Post-Marketing Surveillance of Nirsevimab: Safety Profile and Adverse Event Analysis from Spain’s 2023–2024 RSV Immunisation Campaign
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Population and Period
2.2. Case Definition
2.3. Data Sources
2.4. Study Variables and Statistical Analysis
2.5. Ethical Considerations
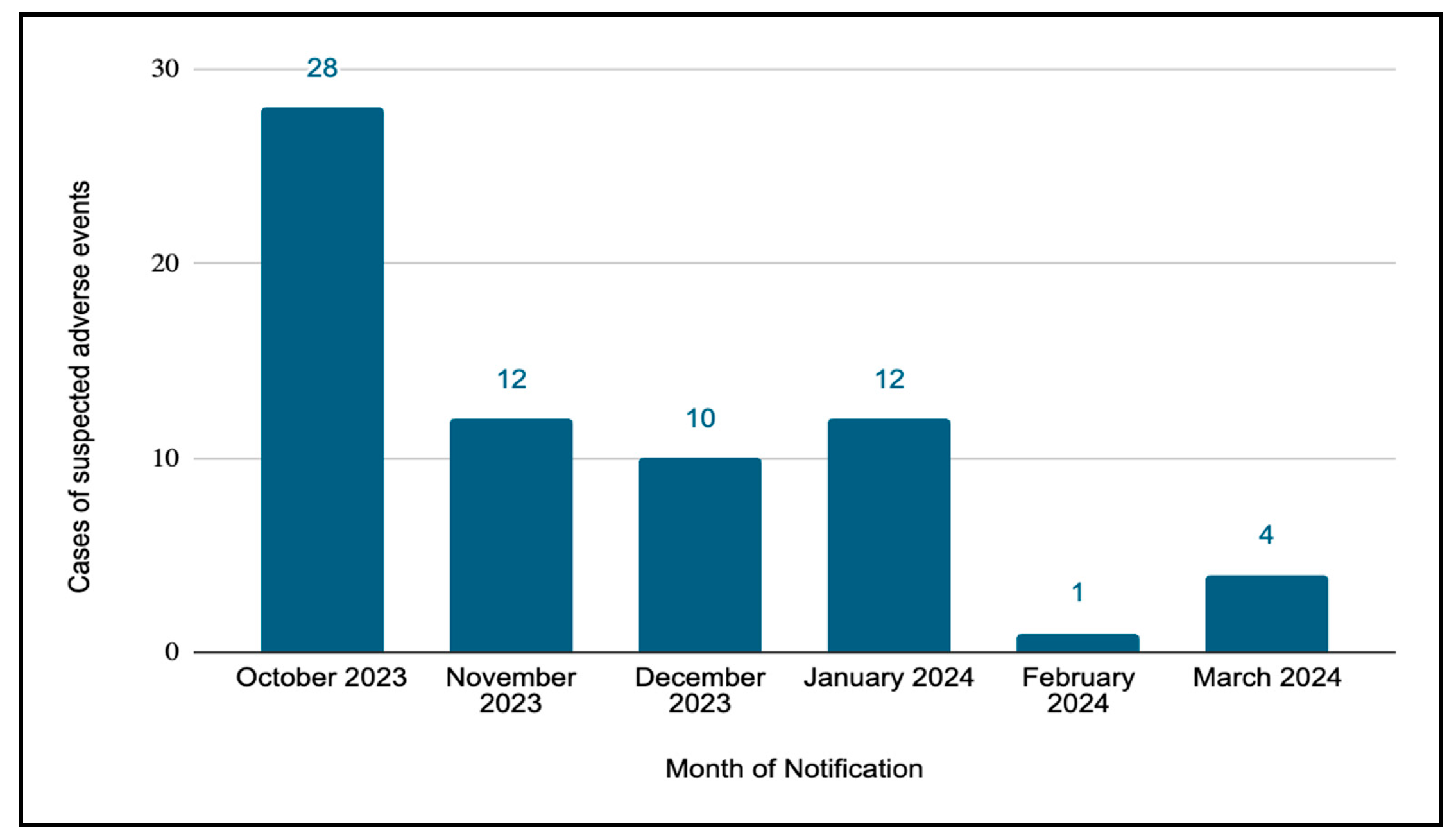
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RSV | Respiratory syncytial virus |
| SAEs | Suspected adverse events |
| LRTI | Lower respiratory tract infection |
| PT | Preferred term |
| SOC | System organ class |
References
- Kieffer, A.; Beuvelet, M.; Sardesai, A.; Musci, R.; Milev, S.; Roiz, J.; Lee, J.K.H. Expected Impact of Universal Immunization with Nirsevimab Against RSV-Related Outcomes and Costs Among All US Infants in Their First RSV Season: A Static Model. J. Infect. Dis. 2022, 226 (Suppl. S2), S282–S292. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). European Union Risk Management Plan (EU RMP) for Beyfortus® (Nirsevimab) [Internet]. 2022. Available online: https://www.ema.europa.eu/en/documents/rmp/beyfortus-epar-risk-management-plan_en.pdf (accessed on 9 August 2024).
- Ministerio de Sanidad. Enfermedad por Virus Respiratorio Sincitial (VRS) [Internet]. 2023. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/Nirsevimab_2023.pdf (accessed on 9 August 2024).
- Gonzales, T.; Bergamasco, A.; Cristarella, T.; Goyer, C.; Wojdyla, M.; Oladapo, A.; Sawicky, J.; Yee, J.; Moride, Y. Effectiveness and Safety of Palivizumab for the Prevention of Serious Lower Respiratory Tract Infection Caused by Respiratory Syncytial Virus: A Systematic Review. Am. J. Perinatol. 2023, 41, e1107–e1115. [Google Scholar] [CrossRef]
- O’Hagan, S.; Galway, N.; Shields, M.D.; Mallett, P.; Groves, H.E. Review of the Safety, Efficacy and Tolerability of Palivizumab in the Prevention of Severe Respiratory Syncytial Virus (RSV) Disease. Drug Healthc. Patient Saf. 2023, 15, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.; Cruz-Cañete, M.; Pérez, C.; Couceiro, J.A.; Otheo, E.; Launes, C.; Rodrigo, C.; Jiménez, A.B.; Llorente, M.; Montesdeoca, A.; et al. Nirsevimab for the prevention of respiratory syncytial virus disease in children. Statement of the Spanish Society of Paediatric Infectious Disease (SEIP). An. Pediatría Engl. Ed. 2023, 99, 257–263. [Google Scholar] [CrossRef]
- López-Lacort, M.; Muñoz-Quiles, C.; Mira-Iglesias, A.; López-Labrador, F.X.; Mengual-Chuliá, B.; Fernández-García, C.; Carballido-Fernández, M.; Pineda-Caplliure, A.; Mollar-Maseres, J.; Benavent, M.S.; et al. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Eurosurveillance 2024, 29, 2400046. [Google Scholar] [CrossRef]
- Ministerio de Sanidad. Recomendaciones de utilización de nirsevimab para la temporada 2024-2025 en España [Internet]. 2024. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/Nirsevimab.pdf (accessed on 9 August 2024).
- Estrella-Porter, P.; Blanco-Calvo, C.; Lameiras-Azevedo, A.S.; Juaneda, J.; Fernández-Martínez, S.; Gómez-Pajares, F.; Tempelsman, R.; Roig-Sena, F.J.; Pérez-Panades, J.; Botella-Rocamora, P.; et al. Effectiveness of nirsevimab introduction against respiratory syncytial virus in the Valencian Community: A preliminary assessment. Vaccine 2024, 42, 126030. Available online: https://www.sciencedirect.com/science/article/pii/S0264410X24006571 (accessed on 31 July 2024). [CrossRef]
- Ezpeleta, G.; Navascués, A.; Viguria, N.; Herranz-Aguirre, M.; Juan Belloc, S.E.; Gimeno Ballester, J.; Carlos Muruzábal, J.; García-Cenoz, M.; Trobajo-Sanmartín, C.; Echeverria, A.; et al. Effectiveness of Nirsevimab Immunoprophylaxis Administered at Birth to Prevent Infant Hospitalisation for Respiratory Syncytial Virus Infection: A Population-Based Cohort Study. Vaccines 2024, 12, 383. [Google Scholar] [CrossRef]
- Ares-Gómez, S.; Mallah, N.; Santiago-Pérez, M.I.; Pardo-Seco, J.; Pérez-Martínez, O.; Otero-Barrós, M.T.; Suárez-Gaiche, N.; Kramer, R.; Jin, J.; Platero-Alonso, L.; et al. Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia, Spain: Initial results of a population-based longitudinal study. Lancet Infect. Dis. 2024, 24, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Barbas Del Buey, J.F.; Íñigo Martínez, J.; Gutiérrez Rodríguez, M.Á.; Alonso García, M.; Sánchez-Gómez, A.; Lasheras Carbajo, M.D.; Bueno, S.J.; Vasallo, M.D.E.; Zambrano, M.A.L.; Rey, C.C.; et al. The effectiveness of nirsevimab in reducing the burden of disease due to respiratory syncytial virus (RSV) infection over time in the Madrid region (Spain): A prospective population-based cohort study. Front. Public Health 2024, 12, 1441786. Available online: https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2024.1441786/full (accessed on 29 January 2025). [CrossRef]
- Agüera, M.; Soler-Garcia, A.; Alejandre, C.; Moussalam-Merino, S.; Sala-Castellví, P.; Pons, G.; Penela-Sánchez, D.; González-Grado, C.; Alsina-Rossell, J.; Climent, C.; et al. Nirsevimab immunization’s real-world effectiveness in preventing severe bronchiolitis: A test-negative case-control study. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2024, 35, e14175. [Google Scholar] [CrossRef]
- López-Lacort, M.; Muñoz-Quiles, C.; Mira-Iglesias, A.; Xavier López-Labrador, F.; Garcés-Sánchez, M.; Escribano-López, B.; Zornoza-Moreno, M.; Pérez-Martín, J.J.; Alfayate-Miguelez, S.; Iofrío-De Arce, A.; et al. Nirsevimab Effectiveness Against Severe Respiratory Syncytial Virus Infection in the Primary Care Setting. Pediatrics. 2025, 155, e2024066393. [Google Scholar] [CrossRef] [PubMed]
- Mazagatos, C.; Mendioroz, J.; Rumayor, M.B.; Gallardo García, V.; Álvarez Río, V.; Cebollada Gracia, A.D.; Rebollo, N.B.; Boada, M.I.B.; Pérez-Martínez, O.; Azevedo, A.S.L.; et al. Estimated Impact of Nirsevimab on the Incidence of Respiratory Syncytial Virus Infections Requiring Hospital Admission in Children < 1 Year, Weeks 40, 2023, to 8, 2024, Spain. Influenza Other Respir. Viruses 2024, 18, e13294. [Google Scholar] [PubMed]
- Dagan, R.; Hammitt, L.L.; Seoane Nuñez, B.; Baca Cots, M.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Chang, Y.; Currie, A.; et al. Infants Receiving a Single Dose of Nirsevimab to Prevent RSV Do Not Have Evidence of Enhanced Disease in Their Second RSV Season. J. Pediatr. Infect. Dis. Soc. 2024, 13, 144–147. [Google Scholar] [CrossRef] [PubMed]
- A Study to Evaluate the Safety and Efficacy of MEDI8897 for the Prevention of Medically Attended Lower Respiratory Tract Infection Due to Respiratory Syncytial Virus in Healthy Late Preterm and Term Infants (MELODY) [Internet]. Available online: https://www.astrazenecaclinicaltrials.com/study/D5290C00004/ (accessed on 2 October 2024).
- A Study to Evaluate the Safety of MEDI8897 for the Prevention of Medically Attended Respiratory Syncytial Virus(RSV) Lower Respiratory Track Infection (LRTI) in High-Risk Children [Internet]. Available online: https://www.astrazenecaclinicaltrials.com/study/D5290C00005/ (accessed on 2 October 2024).
- Domachowske, J.; Madhi, S.A.; Simões, E.A.F.; Atanasova, V.; Cabañas, F.; Furuno, K.; Garcia-Garcia, M.L.; Grantina, I.; Nguyen, K.A.; Brooks, D.; et al. Safety of Nirsevimab for RSV in Infants with Heart or Lung Disease or Prematurity. N. Engl. J. Med. 2022, 386, 892–894. [Google Scholar] [CrossRef]
- Domachowske, J.B.; Chang, Y.; Atanasova, V.; Cabañas, F.; Furuno, K.; Nguyen, K.A.; Banu, I.; Kubiak, R.J.; Leach, A.; Mankad, V.S.; et al. Safety of Re-dosing Nirsevimab Prior to RSV Season 2 in Children With Heart or Lung Disease. J. Pediatr. Infect. Dis. Soc. 2023, 12, 477–480. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios. ¿Qué es el Sistema Español de Farmacovigilancia de Medicamentos de uso humano? [Internet]. ¿Qué es el Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano? 2019. Available online: https://www.aemps.gob.es/medicamentos-de-uso-humano/farmacovigilancia-de-medicamentos-de-uso-humano/que-es-el-sistema-espanol-de-farmacovigilancia-de-medicamentos-de-uso-humano/ (accessed on 4 September 2024).
- Egoavil, C.M.; Tuells, J.; Carreras, J.J.; Montagud, E.; Pastor-Villalba, E.; Caballero, P.; Nolasco, A. Trends of Adverse Events Following Immunization (AEFI) Reports of Human Papillomavirus Vaccine in the Valencian Community—Spain (2008–2018). Vaccines 2020, 8, 117. [Google Scholar] [CrossRef]
- Brown, E.G. Using MedDRA. Drug Saf. 2004, 27, 591–602. [Google Scholar] [CrossRef]
- Laemmle-Ruff, I.; Crawford, N.W. Respiratory syncytial virus prevention is finally here. Aust. J. Gen. Pract. 2024, 53, 704–708. Available online: https://www1.racgp.org.au/ajgp/2024/october/respiratory-syncytial-virus-prevention-is-finally (accessed on 26 January 2025). [CrossRef]
- Turalde-Mapili, M.W.R.; Mapili, J.A.L.; Turalde, C.W.R.; Pagcatipunan, M.R. The efficacy and safety of nirsevimab for the prevention of RSV infection among infants: A systematic review and meta-analysis. Front. Pediatr. 2023, 11, 1132740. Available online: https://www.frontiersin.org/journals/pediatrics/articles/10.3389/fped.2023.1132740/full (accessed on 26 January 2025). [CrossRef]
- European Medicines Agency (EMA). Beyfortus: EPAR—Product Information [Internet]. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/beyfortus-epar-product-information_en.pdf (accessed on 26 January 2025).
- Carcione, D.; Spencer, P.; Pettigrew, G.; Leeb, A.; Drake-Brockman, C.; Ford, T.; Effler, P. Active post-marketing safety surveillance of nirsevimab administered to children in Western Australia, April–July 2024. Pediatr. Infect. Dis. J. 2025, 44, e174–e176. [Google Scholar] [CrossRef]
- Consolati, A.; Farinelli, M.; Serravalle, P.; Rollandin, C.; Apprato, L.; Esposito, S.; Bongiorno, S. Safety and Efficacy of Nirsevimab in a Universal Prevention Program of Respiratory Syncytial Virus Bronchiolitis in Newborns and Infants in the First Year of Life in the Valle d’Aosta Region, Italy, in the 2023-2024 Epidemic Season. Vaccines 2024, 12, 549. [Google Scholar] [CrossRef] [PubMed]
- Mankad, V.S.; Leach, A.; Chang, Y.; Wählby Hamrén, U.; Kiazand, A.; Kubiak, R.J.; Takas, T.; Villafana, T.; Shroff, M. Comprehensive Summary of Safety Data on Nirsevimab in Infants and Children from All Pivotal Randomized Clinical Trials. Pathogens 2024, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.P.; Ding, F.R. Administration of Nirsevimab for RSV Prophylaxis in Infants: A Comprehensive Review. Vaccines 2025, 13, 470. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.B.; Fernandes, E.G.; Miyaji, K.T.; Arantes, B.A.; Valente, M.G.; Sato, H.K.; Sartori, A.M.C. Adverse events following Quadrivalent HPV vaccination reported in Sao Paulo State, Brazil, in the first three years after introducing the vaccine for routine immunization (March 2014 to December 2016). Rev. Inst. Med. Trop. São Paulo 2019, 61, e43. [Google Scholar] [CrossRef]
- Nuñez, O.; Olmedo, C.; Moreno-Perez, D.; Lorusso, N.; Fernández Martínez, S.; Pastor Villalba, P.E.; Gutierrez, Á.; Alonso-García, M.; Latasa, P.; Sancho, R.; et al. Nirsevimab Effectiveness Against Rsv Hospital Admission in Children Under 1 Year of Age: A Spanish Population-Based Case Control Study; Social Science Research Network: Rochester, NY, USA, 2024; Available online: https://papers.ssrn.com/abstract=4925473 (accessed on 21 January 2025).
- Ocana de Sentuary, C.; Testard, C.; Lagrée, M.; Leroy, M.; Gasnier, L.; Enes-Dias, A.; Leruste, C.; Diallo, D.; Génin, M.; Rakza, T.; et al. Acceptance and safety of the RSV-preventive treatment of newborns with nirsevimab in the maternity department: A prospective longitudinal cohort study in France. eClinicalMedicine 2024, 79, 102986. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Caster, O.; Rochon, P.A.; den Ruijter, H. Reported adverse drug reactions in women and men: Aggregated evidence from globally collected individual case reports during half a century. eClinicalMedicine 2019, 17, 100188. Available online: https://www.thelancet.com/journals/eclinm/article/PIIS2589-5370(19)30183-X/fulltext (accessed on 21 January 2025). [CrossRef]
- Fabiano, V.; Mameli, C.; Zuccotti, G.V. Adverse drug reactions in newborns, infants and toddlers: Pediatric pharmacovigilance between present and future. Expert. Opin. 2015, 11, 95–105. [Google Scholar] [CrossRef]
| Characteristic | Total (n = 67) | |
|---|---|---|
| Age at dosing | Mean age in months (SD) | 3.24 (3.25) |
| Median age in months (IQR) | 2.03 (1.0, 5.1) | |
| Age group, n (%) | Infant | 53 (79.1%) |
| Newborn | 14 (20.9%) | |
| Sex, n (%) | Female | 25 (37.3) |
| Male | 42 (62.7) | |
| SAEs per case, n (%) | 1 | 27 (40.3%) |
| 2 | 22 (32.8%) | |
| 3 | 12 (17.9%) | |
| 4 | 2 (3.0%) | |
| 5 | 2 (3.0%) | |
| 7 | 1 (1.5%) | |
| 8 | 1 (1.5%) | |
| Category | Cases | Percentage (%) | Notification Rate (Per 100,000 Doses) |
|---|---|---|---|
| Non-serious | 31 | 46.3% | 10.7 |
| Life-threatening, medically significant condition | 1 | 1.5% | 0.3 |
| Life-threatening, requires hospitalisation | 8 | 11.9% | 2.8 |
| Life-threatening, requires hospitalisation, medically significant condition | 1 | 1.5% | 0.3 |
| Life-threatening, requires hospitalisation, results in persistent/significant disability | 1 | 1.5% | 0.3 |
| Requires hospitalisation | 10 | 14.9% | 3.4 |
| Prolongs hospitalisation | 7 | 10.4% | 2.4 |
| Medically significant condition | 6 | 9.0% | 2.1 |
| Fatal | 1 | 1.5% | 0.3 |
| Fatal, requires hospitalisation | 1 | 1.5% | 0.3 |
| Total | 67 | 100.0% | 23.1 |
| Case Outcome | Number of Cases | Percentage | Notification Rate (Per 100,000 Doses) |
|---|---|---|---|
| Recovered | 37 | 55.2% | 12.8 |
| Recovering | 18 | 26.9% | 6.2 |
| Not recovered | 4 | 6.0% | 1.4 |
| Fatal | 2 | 3.0% | 0.7 |
| Unknown | 6 | 9.0% | 2.1 |
| Total | 67 | 100.0% | 23.1 |
| Medical History | Number of Notifications |
|---|---|
| Premature neonate | 3 |
| Bronchiolitis | 2 |
| Low birth weight neonate | 2 |
| Nephrogenic anaemia | 1 |
| Multiple congenital abnormalities | 1 |
| Central venous catheterization | 1 |
| Patent ductus arteriosus | 1 |
| COVID-19 | 1 |
| Ventricular septal defect | 1 |
| Foetal malnutrition | 1 |
| Bronchopulmonary dysplasia | 1 |
| Epileptic encephalopathy | 1 |
| Congenital pulmonary valve stenosis | 1 |
| Cystic fibrosis | 1 |
| Gastrostomy | 1 |
| Pneumothorax | 1 |
| Foetal growth restriction | 1 |
| Neonatal sepsis | 1 |
| Sturge–Weber syndrome | 1 |
| Foetal distress syndrome | 1 |
| Congenital nephrotic syndrome | 1 |
| VACTERL syndrome | 1 |
| Total | 26 |
| Preferred Terms (PT) ** | Number of SAEs | Percentages (%) | Notification Rate (Per 100,000 Doses) | Median Latency (in Days) After the First Dose |
|---|---|---|---|---|
| Rash | 12 | 8.51% | 4.1 | 6.0 |
| Drug ineffective | 10 | 7.09% | 3.4 | 52.5 |
| Pyrexia | 10 | 7.09% | 3.4 | 2.0 |
| Respiratory syncytial virus bronchiolitis | 9 | 6.38% | 3.1 | 64.0 |
| Bronchiolitis | 4 | 2.84% | 1.4 | 4.0 |
| Acute respiratory failure | 3 | 2.13% | 1 | 6.0 |
| Urticaria | 3 | 2.13% | 1 | 14 |
| Vomiting | 3 | 2.13% | 1 | 1.0 |
| Metabolic acidosis | 2 | 1.42% | 0.7 | 21.8 |
| Diarrhoea | 2 | 1.42% | 0.7 | 3.0 |
| Maculo-papular rash | 2 | 1.42% | 0.7 | 3.5 |
| Gastroenteritis | 2 | 1.42% | 0.7 | 37.0 |
| Upper respiratory tract infection | 2 | 1.42% | 0.7 | 13.0 |
| Lethargy | 2 | 1.42% | 0.7 | 0.8 |
| Skin reaction | 2 | 1.42% | 0.7 | 3.5 |
| Oxygen saturation decreased | 2 | 1.42% | 0.7 | 2.0 |
| Injection site urticaria | 2 | 1.42% | 0.7 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrella-Porter, P.; Correcher-Martínez, E.; Orrico-Sánchez, A.; Carreras, J.J. Post-Marketing Surveillance of Nirsevimab: Safety Profile and Adverse Event Analysis from Spain’s 2023–2024 RSV Immunisation Campaign. Vaccines 2025, 13, 623. https://doi.org/10.3390/vaccines13060623
Estrella-Porter P, Correcher-Martínez E, Orrico-Sánchez A, Carreras JJ. Post-Marketing Surveillance of Nirsevimab: Safety Profile and Adverse Event Analysis from Spain’s 2023–2024 RSV Immunisation Campaign. Vaccines. 2025; 13(6):623. https://doi.org/10.3390/vaccines13060623
Chicago/Turabian StyleEstrella-Porter, Pablo, Elisa Correcher-Martínez, Alejandro Orrico-Sánchez, and Juan José Carreras. 2025. "Post-Marketing Surveillance of Nirsevimab: Safety Profile and Adverse Event Analysis from Spain’s 2023–2024 RSV Immunisation Campaign" Vaccines 13, no. 6: 623. https://doi.org/10.3390/vaccines13060623
APA StyleEstrella-Porter, P., Correcher-Martínez, E., Orrico-Sánchez, A., & Carreras, J. J. (2025). Post-Marketing Surveillance of Nirsevimab: Safety Profile and Adverse Event Analysis from Spain’s 2023–2024 RSV Immunisation Campaign. Vaccines, 13(6), 623. https://doi.org/10.3390/vaccines13060623







