A Multi-Center Study on Sensitization to Thimerosal in North-Eastern Italy, 1997–2023: Prevalence, Risk Factors, the Role of Occupation and the Impact of Vaccinations
Abstract
1. Introduction
1.1. Thimerosal in Human Vaccines and Medications
1.2. Thimerosal as a Hapten
2. Methods
2.1. Evaluation of Patients and Patch Testing
- Reactions degree +, ++ and +++ were considered positive.
- Doubtful reactions (?+) were considered negative.
2.2. Statistical Analysis
- Years 1997–2004 in all centers but Trieste;
- Across 1997–2015, limited to Padua and Pordenone;
- Across 2010–2023, limited to Trieste and Pordenone.
- 1904–1938: when only vaccination against small pox was mandatory in Italy since 1888.
- 1939–1965: since diphtheria vaccination was mandatorily introduced in 1939.
- 1966–1980: since polio vaccination was enforced in 1966 and tetanus in 1968.
- 1981–1990: since small pox immunization was abolished in 1981.
- 1991–1998: since HBV vaccination was made mandatory for all newborns.
- 1999 onward: when a call for removal of Thimerosal from all new vaccine formulations was launched in USA.
- Model 1: across 1997–2004, including all centers but Trieste;
- Model 2: across 1997–2015, limited to Padua and Pordenone;
- Model 3: across 2010–2023, limited to Trieste and Pordenone.
3. Results
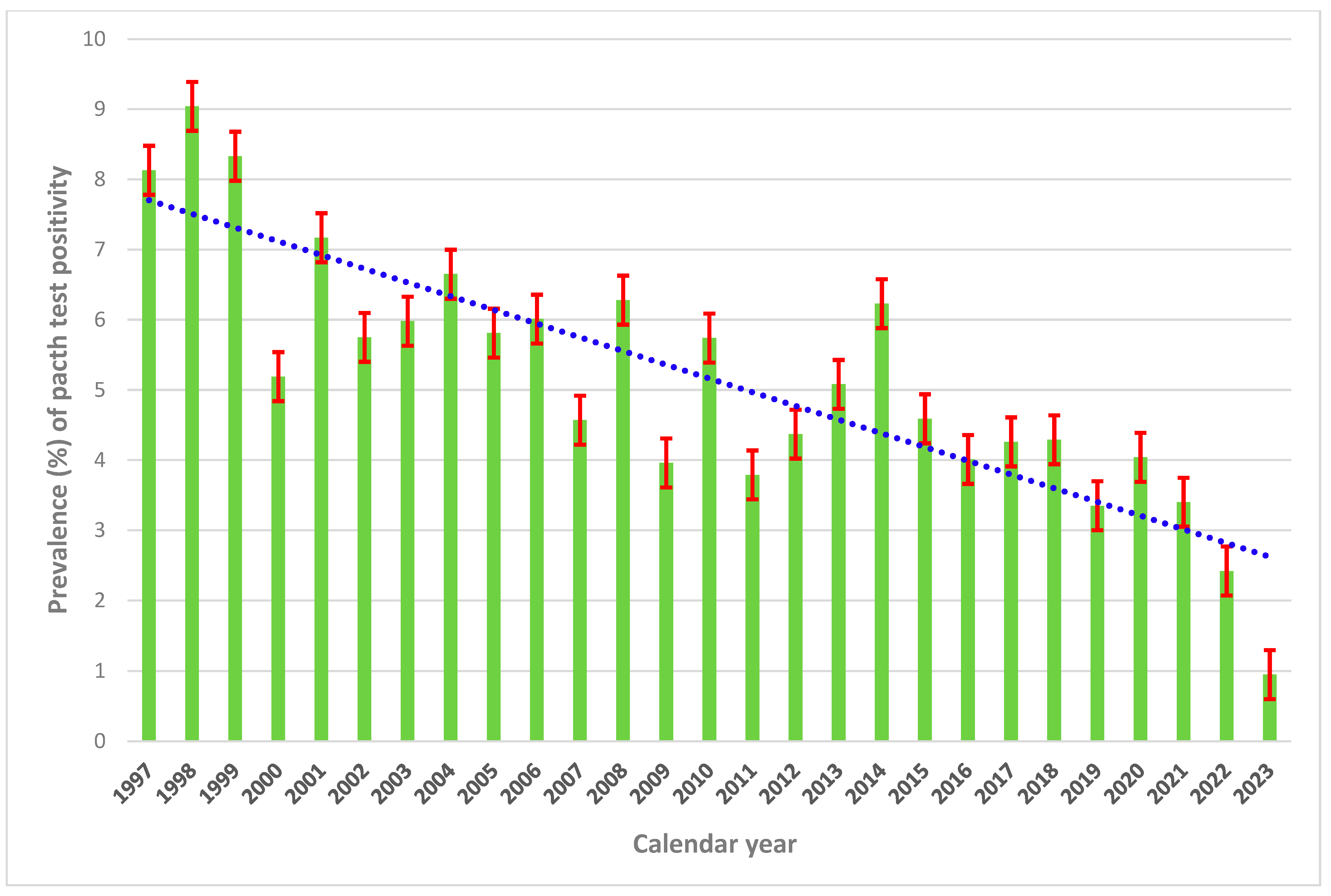
3.1. Calendar Years 1997–2004 (Considering All Centers but Trieste)
3.2. Calendar Years 1997–2015 (Considering Only Padua and Pordenone)
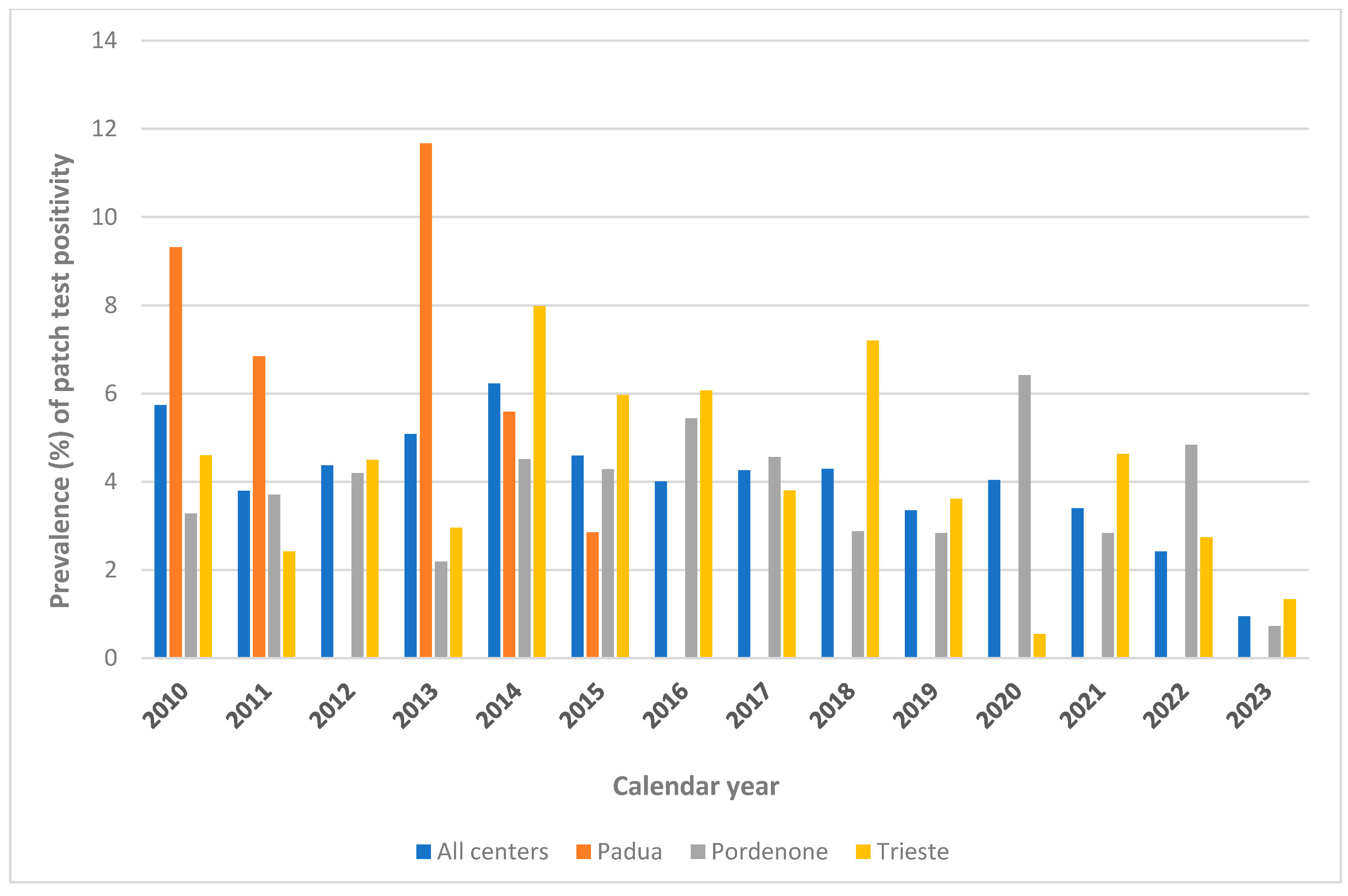
3.3. Calendar Years 2010–2023 (Considering Only Trieste and Pordenone)
4. Discussion
4.1. Prevalence of Sensitization
4.2. The Impact of Vaccination
4.3. Strength and Weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Migdal, C.; Foggia, L.; Tailhardat, M.; Courtellemont, P.; Haftek, M.; Serres, M. Sensitization effect of Thimerosal is mediated in vitro via reactive oxygen species and calcium signaling. Toxicology 2010, 274, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Sykes, L.K.; Geier, M.R. A review of Thimerosal (Merthiolate) and its ethylmercury breakdown product: Specific historical considerations regarding safety and effectiveness. J. Toxicol. Environ. Health B Crit. Rev. 2007, 10, 575–596. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; King, P.G.; Hooker, B.S.; Dórea, J.G.; Kern, J.K.; Sykes, L.K.; Geier, M.R. Thimerosal: Clinical, epidemiologic and biochemical studies. Clin. Chim. Acta 2015, 444, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Yeter, D.; Deth, R. ITPKC susceptibility in Kawasaki syndrome as a sensitizing factor for autoimmunity and coronary arterial wall relaxation induced by thimerosal’s effects on calcium signaling via IP3. Autoimmun. Rev. 2012, 11, 903–908. [Google Scholar] [CrossRef]
- Center for Disease Control and Preventoin (CDC). Thimerosal in Flu Vaccines. Available online: https://www.cdc.gov/flu/vaccine-safety/thimerosal.html (accessed on 9 March 2025).
- Food and Drug Administration (FDA). Thimerosal and Vaccines. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/thimerosal-and-vaccines (accessed on 20 February 2025).
- Madsen, K.M.; Lauritsen, M.B.; Pedersen, C.B.; Thorsen, P.; Plesner, A.M.; Andersen, P.H.; Mortensen, P.B. Thimerosal and the occurrence of autism: Negative ecological evidence from Danish population-based data. Pediatrics 2003, 112, 604–606. [Google Scholar] [CrossRef]
- Stehr-Green, P.; Tull, P.; Stellfeld, M.; Mortenson, P.B.; Simpson, D. Autism and thimerosal-containing vaccines: Lack of consistent evidence for an association. Am. J. Prev. Med. 2003, 25, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Hviid, A.; Stellfeld, M.; Wohlfahrt, J.; Melbye, M. Association between thimerosal-containing vaccine and autism. J. Am. Med. Assoc. 2003, 290, 1763–1766. [Google Scholar] [CrossRef]
- Joint statement of the American Academy of Pediatrics (AAP) and the United States Public Health Service (USPHS). Pediatrics 1999, 104 Pt 1, 568–569. [CrossRef] [PubMed]
- Baker, J.P. Mercury, vaccines, and autism: One controversy, three histories. Am. J. Public Health 2008, 98, 244–253. [Google Scholar] [CrossRef]
- European Parliament. Parliamentary Question. Available online: https://www.europarl.europa.eu/doceo/document/E-6-2008-4469-ASW_EN.html (accessed on 22 April 2025).
- United States Environmental Protection Agency. Mercury Study Report to Congress: Volume 1: Executive Summary. Available online: https://www.epa.gov/sites/default/files/2015-09/documents/volume1.pdf (accessed on 28 May 2025).
- Ball, L.K.; Ball, R.; Pratt, R.D. An assessment of Thimerosal use in childhood vaccines. Pediatrics 2001, 107, 1147–1154. [Google Scholar] [CrossRef]
- World Health Organization. Thimerosal. Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/norms-and-standards/vaccine-standardization/thiomersal (accessed on 25 May 2025).
- European Medicine (EMA). Thiomersal in Vaccines for Human Use—Recent Evidence Supports Safety of Thiomersal-Containing Vaccines—Scientific Guideline. 2004. Available online: https://www.ema.europa.eu/en/thiomersal-vaccines-human-use-recent-evidence-supports-safety-thiomersal-containing-vaccines-scientific-guideline (accessed on 28 May 2025).
- European Agency for the Evaluation of Medicinal Products (EMEA). CPMP Position Paper on Thiomersal Implementation of the Warning Statement Relating to Sensitisation. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/chmp-position-paper-thiomersal-implementation-warning-statement-relating-sensitisation_en.pdf (accessed on 28 May 2025).
- Stratton, K.; Gable, A.; McCormick, M.C. Immunization Safety Review: Health Effects of Low-Dose Exposures to Thimerosal and Methylmercury; National Academy Press: Cambridge, MA, USA, 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK223725/pdf/Bookshelf_NBK223725.pdf (accessed on 22 April 2025).
- Breithaupt, A.; Jacob, S.E. Thimerosal and the relevance of patch-test reactions in children. Dermatitis 2008, 19, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Che, D.; Hao, Y.; Zheng, Y.; Liu, R.; Qian, Y.; Cao, J.; Wang, J.; Zhang, Y.; He, L.; et al. Thimerosal induces skin pseudo-allergic reaction via Mas-related G-protein coupled receptor B2. J. Dermatol. Sci. 2019, 95, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Belsito, D.V. Thimerosal: Contact (non)-allergen of the year. Am. J. Contact Dermat. 2002, 13, 1–2. [Google Scholar] [CrossRef]
- Elmobody, K.; Mailbach, J.; Mailbach, H.; Dung Do, L.H. Long-Term North American Trend in Patch Test Reactions: A 32-Year Statistical Overview (1984–2016). Dermatitis 2023, 34, 36–41. [Google Scholar] [CrossRef]
- Dórea, J.G. Abating Mercury Exposure in Young Children Should Include Thimerosal-Free Vaccines. Neurochem. Res. 2017, 42, 2673–2685. [Google Scholar] [CrossRef]
- Garg, T.; Agarwal, S.; Chander, R.; Singh, A.; Yadav, P. Patch testing in patients with suspected cosmetic dermatitis: A retrospective study. J. Cosmet. Dermatol. 2018, 17, 95–100. [Google Scholar] [CrossRef]
- Zhao, J.; Li, L.F. Contact sensitization to cosmetic series of allergens in a general population in Beijing. J. Cosmet. Dermatol. 2014, 13, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Hong, D.K.; Jeong, N.J.; Lee, J.H.; Choi, Y.; Lee, A.Y.; Lee, C.; Kim, K.J.; Park, H.Y.; Yang, J.; et al. Multicenter study of preservative sensitivity in patients with suspected cosmetic contact dermatitis in Korea. J. Dermatol. 2012, 39, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.A.; Uter, W.; Pirker, C.; Geier, J.; Frosch, P.J. Allergic and non-allergic periorbital dermatitis: Patch test results of the Information Network of the Departments of Dermatology during a 5-year period. Contact Derm. 2004, 51, 13–19. [Google Scholar] [CrossRef]
- Suneja, D.V. Belsito, Thimerosal in the detection of clinically relevant allergic contact reactions. J. Am. Acad. Dermatol. 2001, 45, 23–27. [Google Scholar] [CrossRef]
- Uter, W.; Gefeller, O.; Geier, J.; Schnuch, A. Changes of the patch test population (MOAHLFA index) in long term participants of the Information Network of Departments of Dermatology, 1999–2006. Contact Dermat. 2008, 59, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.D.; Aalto-Korte, K.; Agner, T.; Andersen, K.E.; Bircher, A.; Bruze, M.; Cannavó, A.; Giménez-Arnau, A.; Gonçalo, M.; Goossens, A.; et al. European Society of Contact Dermatitis guideline for diagnostic patch testing—Recommendations on best practice. Contact Dermat. 2015, 73, 195–221. [Google Scholar] [CrossRef]
- Istituto Superiore di Sanità (ISS). Obbligo Vaccinale: Un po’ di Storia. Available online: https://www.epicentro.iss.it/vaccini/obbligovaccinalestoria (accessed on 20 April 2025).
- Anonymous. The frequency of contact sensitivity in North America 1972–1974. Contact Dermat. 1975, 1, 277–280. [Google Scholar]
- European Center for Disease Control and Prevention (ECDC). Childhood Immunization. Available online: https://www.ecdc.europa.eu/en/immunisation-vaccines/childhood-vaccination (accessed on 27 May 2025).
- Council of the European Union. Council Conclusions on the Community Strategy Concerning Mercury. Available online: https://www.eu2005.lu/en/actualites/conseil/2005/06/24env/mercure.pdf (accessed on 28 May 2025).
- Eur Lex. Communication From The Commission To The Council And The European Parliament—Community Strategy Concerning Mercury. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:52005DC0020 (accessed on 28 May 2025).
- European Parliament. European Parliament Resolution on the Community Strategy Concerning Mercury (2005/2050(INI)); P6-TA(2006)0078; A6-0044/2006. Available online: https://www.europarl.europa.eu/doceo/document/TA-6-2006-0078_EN.html (accessed on 28 May 2025).
- Freed, G.L.; Andreae, M.C.; Cowan, A.E.; Katz, S.L. Vaccine safety policy analysis in three European countries: The case of thimerosal. Health Policy 2022, 62, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Hessel, L. Le mercure et les vaccins [Mercury in vaccines]. Bull. Acad. Natl. Med. 2003, 187, 1501–1510. [Google Scholar]
- Minton, K. UK 5-in-1 baby vaccine. Nat. Rev. Immunol. 2004, 4, 659. [Google Scholar] [CrossRef]
- Official Gazette of the Italian Republic. Decree 13 November 2001. Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2002-03-19&atto.codiceRedazionale=02A03137&elenco30giorni=false (accessed on 25 May 2025).
- Uter, W.; Bauer, A.; Fortina, A.B.; Bircher, A.J.; Brans, R.; Buhl, T.; Cooper, S.M.; Czarnecka-Operacz, M.; Dickel, H.; Dugonik, A.; et al. Patch test results with the European baseline series and additions thereof in the ESSCA network, 2015–2018. Contact Dermat. 2021, 84, 109–120. [Google Scholar] [CrossRef]
- Aristizabal-Torres, M.A.; Youssef, M.; Yang, Y.W.; Yiannias, J.A.; Davis, M.D.P.; Hall, M.R. Trends in Patch Test Results with the Mayo Clinic Extended Standard Series: A 14-Year Retrospective Review (2010–2023). In Dermatitis; Mary Ann Liebert: New York, NY, USA, 2025. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Hou, A.; Warshaw, E.M.; DeKoven, J.G.; Maibach, H.I.; Belsito, D.V.; Taylor, J.S.; Zug, K.A.; Sasseville, D.; Fransway, A.F.; et al. Prevalence and Trend of Allergen Sensitization in Adults and Children with Atopic Dermatitis Referred for Patch Testing, North American Contact Dermatitis Group Data, 2001–2016. J. Allergy Clin. Immunol. Pract. 2021, 9, 2853–2866.e14. [Google Scholar] [CrossRef]
- Center for Disease Preventions and Control. Thimerosal and Vaccines. Available online: https://www.cdc.gov/vaccine-safety/about/thimerosal.html#:~:text=Thimerosal%20hasn’t%20been%20used,a%20flu%20vaccine%20without%20it (accessed on 28 May 2025).
- European Commission. Request for a Scientific Advice on the Safety of Thimerosal (CAS No. 54-64-8, EC No. 200-210-4) and Phenylmercuriclt Salts as Preservatives in Cosmetic Products. Available online: https://health.ec.europa.eu/document/download/ab925cb3-fd22-4b72-9479-320c795dda14_en?filename=sccs2022_q_037.pdf&prefLang=ga (accessed on 27 May 2025).
- Rubegni, G.; Padula, T.; Calabrese, L.; D’Onghia, M.; Tognetti, L.; Cinotti, E.; Lazzeri, L.; Ermini, G.; Cartocci, A.; Tosi, G.M. Eyelid Contact Dermatitis: 25-Year Single-Center Retrospective Study. J. Clin. Med. 2025, 14, 823. [Google Scholar] [CrossRef]
- World Health Organization. Diphtheria Tetanus Toxoid and Pertussis (DTP) Vaccination Coverage. Available online: https://immunizationdata.who.int/global/wiise-detail-page/diphtheria-tetanus-toxoid-and-pertussis-(dtp)-vaccination-coverage?CODE=ITA&ANTIGEN=&YEAR= (accessed on 23 April 2025).
- Epicentro-Istituto Superiore di Sanità. Le Vaccinazioni in Italia. Available online: https://www.epicentro.iss.it/vaccini/dati_ita#parotite (accessed on 19 May 2025).
- Cegolon, L.; Larese Filon, F. Sensitization to Lanolin in North-Eastern Italy, 1997–2021: Prevalence, Risk Factors and the Impact of Occupation. On Behalf Of The North-East Research Group On Contact Dermatitis. Life 2024, 14, 916. [Google Scholar] [CrossRef]
- Cegolon, L.; Larese Filon, F. Prevalence and determinants of sensitization to neomycin in North-Eastern Italy, 1997–2021. Contact Dermat. 2025, 92, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Badalini, A.; Larese Filon, F. Epidemiological and Occupational Pattern of Patch-Test Reactions to p-Tert-butylphenol-formaldehyde Resin in North-Eastern Italy, 1997–2021. Life 2025, 15, 698. [Google Scholar] [CrossRef] [PubMed]
- Uter, W.; Gefeller, O.; Mahler, V.; Geier, J. Trends and current spectrum of contact allergy in Central Europe: Results of the Information Network of Departments of Dermatology (IVDK) 2007–2018. Br. J. Dermatol. 2020, 183, 857–865. [Google Scholar] [CrossRef] [PubMed]

| Calendar Year of Birth | Patients’ Age at Patch Test (Years) | ||||
|---|---|---|---|---|---|
| Median (IQR) | N (%) | ||||
| <30 | 30–42 | 43–57 | 58+ | ||
| 1904–1938 | 72 (67; 77) | 0 | 0 | 0 | 3383 (100) |
| 1939–1965 | 52 (44; 59) | 0 | 2600 (20.18) | 6240 (48.43) | 4045 (31.39) |
| 1966–1980 | 32 (27; 39) | 4110 (41.39) | 4278 (43.09) | 1541 (15.52) | 0 |
| 1981–1990 | 24 (19; 29) | 2651 (79.87) | 668 (20.13) | 0 | 0 |
| 1991–1998 | 21 (18; 25) | 1205 (97.41) | 32 (2.59) | 0 | 0 |
| 1999–2015 | 18 (16; 21) | 446 (100) | 0 | 0 | 0 |
| TERMS | Period 1997–2004 (Padua, Pordenone, Trento–Bolzano–Rovigo) | Period 1997–2015 (Padua and Pordenone) | Period 2010–2023 (Trieste and Pordenone) | ||||||||
| Total Tested (N) | Thimerosal + N (Row %) | p- Value | Total Tested (N) | Thimerosal + N (Row %) | p- Value | Total Tested (N) | Thimerosal + N (Row %) | p-Value | |||
| Total Patients Examined for Suspected ACD | 10,962 | 1040 (9.49) | 14,596 | 1227 (8.41) | 8305 | 333 (4.01) | |||||
| Center | Padua | 5085 | 11.84 | <0.001 | 8619 | 10.79 | <0.001 | - | - | 0.347 | |
| Pordenone | 2243 | 7.53 | 5976 | 4.97 | 3700 | 3.78 | |||||
| Trieste | - | - | - | - | 4605 | 4.19 | |||||
| Trento/Bolzano/Rovigo | 3633 | 7.40 | - | - | - | - | |||||
| Sex | Females | 7388 | 9.54 | 0.780 | 9737 | 8.64 | 0.156 | 5665 | 4.31 | 0.043 | |
| Males | 3573 | 9.38 | 4858 | 7.95 | 2640 | 3.37 | |||||
| Age (years) (M: 4) | M (SE) | 41.10 (0.16) | 34.2 (0.42) | 42.6 (0.14) | 34.8 (0.39) | 45.7 (0.19) | 39.6 (0.73) | ||||
| Median (IQR) | 38 (28; 53) | 33 (23; 43) | <0.001 * | 40 (29; 55) | 33 (24; 44) | <0.001 * | 45 (32; 59) | 38 (30; 48) | <0.001 * | ||
| ≤40 | 5813 | 12.16 | <0.001 | 7114 | 11.41 | <0.001 | 3228 | 5.45 | <0.001 | ||
| 41+ | 5148 | 6.47 | 7481 | 5.55 | 5076 | 3.09 | |||||
| Birth year | 1904–1938 | 1537 | 2.80 | <0.001 | 1613 | 2.67 | <0.001 | 320 | 1.25 | <0.001 | |
| 1939–1965 | 4858 | 8.95 | 6106 | 7.37 | 3019 | 2.12 | |||||
| 1966–1980 | 3884 | 10.43 | 4804 | 9.18 | 2429 | 5.02 | |||||
| 1981–1990 | 682 | 23.02 | 593 | 17.14 | 1279 | 8.68 | |||||
| 1991–1998 | - | - | 433 | 4.62 | 879 | 3.07 | |||||
| 1999–2015 | - | - | 45 | 0 | 377 | 1.33 | |||||
| Atopic dermatitis (M: 3140) | No | 8856 | 9.85 | 0.008 | 13,134 | 8.44 | 0.713 | 6710 | 3.71 | 0.002 | |
| Yes | 412 | 13.83 | 1289 | 8.15 | 1485 | 5.45 | |||||
| Occupational dermatitis (M: 31) | No | 9900 | 9.22 | 0.004 | 13,641 | 8.24 | 0.006 | 7504 | 3.84 | 0.007 | |
| Yes | 1061 | 11.97 | 954 | 10.80 | 770 | 5.84 | |||||
| Body area affected by dermatitis | Hand (M: 4432) | No | 5273 | 9.63 | 0.551 | 8798 | 8.24 | 0.573 | 5530 | 3.87 | 0.102 |
| Yes | 3938 | 10.01 | 5097 | 8.51 | 2539 | 4.65 | |||||
| Leg (M: 4430) | No | 8574 | 9.80 | 0.957 | 12,849 | 8.34 | 0.977 | 7286 | 4.24 | 0.079 | |
| Yes | 637 | 9.73 | 1046 | 8.32 | 785 | 2.93 | |||||
| Face (M: 4430) | No | 7476 | 9.59 | 0.176 | 10.921 | 8.35 | 0.936 | 6355 | 3.84 | 0.017 | |
| Yes | 1735 | 10.66 | 2974 | 8.31 | 1716 | 5.13 | |||||
| Occupation (M: 65) | Clerks | 2428 | 12.11 | <0.001 | 2852 | 10.38 | <0.001 | 1595 | 4.70 | <0.001 | |
| Health care workers | 1641 | 14.20 | 1397 | 14.03 | 680 | 8.53 | |||||
| Teachers | - | - | 126 | 8.73 | 312 | 5.13 | |||||
| Cashiers | - | - | 12 | 8.33 | 16 | 6.25 | |||||
| Sellers | - | - | 145 | 3.45 | 222 | 6.76 | |||||
| Restaurant workers | 478 | 12.13 | 520 | 9.81 | 470 | 2.34 | |||||
| Hairdressers | 98 | 8.16 | 116 | 7.76 | 131 | 3.82 | |||||
| Farmers | 139 | 12.23 | 128 | 10.16 | 46 | 2.17 | |||||
| Construction workers | 682 | 10.85 | 556 | 9.35 | 193 | 3.63 | |||||
| Painters | 12 | 8.33 | 40 | 10.00 | 48 | 10.42 | |||||
| Mechanics | 528 | 8.52 | 685 | 6.13 | 481 | 4.78 | |||||
| Workers of wood industry | 218 | 8.26 | 291 | 6.87 | 100 | 4.00 | |||||
| Artisans general | 335 | 7.16 | 270 | 8.15 | 34 | 8.82 | |||||
| Leather artisans | 94 | 7.45 | 81 | 7.41 | 5 | 0 | |||||
| Chemistry workers | 146 | 13.01 | 155 | 11.61 | 36 | 13.89 | |||||
| Drivers | 161 | 7.45 | 148 | 9.46 | 53 | 3.77 | |||||
| Cleaners | 95 | 6.32 | 126 | 0.79 | 107 | 2.80 | |||||
| Housewives | 1831 | 6.01 | 1700 | 6.06 | 711 | 3.52 | |||||
| Students | - | - | 421 | 5.23 | 605 | 3.14 | |||||
| Retirees | 1326 | 3.39 | 1809 | 2.76 | 1232 | 1.14 | |||||
| Unemployed | 198 | 10.10 | 304 | 8.22 | 323 | 1.86 | |||||
| Other | 551 | 8.89 | 2713 | 9.80 | 881 | 3.86 | |||||
| Study Period | Terms | aOR (95%CI) | p- Value | BH p Value | |
|---|---|---|---|---|---|
| Years 1997–2004 (Padua, Pordenone, Trento–Bolzano–Rovigo) (10,962 obs.) | BH p ≤ 0.0130 | ||||
| Center | Padua | 1.69 (1.40; 2.04) | <0.001 | 0.0022 | |
| Pordedone | reference | ||||
| Trento–Bolzano–Rovigo | 1.00 (0.82; 1.23) | 0.976 | - | ||
| Birth year | 1904–1938 | reference | |||
| 1939–1965 | 2.49 (1.72; 3.62) | <0.001 | 0.0043 | ||
| 1966–1980 | 2.74 (1.85; 4.06) | <0.001 | 0.0065 | ||
| 1981–1990 | 8.13 (5.28; 12.51) | <0.001 | 0.0087 | ||
| 1991–1998 | omitted | ||||
| 1999–2015 | omitted | ||||
| Occupation | Clerks | reference | |||
| HCWs | 1.50 (1.22; 1.83) | <0.001 | 0.00130 | ||
| Housewives | 0.77 (0.60; 0.99) | 0.045 | 0.0174 (NS) | ||
| Retirees | 0.62 (0.42; 0.92) | 0.018 | 0.0152 (NS) | ||
| Calendar year (1997–2004)—linear term | 0.95 (0.91; 0.98) | 0.003 | 0.0130 | ||
| Years 1997–2015 (Limited to Padua and Pordenone) (14,550 obs.) | BH p ≤ 0.0148 | ||||
| Center | Padua | 2.01 (1.73; 2.33) | <0.001 | 0.0037 | |
| Pordedone | reference | - | |||
| Birth year | 1904–1938 | reference | |||
| 1939–1965 | 2.52 (1.77; 3.58) | <0.001 | 0.0056 | ||
| 1966–1980 | 2.98 (2.07; 4.31) | <0.001 | 0.0074 | ||
| 1981–1990 | 8.17 (5.56; 12.01) | <0.001 | 0.0093 | ||
| 1991–1998 | 2.89 (1.55; 5.40) | 0.001 | 0.0130 | ||
| 1999–2015 | - | - | - | ||
| Occupation | Clerks | reference | |||
| HCWs | 1.45 (1.18; 1.77) | <0.001 | 0.0011 | ||
| Cleaners | 0.13 (0.02; 0.92) | 0.041 | 0.0167 (NS) | ||
| Retirees | 0.62 (0.43; 0.88) | 0.007 | 0.0148 | ||
| Calendar year (1997–2015)—linear term | 0.94 (0.92; 0.95) | <0.001 | 0.0019 | ||
| Years 2010–2023 (limited to Pordenone and Trieste) (7936 obs.) | BH p ≤ 0.0019 | ||||
| Center | Trieste | 1.12 (0.88; 1.42) | 0.346 | - | |
| Pordedone | reference | ||||
| Face dermatitis | No | reference | |||
| Yes | 1.37 (1.06; 1.78) | 0.036 | 0.0179 (NS) | ||
| Atopic dermatitis | No | reference | |||
| Yes | 1.29 (0.98; 1.68) | 0.065 | - | ||
| Birth year | 1904–1938 | reference | |||
| 1939–1965 | 1.03 (0.35; 2.99) | 0.962 | - | ||
| 1966–1980 | 1.95 (0.65; 5.84) | 0.235 | - | ||
| 1981–1990 | 3.59 (1.19; 10.82) | 0.023 | 0.0071 (NS) | ||
| 1991–1998 | 1.33 (0.41; 4.29) | 0.632 | - | ||
| 1999–2015 | 0.67 (0.16; 2.89) | 0.593 | - | ||
| Occupation | Clerks | reference | |||
| HCWs | 1.84 (1.28; 2.64) | 0.001 | 0.0018 | ||
| Restaurant workers | 0.51 (0.27; 0.97) | 0.041 | 0.0143 (NS) | ||
| Chemistry workers | 3.22 (1.19; 8.67) | 0.021 | 0.0054 (NS) | ||
| Retirees | 0.49 (0.26; 0.95) | 0.035 | 0.0089 (NS) | ||
| Unemployed | 0.40 (0.17; 0.94) | 0.036 | 0.0089 (NS) | ||
| Calendar year (2010–2023)—linear term | 0.97 (0.94; 1.00) | 0.036 | 0.0107 (NS) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegolon, L.; Patriarca, E.; Larese Filon, F. A Multi-Center Study on Sensitization to Thimerosal in North-Eastern Italy, 1997–2023: Prevalence, Risk Factors, the Role of Occupation and the Impact of Vaccinations. Vaccines 2025, 13, 622. https://doi.org/10.3390/vaccines13060622
Cegolon L, Patriarca E, Larese Filon F. A Multi-Center Study on Sensitization to Thimerosal in North-Eastern Italy, 1997–2023: Prevalence, Risk Factors, the Role of Occupation and the Impact of Vaccinations. Vaccines. 2025; 13(6):622. https://doi.org/10.3390/vaccines13060622
Chicago/Turabian StyleCegolon, Luca, Emilia Patriarca, and Francesca Larese Filon. 2025. "A Multi-Center Study on Sensitization to Thimerosal in North-Eastern Italy, 1997–2023: Prevalence, Risk Factors, the Role of Occupation and the Impact of Vaccinations" Vaccines 13, no. 6: 622. https://doi.org/10.3390/vaccines13060622
APA StyleCegolon, L., Patriarca, E., & Larese Filon, F. (2025). A Multi-Center Study on Sensitization to Thimerosal in North-Eastern Italy, 1997–2023: Prevalence, Risk Factors, the Role of Occupation and the Impact of Vaccinations. Vaccines, 13(6), 622. https://doi.org/10.3390/vaccines13060622







