T.P.; Wiethoff, C.M.; Blackbourne, J.L.; Heinz, B.A.; Foster, D.; et al. The neutralizing antibody, LY-CoV555, protects against SARS-CoV-2 infection in nonhuman primates. Sci. Transl. Med. 2021, 13, eabf1906. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Zhang, L.; Wan, S.; Zhang, L.; Zhou, F. SARS-CoV-2 Omicron variant: Recent progress and future perspectives. Signal Transduct. Target. Ther. 2022, 7, 141. [Google Scholar] [CrossRef]
- Fang, Y.; Sun, P.; Xie, X.; Du, M.; Du, F.; Ye, J.; Kalveram, B.K.; Plante, J.A.; Plante, K.S.; Li, B.; et al. An antibody that neutralizes SARS-CoV-1 and SARS-CoV-2 by binding to a conserved spike epitope outside the receptor binding motif. Sci. Immunol. 2022, 7, eabp9962. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, C.; Huang, A.; Xia, S.; Lu, S.; Shi, Z.; Lu, L.; Jiang, S.; Yang, Z.; Wu, Y.; et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020, 9, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, L.; Jiang, S. SARS-CoV-2 evolution from the BA.2.86 to JN.1 variants: Unexpected consequences. Trends Immunol. 2024, 45, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wu, H.; Hu, Y.; Zheng, X.; Chang, F.; Liu, Y.; Pan, Z.; Wang, Q.; Tang, F.; Qian, J.; et al. Immune evasion of Omicron variants JN.1, KP.2, and KP.3 to the polyclonal and monoclonal antibodies from COVID-19 convalescents and vaccine recipients. Antivir. Res. 2025, 235, 106092. [Google Scholar] [CrossRef]
- Li, P.; Faraone, J.N.; Hsu, C.C.; Chamblee, M.; Zheng, Y.M.; Carlin, C.; Bednash, J.S.; Horowitz, J.C.; Mallampalli, R.K.; Saif, L.J.; et al. Neutralization escape, infectivity, and membrane fusion of JN.1-derived SARS-CoV-2 SLip, FLiRT, and KP.2 variants. Cell Rep. 2024, 43, 114520. [Google Scholar] [CrossRef]
- Zhou, B.; Gui, Q.; Liu, C.; Guo, H.; Wang, H.; Cheng, L.; Fan, Q.; Ge, X.; Zhang, Z.; Ju, B. Structure and function of an unusual R452-dependent monoclonal antibody against SARS-CoV-2. J. Virol. 2025, 99, e0184424. [Google Scholar] [CrossRef]
- Chi, X.; Yan, R.; Zhang, J.; Zhang, G.; Zhang, Y.; Hao, M.; Zhang, Z.; Fan, P.; Dong, Y.; Yang, Y.; et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020, 369, 650–655. [Google Scholar] [CrossRef]
- Noy-Porat, T.; Mechaly, A.; Levy, Y.; Makdasi, E.; Alcalay, R.; Gur, D.; Aftalion, M.; Falach, R.; Leviatan Ben-Arye, S.; Lazar, S.; et al. Therapeutic antibodies, targeting the SARS-CoV-2 spike N-terminal domain, protect lethally infected K18-hACE2 mice. iScience 2021, 24, 102479. [Google Scholar] [CrossRef]
- Liu, B.; Liu, H.; Han, P.; Wang, X.; Wang, C.; Yan, X.; Lei, W.; Xu, K.; Zhou, J.; Qi, J.; et al. Enhanced potency of an IgM-like nanobody targeting conserved epitope in SARS-CoV-2 spike N-terminal domain. Signal Transduct. Target. Ther. 2024, 9, 131. [Google Scholar] [CrossRef]
- Liu, L.; Wang, P.; Nair, M.S.; Yu, J.; Rapp, M.; Wang, Q.; Luo, Y.; Chan, J.F.; Sahi, V.; Figueroa, A.; et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020, 584, 450–456. [Google Scholar] [CrossRef]
- Cao, Y.; Song, W.; Wang, L.; Liu, P.; Yue, C.; Jian, F.; Yu, Y.; Yisimayi, A.; Wang, P.; Wang, Y.; et al. Characterization of the enhanced infectivity and antibody evasion of Omicron BA.2.75. Cell Host Microbe 2022, 30, 1527–1539.e5. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody resistance of SARS-CoV-2 variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, G.; Guo, Y.; Wang, P.; Nair, M.S.; Wang, M.; Huang, Y.; Yu, J.; Liu, L.; Katsamba, P.S.; Bahna, F.; et al. Neutralizing antibody 5–7 defines a distinct site of vulnerability in SARS-CoV-2 spike N-terminal domain. Cell Rep. 2021, 37, 109928. [Google Scholar] [CrossRef] [PubMed]
- Kaku, Y.; Okumura, K.; Kawakubo, S.; Uriu, K.; Chen, L.; Kosugi, Y.; Uwamino, Y.; Begum, M.M.; Leong, S.; Ikeda, T.; et al. Virological characteristics of the SARS-CoV-2 XEC variant. Lancet Infect. Dis. 2024, 24, e736. [Google Scholar] [CrossRef]
- Li, P.; Faraone, J.N.; Hsu, C.C.; Chamblee, M.; Liu, Y.; Zheng, Y.M.; Xu, Y.; Carlin, C.; Horowitz, J.C.; Mallampalli, R.K.; et al. Role of glycosylation mutations at the N-terminal domain of SARS-CoV-2 XEC variant in immune evasion, cell-cell fusion, and spike stability. J. Virol. 2025, 99, e0024225. [Google Scholar] [CrossRef]
- Kaku, Y.; Uriu, K.; Okumura, K.; Genotype to Phenotype Japan, C.; Ito, J.; Sato, K. Virological characteristics of the SARS-CoV-2 KP.3.1.1 variant. Lancet Infect. Dis. 2024, 24, e609. [Google Scholar] [CrossRef]
- Alshahrani, M.; Parikh, V.; Foley, B.; Raisinghani, N.; Verkhivker, G. Mutational Scanning and Binding Free Energy Computations of the SARS-CoV-2 Spike Complexes with Distinct Groups of Neutralizing Antibodies: Energetic Drivers of Convergent Evolution of Binding Affinity and Immune Escape Hotspots. Int. J. Mol. Sci. 2025, 26, 1507. [Google Scholar] [CrossRef]
- Feng, Z.; Huang, J.; Baboo, S.; Diedrich, J.K.; Bangaru, S.; Paulson, J.C.; Yates, J.R., 3rd; Yuan, M.; Wilson, I.A.; Ward, A.B. Structural and Functional Insights into the Evolution of SARS-CoV-2 KP.3.1.1 Spike Protein. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cai, Z.; Ni, W.; Li, W.; Wu, Z.; Yao, X.; Zheng, Y.; Zhao, Y.; Yuan, W.; Liang, S.; Wang, Q.; et al. SARS-CoV-2 S protein disrupts the formation of ISGF3 complex through conserved S2 subunit to antagonize type I interferon response. J. Virol. 2025, 99, e0151624. [Google Scholar] [CrossRef]
- Brouwer, P.J.M.; Caniels, T.G.; van der Straten, K.; Snitselaar, J.L.; Aldon, Y.; Bangaru, S.; Torres, J.L.; Okba, N.M.A.; Claireaux, M.; Kerster, G.; et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 2020, 369, 643–650. [Google Scholar] [CrossRef]
- Guo, L.; Lin, S.; Chen, Z.; Cao, Y.; He, B.; Lu, G. Targetable elements in SARS-CoV-2 S2 subunit for the design of pan-coronavirus fusion inhibitors and vaccines. Signal Transduct. Target. Ther. 2023, 8, 197. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.; Zhang, X. Neutralizing antibodies for the prevention and treatment of COVID-19. Cell. Mol. Immunol. 2021, 18, 2293–2306. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Liu, Y.; Mao, Q.; Li, J.; Lu, Y.; Shi, J.; Li, C.; Yu, J.; Gong, J.; Wang, X.; et al. Novel Trispecific Neutralizing Antibodies with Enhanced Potency and Breadth Against Pan-Sarbecoviruses. MedComm 2025, 6, e70191. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Li, C.; Zhang, Z.; Huang, Y.; Liu, B.; Zhang, Z.; Luo, J.; Wang, Q.; Yin, L.; Chen, S.; et al. A shark-derived broadly neutralizing nanobody targeting a highly conserved epitope on the S2 domain of sarbecoviruses. J. Nanobiotechnol. 2025, 23, 110. [Google Scholar] [CrossRef]
- Li, M.; Lou, F.; Fan, H. SARS-CoV-2 variant Omicron: Currently the most complete “escapee” from neutralization by antibodies and vaccines. Signal Transduct. Target. Ther. 2022, 7, 28. [Google Scholar] [CrossRef]
- Volz, E.M.; Koelle, K.; Bedford, T. Viral phylodynamics. PLoS Comput. Biol. 2013, 9, e1002947. [Google Scholar] [CrossRef]
- Hu, Y.F.; Zhang, B.Z.; Chu, H.; Huang, J.D. Distinct evolution patterns of influenza viruses and implications for vaccine development. Innovation 2025, 6, 100739. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, J.; Yuan, R.Y.; Wang, M.Y.; He, P.; Su, J.G.; Han, Z.B.; Jin, Y.Q.; Hou, J.W.; Zhang, H.; et al. Design of a mutation-integrated trimeric RBD with broad protection against SARS-CoV-2. Cell Discov. 2022, 8, 17. [Google Scholar] [CrossRef]
- Guthmiller, J.J.; Yu-Ling Lan, L.; Li, L.; Fu, Y.; Nelson, S.A.; Henry, C.; Stamper, C.T.; Utset, H.A.; Freyn, A.W.; Han, J.; et al. Long-lasting B cell convergence to distinct broadly reactive epitopes following vaccination with chimeric influenza virus hemagglutinins. Immunity 2025, 58, 980–996.e7. [Google Scholar] [CrossRef]
- Cohen, A.A.; van Doremalen, N.; Greaney, A.J.; Andersen, H.; Sharma, A.; Starr, T.N.; Keeffe, J.R.; Fan, C.; Schulz, J.E.; Gnanapragasam, P.N.P.; et al. Mosaic RBD nanoparticles protect against challenge by diverse sarbecoviruses in animal models. Science 2022, 377, eabq0839. [Google Scholar] [CrossRef]
- Yang, J.; Liu, M.Q.; Liu, L.; Li, X.; Xu, M.; Lin, H.; Liu, S.; Hu, Y.; Li, B.; Liu, B.; et al. A triple-RBD-based mucosal vaccine provides broad protection against SARS-CoV-2 variants of concern. Cell. Mol. Immunol. 2022, 19, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.P.; Hassler, H.B.; Sah, P.; Galvani, A.P.; Dornburg, A. The durability of natural infection and vaccine-induced immunity against future infection by SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2022, 119, e2204336119. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Abolhassani, H.; Du, L.; Piralla, A.; Bertoglio, F.; de Campos-Mata, L.; Wan, H.; Schubert, M.; Cassaniti, I.; Wang, Y.; et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat. Commun. 2022, 13, 2670. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Walker, L.S.K.; Liu, Z.; Linterman, M.A.; Li, Z. Targeting T(FH) cells in human diseases and vaccination: Rationale and practice. Nat. Immunol. 2022, 23, 1157–1168. [Google Scholar] [CrossRef]
- Lin, G.; Tang, Y.L.; Fu, Z.; Chen, R.; Liu, Y.; Liu, Z.; Kuang, X.; Sun, J.; Zhao, J.; Zhang, Y. Enhancing protective immunity against SARS-CoV-2 with a self-amplifying RNA lipid nanoparticle vaccine. J. Control. Release 2025, 378, 250–265. [Google Scholar] [CrossRef]
- Low, J.G.; de Alwis, R.; Chen, S.; Kalimuddin, S.; Leong, Y.S.; Mah, T.K.L.; Yuen, N.; Tan, H.C.; Zhang, S.L.; Sim, J.X.Y.; et al. A phase I/II randomized, double-blinded, placebo-controlled trial of a self-amplifying COVID-19 mRNA vaccine. NPJ Vaccines 2022, 7, 161. [Google Scholar] [CrossRef]
- Oda, Y.; Kumagai, Y.; Kanai, M.; Iwama, Y.; Okura, I.; Minamida, T.; Yagi, Y.; Kurosawa, T.; Greener, B.; Zhang, Y.; et al. Immunogenicity and safety of a booster dose of a self-amplifying RNA COVID-19 vaccine (ARCT-154) versus BNT162b2 mRNA COVID-19 vaccine: A double-blind, multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet Infect. Dis. 2024, 24, 351–360. [Google Scholar] [CrossRef]
- Ren, C.; Gao, Y.; Zhang, C.; Zhou, C.; Hong, Y.; Qu, M.; Zhao, Z.; Du, Y.; Yang, L.; Liu, B.; et al. Respiratory Mucosal Immunity: Kinetics of Secretory Immunoglobulin A in Sputum and Throat Swabs From COVID-19 Patients and Vaccine Recipients. Front. Microbiol. 2022, 13, 782421. [Google Scholar] [CrossRef]
- Lapuente, D.; Winkler, T.H.; Tenbusch, M. B-cell and antibody responses to SARS-CoV-2: Infection, vaccination, and hybrid immunity. Cell. Mol. Immunol. 2024, 21, 144–158. [Google Scholar] [CrossRef]
- Kaetzel, C.S.; Robinson, J.K.; Chintalacharuvu, K.R.; Vaerman, J.P.; Lamm, M.E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: A local defense function for IgA. Proc. Natl. Acad. Sci. USA 1991, 88, 8796–8800. [Google Scholar] [CrossRef]
- Li, J.X.; Wu, S.P.; Guo, X.L.; Tang, R.; Huang, B.Y.; Chen, X.Q.; Chen, Y.; Hou, L.H.; Liu, J.X.; Zhong, J.; et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: A randomised, open-label, single-centre trial. Lancet Respir. Med. 2022, 10, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Liu, Y.; He, Q.; Pan, H.; Liang, Z.; Zhou, J.; Pan, Y.; Liu, S.; Wu, J.; Yang, K.; et al. Effectiveness of a booster dose of aerosolized or intramuscular adenovirus type 5 vectored COVID-19 vaccine in adults: A multicenter, partially randomized, platform trial in China. Nat. Commun. 2025, 16, 2969. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Verma, S.; Reddy, P.; Diamond, M.S.; Curiel, D.T.; Patel, C.; Jain, M.K.; Redkar, S.V.; Bhate, A.S.; Gundappa, V.; et al. Phase III Pivotal comparative clinical trial of intranasal (iNCOVACC) and intramuscular COVID 19 vaccine (Covaxin((R))). NPJ Vaccines 2023, 8, 125. [Google Scholar] [CrossRef]
- Pitcovski, J.; Gruzdev, N.; Abzach, A.; Katz, C.; Ben-Adiva, R.; Brand-Shwartz, M.; Yadid, I.; Ratzon-Ashkenazi, E.; Emquies, K.; Israeli, H.; et al. Oral subunit SARS-CoV-2 vaccine induces systemic neutralizing IgG, IgA and cellular immune responses and can boost neutralizing antibody responses primed by an injected vaccine. Vaccine 2022, 40, 1098–1107. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, E.; Yang, J.; Yang, Y.; Yu, J.; Xiao, Y.; Li, W.; Zhou, D.; Li, Y.; Zhao, B.; et al. Frontline Science: Nasal epithelial GM-CSF contributes to TLR5-mediated modulation of airway dendritic cells and subsequent IgA response. J. Leukoc. Biol. 2017, 102, 575–587. [Google Scholar] [CrossRef]
- Watson, A.; Wilkinson, T.M.A. Respiratory viral infections in the elderly. Ther. Adv. Respir. Dis. 2021, 15, 1753466621995050. [Google Scholar] [CrossRef]
- Ye, Q.; Wu, M.; Zhou, C.; Lu, X.; Huang, B.; Zhang, N.; Zhao, H.; Chi, H.; Zhang, X.; Ling, D.; et al. Rational development of a combined mRNA vaccine against COVID-19 and influenza. NPJ Vaccines 2022, 7, 84. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, H.; Forgacs, D.; Ross, T.M. Flu-COVID combo recombinant protein vaccines elicited protective immune responses against both influenza and SARS-CoV-2 viruses infection. Vaccine 2024, 42, 1184–1192. [Google Scholar] [CrossRef]
- Dulin, H.; Barre, R.S.; Xu, D.; Neal, A.; Vizcarra, E.; Chavez, J.; Ulu, A.; Yang, M.S.; Khan, S.R.; Wuang, K.; et al. Harnessing preexisting influenza virus-specific immunity increases antibody responses against SARS-CoV-2. J. Virol. 2024, 98, e0157123. [Google Scholar] [CrossRef]
- Aliprantis, A.O.; Shaw, C.A.; Griffin, P.; Farinola, N.; Railkar, R.A.; Cao, X.; Liu, W.; Sachs, J.R.; Swenson, C.J.; Lee, H.; et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum. Vaccines Immunother. 2021, 17, 1248–1261. [Google Scholar] [CrossRef]
- Bonam, S.R.; Hu, H. Next-Generation Vaccines Against COVID-19 Variants: Beyond the Spike Protein. Zoonoses 2023, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rice, A.; Verma, M.; Shin, A.; Zakin, L.; Sieling, P.; Tanaka, S.; Balint, J.; Dinkins, K.; Adisetiyo, H.; Morimoto, B.; et al. Intranasal plus subcutaneous prime vaccination with a dual antigen COVID-19 vaccine elicits T-cell and antibody responses in mice. Sci. Rep. 2021, 11, 14917. [Google Scholar] [CrossRef] [PubMed]
- Hajnik, R.L.; Plante, J.A.; Liang, Y.; Alameh, M.G.; Tang, J.; Bonam, S.R.; Zhong, C.; Adam, A.; Scharton, D.; Rafael, G.H.; et al. Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models. Sci. Transl. Med. 2022, 14, eabq1945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liang, Q.; He, X.; Zhao, C.; Ren, W.; Yang, Z.; Wang, Z.; Ding, Q.; Deng, H.; Wang, T.; et al. Loss of Spike N370 glycosylation as an important evolutionary event for the enhanced infectivity of SARS-CoV-2. Cell Res. 2022, 32, 315–318. [Google Scholar] [CrossRef]
- Madan-Lala, R.; Pradhan, P.; Roy, K. Combinatorial Delivery of Dual and Triple TLR Agonists via Polymeric Pathogen-like Particles Synergistically Enhances Innate and Adaptive Immune Responses. Sci. Rep. 2017, 7, 2530. [Google Scholar] [CrossRef]
- Antanasijevic, A.; Bowman, C.A.; Kirchdoerfer, R.N.; Cottrell, C.A.; Ozorowski, G.; Upadhyay, A.A.; Cirelli, K.M.; Carnathan, D.G.; Enemuo, C.A.; Sewall, L.M.; et al. From structure to sequence: Antibody discovery using cryoEM. Sci. Adv. 2022, 8, eabk2039. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Lin, A.; Xu, C.; Li, Z.; Liu, K.; Liu, B.; Ma, X.; Zhao, F.; Jiang, H.; et al. Algorithm for optimized mRNA design improves stability and immunogenicity. Nature 2023, 621, 396–403. [Google Scholar] [CrossRef]
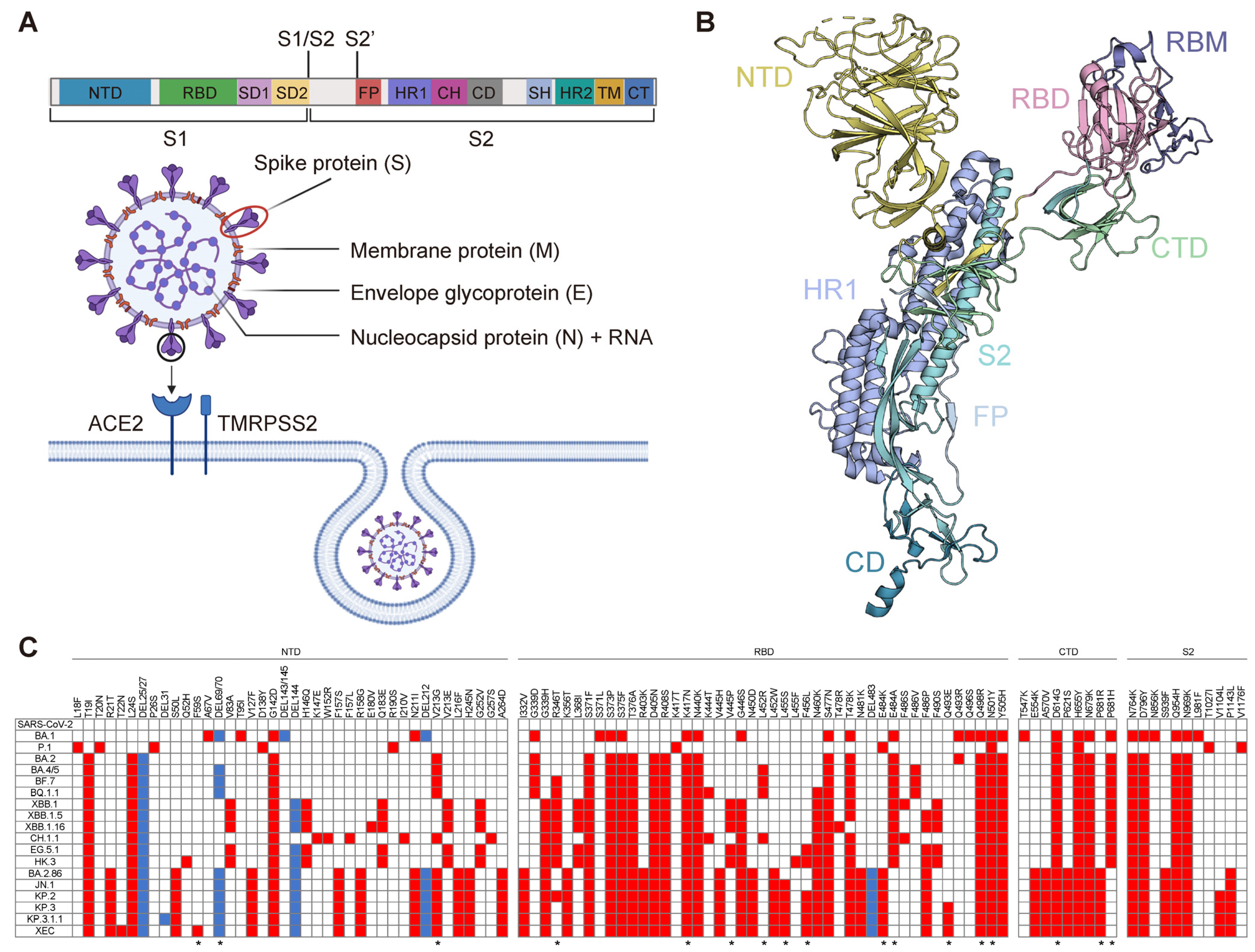
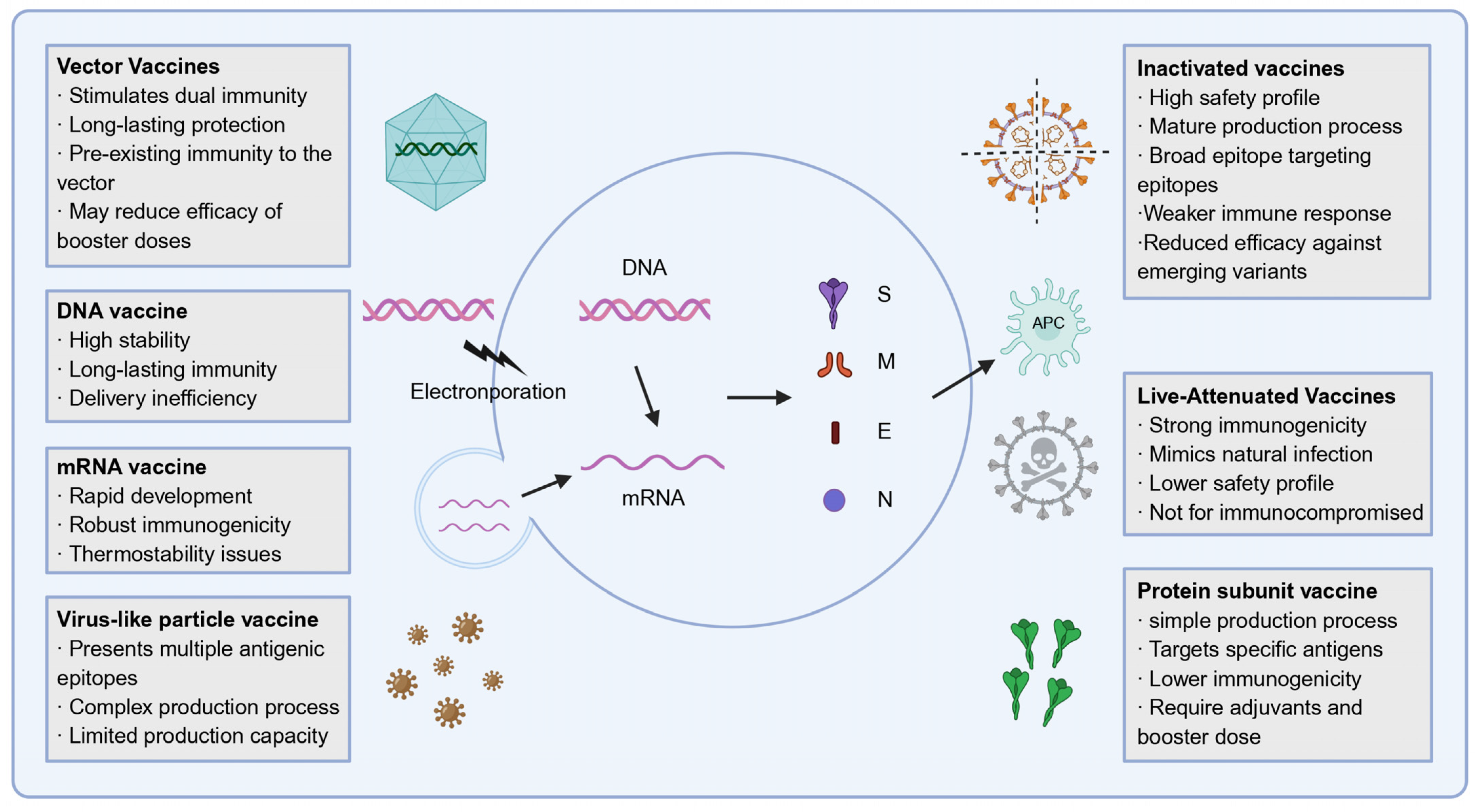
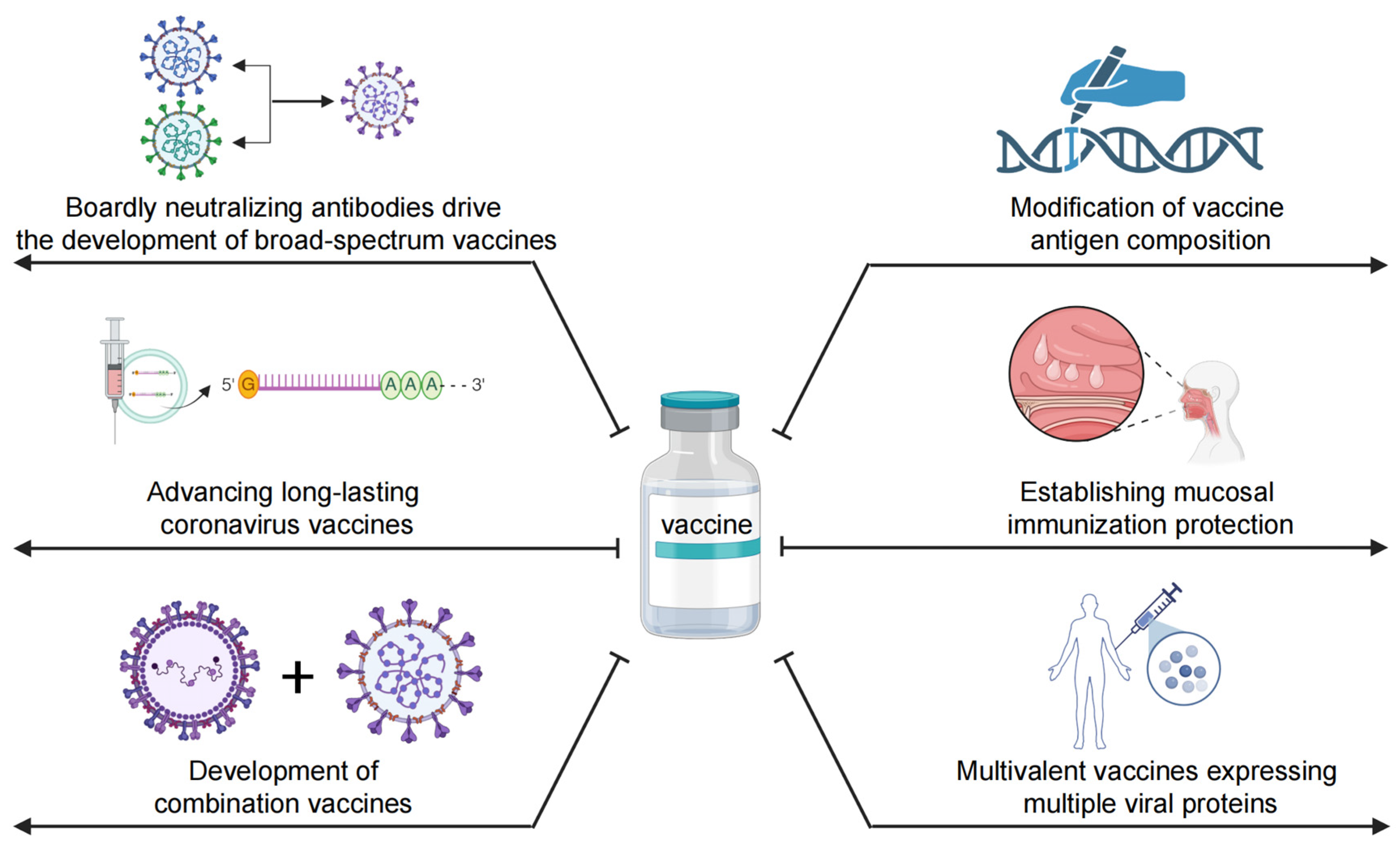
| No. | Name | Epitope | Detailed Information | Antibody Type | Developers | Article |
|---|---|---|---|---|---|---|
| 1 | Bamlanivimab | RBD | Isolated from PBMCs, obtained EUA in November 2020, restricted in April 2022 due to reduced Omicron efficacy | IgG | Chen P, et al. | [161] |
| 2 | Etesevimab | Isolated from PBMCs, obtained EUA in Feb 2021, modified CB6 enhanced neutralization of Omicron sublineages | IgG | Shi R, et al. | [162] | |
| 3 | REGN10933/10987 | Cocktail therapy with antibodies binding to RBD is ineffective against Omicron | IgG | Baum A, et al. | [163] | |
| 4 | Sotrovimab | Derived from S309, targets a conserved epitope on the SARS-CoV-2 RBD | IgG | Pinto D, et al. | [164] | |
| 5 | Tixagevimab | Combines with Cilgavimab enhances broad-spectrum neutralization against SARS-CoV-2 variants | IgG | Zost SJ, et al. | [165] | |
| 6 | Cilgavimab | Has strong neutralization against SARS-CoV-2 variants, binds non-overlapping epitopes with Tixagevimab | ||||
| 7 | Bebtelovimab | Neutralizes SARS-CoV-2 variants (Omicron, BA.2, Delta), binds key RBD residues for ACE2 interaction | IgG | Westendorf K, et al. | [166] | |
| 8 | Regdanvimab | Neutralizes SARS-CoV-2 and VOCs variants, targets key RBD overlapping ACE2 region | IgG | Kim C, et al. | [167] | |
| 9 | CR9 | Neutralizes SARS-CoV-2 and Omicron subvariants, inhibits viral replication | IgG | Chen Z, et al. | [168] | |
| 10 | BRII-196 | Targets SARS-CoV-2 RBD, blocking virus-ACE2 interaction | IgG | Ju B, et al. | [169] | |
| 11 | BRII-198 | Targets SARS-CoV-2 RBD, blocking virus-ACE2 interaction | ||||
| 12 | 7F | Targets conserved RBD, neutralizes SARS-CoV-2, SARS-CoV-1, WIV16 | VHH | Swart IC, et al. | [170] | |
| 13 | RBD-chAb-45 | Chimeric antibody targeting SARS-CoV-2 RBD, effectively neutralize SARS-CoV-2 | Humanized mAb | Liang KH, et al. | [171] | |
| 14 | C1596 | NTD | Recognizes NTD epitope, binds multiple Omicron subvariants | IgG | Rubio AA, et al. | [172] |
| 15 | SARS2-57 | Binds NTD loops, binds Alpha, Gamma, Delta, but not Beta, Omicron BA.1 | Murine mAb | Adams LJ, et al. | [173] | |
| 16 | C1717 | Recognizes NTD and SD2 near viral membrane, neutralizes Beta, Gamma, and Omicron | IgG | Wang Z, et al. | [174] | |
| 17 | BD58-0730/0771/ 0784/0786/0790 | Bind a unique epitope on the N1/N2 loop of NTD, neutralize Omicron sub-lineages | IgG | Niu X, et al. | [175] | |
| 18 | 3711 | Targets the silent face of the NTD, shows efficient neutralization against SARS-CoV-2 | IgG | Zhang Z, et al. | [176] | |
| 19 | NT-193 | Binds NTD N3/N5 loops, neutralizes WA1, Beta, Alpha, Gamma and original Wuhan strain | Humanized mAb | Onodera T, et al. | [177] | |
| 20 | WS6 | S2 | Targets conserved S2 epitope, neutralizes SARS-CoV-1 and SARS-CoV-2 | Murine mAb | Shi W, et al. | [178] |
| 21 | CC40.8 | Targets S2 stem helix, neutralizes SARS-CoV-2 variants and SARS-CoV-1 | IgG | Zhou P, et al. | [179] | |
| 22 | S2-4D/5D/8D | Recognizes a conserved epitope in the S2 subunit, neutralizes of diverse SARS-CoV-2 variants | Murine mAb | Li C, et al. | [180] | |
| 23 | S2-4A | Inhibits membrane fusion, neutralizes SARS-CoV-2 variants, Targets residues such as E1144 and F1148 |
| No. | Name | Epitope | Detailed Information | Antibody Type | Developers | Article |
|---|---|---|---|---|---|---|
| 1 | M8a-3/-31/-34 | RBD | Show cross-reactive against SARS-CoV-2 variants and animal sarbecoviruses | Humanized mAb | Fan C, et al. | [181] |
| 2 | SCM12-61/13-65 VSM9-12/44/8-83/16-12 | IC50 values for 8 Sarbecoviruses in clade 1a and 1b range from 0.001 to 1.65 μg/mL | IgG | Hu Y, et al. | [182] | |
| 3 | 2-36 | Neutralizes clade 1 and 2 sarbecovirus, IC50 values range from 0.002 to 0.658 μg/mL | IgG | Wang P, et al. | [183] | |
| 4 | E7 | Shows ultrapotent activity against all sarbecoviruses via quaternary structure | IgG | Chia WN, et al. | [184] | |
| 5 | 2-10, 2-31, 2-45, 2-67 | Neutralization potency varies, with 2-67 showing reduced efficacy against clade 1b sarbecoviruses | VHH | Xiang Y, et al. | [185] | |
| 6 | CYFN1006-1/1006-2 | CYFN1006-1 neutralizes SARS-CoV-2 variants, SARS-CoV-1 and JN.1 subvariant; CYFN1006-2 neutralizes SARS-CoV-2 variants less effectively than SARS-CoV-1 | IgG | Yu L, et al. | [186] | |
| 7 | DH1047 | Neutralizes SARS-CoV-2 2AA MA, SARS-CoV, bat coronaviruses WIV-1 and RsSHC014 | IgG | Martinez DR, et al. | [187] | |
| 8 | 10-40 | Broadly neutralizes clade 1 sarbecovirus, provides protection against SARS-CoV-2 and SARS-CoV | IgG | Liu L, et al. | [188] | |
| 9 | PW5-4, 5-5, 5-535 | show neutralizing activity against SARS-CoV-2 variants, SARS-CoV and clade 1 sarbecoviruses | IgG | Zhao X, et al. | [22] | |
| 10 | Tnb04-01, Tnb03 | Targets the SARS-CoV-2 RBD, showing potent activity via a conserved pocket | VHH | Dong H, et al. | [189] | |
| 11 | 6D6, 7D6 | Target cryptic RBD site, cross-neutralize clade 1/2 and clade 3 | Murine mAb | Li T, et al. | [190] | |
| 12 | S2H97 | Targets the core RBD and neutralizes GD-Pangolin, GX-Pangolin, WIV1 and SARS-CoV-1 | IgG | Starr, T.N, et al. | [191] | |
| 13 | TXG-0078 | NTD | Binds N3/N5 loops of NTD, recognizes α/β-coronaviruses, shows broad binding and neutralization | IgG | Jonathan H, et al. | [192] |
| 14 | S2L28/S2M28/S2X333 | Bind antigenic supersite on the pinnacle of the NTD, and neutralize SARS-CoV-2 and RaTG13 | IgG | McCallum M, et al. | [193] | |
| 15 | 76E1 | S2 | Neutralizes SARS-CoV-2 and variants, cross-binds other human and γ/δ-coronaviruses | IgG | Sun X, et al. | [37] |
| 16 | CV3-25 | Binds conserved stem-helix, neutralizes SARS CoV-1, SARS-CoV-2 and WIV1 | IgG | Hurlburt NK, et al. | [194] | |
| 17 | 1249A8 | Binds S2 domain (residues 1131–1171), neutralizes MERS-CoV, SARS CoV-1 and SARS-CoV-2 | IgG | Piepenbrink MS, et al. | [195] | |
| 18 | B6 | Recognizes linear epitope in S2 stem helix, shows cross-reactivity from lineages A, B and C | Murine mAb | Sauer MM, et al. | [196] | |
| 19 | S2P6 | Targets stem helix, shows broad neutralization against sarbecoviruses, merbecovirus and embecovirus | IgG | Pinto D, et al. | [197] | |
| 20 | 28D9 | Displayed cross-reactivity and reacted with merbecovirus, sarbecovirus and embecovirus | Humanized mAb | Wang C, et al. | [198] | |
| 21 | COV44-62 | Inhibits membrane fusion, neutralizes MERS-CoV, α-CoVs and β-CoVs | IgG | Dacon C, et al. | [38] | |
| 22 | COV44-79 | Inhibits membrane fusion, neutralizes α-CoVs and β-CoVs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, R.; Li, J.; Gong, J.; Shao, Y.; Yu, J.; Chen, Y.; Lu, Y.; Yang, L.; Lin, L.; Hu, Z.; et al. Evolving SARS-CoV-2 Vaccines: From Current Solutions to Broad-Spectrum Protection. Vaccines 2025, 13, 635. https://doi.org/10.3390/vaccines13060635
Qiao R, Li J, Gong J, Shao Y, Yu J, Chen Y, Lu Y, Yang L, Lin L, Hu Z, et al. Evolving SARS-CoV-2 Vaccines: From Current Solutions to Broad-Spectrum Protection. Vaccines. 2025; 13(6):635. https://doi.org/10.3390/vaccines13060635
Chicago/Turabian StyleQiao, Rui, Jiayan Li, Jiami Gong, Yuchen Shao, Jizhen Yu, Yumeng Chen, Yinying Lu, Luxuan Yang, Luanfeng Lin, Zixin Hu, and et al. 2025. "Evolving SARS-CoV-2 Vaccines: From Current Solutions to Broad-Spectrum Protection" Vaccines 13, no. 6: 635. https://doi.org/10.3390/vaccines13060635
APA StyleQiao, R., Li, J., Gong, J., Shao, Y., Yu, J., Chen, Y., Lu, Y., Yang, L., Lin, L., Hu, Z., Wang, P., Zhao, X., & Zhang, W. (2025). Evolving SARS-CoV-2 Vaccines: From Current Solutions to Broad-Spectrum Protection. Vaccines, 13(6), 635. https://doi.org/10.3390/vaccines13060635







