Target Trial Emulation of the Modified Vaccinia Ankara-Bavarian Nordic Vaccine for Pre-Exposure Mpox Prevention in At-Risk Populations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Outcomes
2.2. Participants and Matching for Trial Emulation
2.3. Study Procedures and Definitions
2.4. Sample Size and Statistical Methods
3. Results
3.1. Study Participants and Follow-Up
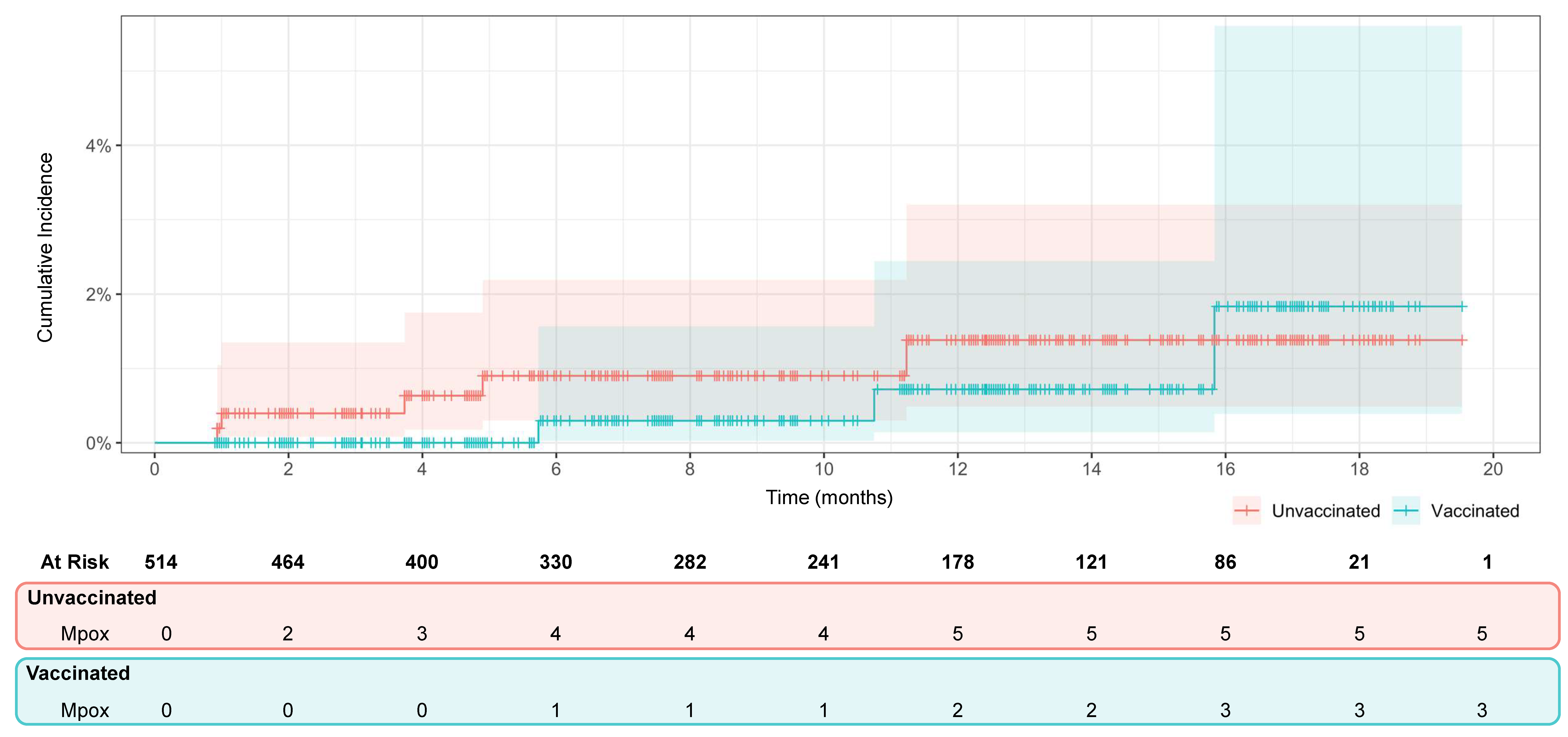
3.2. Vaccine Efficacy
3.3. Vaccine Safety
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| HIV-PrEP | HIV pre-exposure prophylaxis |
| HR | Hazard ratio |
| IEC | Independent Ethics Committee |
| IQR | Interquartile range |
| MPXV | Monkeypox virus |
| MVA-BN | Modified Vaccinia Ankara-Bavarian Nordic |
| mpox | Monkeypox |
| PCR | Protein chain reaction |
| REDcap | Research Electronic Data Capture |
| SD | Standard deviation |
| SMD | Standardized mean difference |
| STI | Sexually transmitted infection |
References
- Mitjà, O.; Ogoina, D.; Titanji, B.K.; Galvan, C.; Muyembe, J.J.; Marks, M.; Orkin, C.M. Monkeypox. Lancet 2023, 401, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Vicente, E.J.; Alemany, A.; Agud-Dios, M.; Ubals, M.; Suñer, C.; Antón, A.; Arando, M.; Arroyo-Andrés, J.; Calderón-Lozano, L.; Casañ, C.; et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: A prospective observational cohort study. Lancet 2022, 400, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Shafaati, M.; Forghani, S.; Shahsavand Davoudi, A.; Samiee, R.; Mohammadi, K.; Akbarpour, S.; Seifi, A.; Salehi, M.; Zare, M. Current advances and challenges in mpox vaccine development: A global landscape. Ther. Adv. Vaccines Immunother. 2025, 13. [Google Scholar] [CrossRef] [PubMed]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.H.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef]
- European Medicines Agency. Emergency Task Force. Possible Use of the Vaccine Jynneos Against Infection by Monkeypox Virus. Available online: https://www.ema.europa.eu/en/documents/public-statement/possible-use-vaccine-jynneos-against-infection-monkeypox-virus_en.pdf (accessed on 18 January 2025).
- Frey, S.E.; Goll, J.B.; Beigel, J.H. Erythema and induration after mpox (JYNNEOS) vaccination revisited. N. Engl. J. Med. 2023, 388, 1432–1435. [Google Scholar] [CrossRef]
- European Medicines Agency. EMA’s Emergency Task Force Advises on Intradermal Use of Imvanex/Jynneos Against Monkeypox. 2022. Available online: https://www.ema.europa.eu/en/news/emas-emergency-task-force-advises-intradermal-use-imvanex-jynneos-against-monkeypox (accessed on 12 March 2025).
- World Health Organization. Smallpox and Mpox (Orthopoxviruses): WHO Position Paper, August 2024. Available online: https://www.who.int/publications/i/item/who-wer-9934-429-456 (accessed on 26 May 2025).
- Pang, Y.; Cao, D.; Zhu, X.; Long, Q.; Tian, F.; Long, X.; Li, Y. Safety and Efficacy of the Modified Vaccinia Ankara-Bavaria Nordic Vaccine Against Mpox in the Real World: Systematic Review and Meta-Analysis. Viral Immunol. 2024, 37, 216–219. [Google Scholar] [CrossRef]
- Yeganeh, N.; Yin, S.; Moir, O.; Danza, P.; Kim, M.; Finn, L.; Fisher, R.; Kulkarni, S.; Perez, M.; Poortinga, K.; et al. Effectiveness of JYNNEOS vaccine against symptomatic mpox disease in adult men in Los Angeles County, August 29, 2022 to January 1, 2023. Vaccine 2024, 42, 125987. [Google Scholar] [CrossRef]
- Ramchandani, M.S.; Berzkalns, A.; Cannon, C.A.; Dombrowski, J.C.; Brown, E.; Chow, E.J.; Barash, E.; Pogosjans, S.; Smith, D.; Golden, M.R. Effectiveness of the Modified Vaccinia Ankara Vaccine Against Mpox in Men Who Have Sex With Men: A Retrospective Cohort Analysis, Seattle, Washington. Open Forum Infect. Dis. 2023, 10, ofad528. [Google Scholar] [CrossRef]
- Brousseau, N.; Carazo, S.; Febriani, Y.; Padet, L.; Hegg-Deloye, S.; Cadieux, G.; Bergeron, G.; Fafard, J.; Charest, H.; Lambert, G.; et al. Single-dose Effectiveness of Mpox Vaccine in Quebec, Canada: Test-negative Design With and Without Adjustment for Self-reported Exposure Risk. Clin. Infect. Dis. 2024, 78, 461–469. [Google Scholar] [CrossRef]
- Hillus, D.; Le, N.H.; Tober-Lau, P.; Fietz, A.-K.; Hoffmann, C.; Stegherr, R.; Huang, L.; Baumgarten, A.; Voit, F.; Bickel, M.; et al. Safety and effectiveness of MVA-BN vaccination against mpox in at-risk individuals in Germany (SEMVAc and TEMVAc): A combined prospective and retrospective cohort study. Lancet Infect. Dis. 2025. [Google Scholar] [CrossRef]
- Sauer, C.M.; Chen, L.C.; Hyland, S.L.; Girbes, A.; Elbers, P.; Celi, L.A. Leveraging electronic health records for data science: Common pitfalls and how to avoid them. Lancet Digit. Health 2022, 4, e893–e898. [Google Scholar] [CrossRef]
- Scola, G.; Chis Ster, A.; Bean, D.; Pareek, N.; Emsley, R.; Landau, S. Implementation of the trial emulation approach in medical research: A scoping review. BMC Med. Res. Methodol. 2023, 23, 186. [Google Scholar] [CrossRef]
- Matthews, A.A.; Danaei, G.; Islam, N.; Kurth, T. Target trial emulation: Applying principles of randomised trials to observational studies. BMJ 2022, 378, e071108. [Google Scholar] [CrossRef] [PubMed]
- Hernán, M.A.; Wang, W.; Leaf, D.E. Target Trial Emulation: A Framework for Causal Inference from Observational Data. JAMA 2022, 328, 2446–2467. [Google Scholar] [CrossRef] [PubMed]
- Fontán-Vela, M.; Hernando, V.; Olmedo, C.; Coma, E.; Martínez, M.; Moreno-Perez, D.; Lorusso, N.; Torres, M.N.; del Buey, J.F.B.; Roig-Sena, J.; et al. Effectiveness of Modified Vaccinia Ankara-Bavaria Nordic Vaccination in a Population at High Risk of Mpox: A Spanish Cohort Study. Clin. Infect. Dis. 2024, 78, 476–483. [Google Scholar] [CrossRef]
- Navarro, C.; Lau, C.; Buchan, S.A.; Burchell, A.N.; Nasreen, S.; Friedman, L.; Okpokoro, E.; Austin, P.C.; Tan, D.H.S.; Gubbay, J.B.; et al. Effectiveness of modified vaccinia Ankara-Bavarian Nordic vaccine against mpox infection: Emulation of a target trial. BMJ 2024, 386, e078243. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Ulm, K. Simple method to calculate the confidence interval of a standardized mortality ratio (SMR). Am. J. Epidemiol. 1990, 131, 373–375. [Google Scholar] [CrossRef]
- Rosen, J.B.; Arciuolo, R.J.; Pathela, P.; Boyer, C.B.; Baumgartner, J.; Latash, J.; Malec, L.; Lee, E.H.; Reddy, V.; King, R.; et al. JYNNEOSTM effectiveness as post-exposure prophylaxis against mpox: Challenges using real-world outbreak data. Vaccine 2024, 42, 548–555. [Google Scholar] [CrossRef]
- Mason, L.M.K.; Betancur, E.; Riera-Montes, M.; Lienert, F.; Scheele, S. MVA-BN vaccine effectiveness: A systematic review of real-world evidence in outbreak settings. Vaccine 2024, 42, 126409. [Google Scholar] [CrossRef] [PubMed]
- Pischel, L.; Martini, B.A.; Yu, N.; Cacesse, D.; Tracy, M.; Kharbanda, K.; Ahmed, N.; Patel, K.M.; Grimshaw, A.A.; Malik, A.A.; et al. Vaccine effectiveness of 3rd generation mpox vaccines against mpox and disease severity: A systematic review and meta-analysis. Vaccine 2024, 42, 126053. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Lopez, L.K.; Glover, C.; Cashman, P.; Reynolds, R.; Macartney, K.; Wood, N. Short-term Adverse Events Following Immunization With Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) Vaccine for Mpox. JAMA 2023, 329, 2091. [Google Scholar] [CrossRef] [PubMed]
- Montalti, M.; Di Valerio, Z.; Angelini, R.; Bovolenta, E.; Castellazzi, F.; Cleva, M.; Pandolfi, P.; Reali, C.; Resi, D.; Todeschini, R.; et al. Safety of Monkeypox Vaccine Using Active Surveillance, Two-Center Observational Study in Italy. Vaccines 2023, 11, 1163. [Google Scholar] [CrossRef]
- Chang, E.M.; Gillespie, E.F.; Shaverdian, N. Truthfulness in patient-reported outcomes: Factors affecting patients’ responses and impact on data quality. Patient Relat. Outcome Meas. 2019, 10, 171–186. [Google Scholar] [CrossRef]

| Vaccinated n = 514 | Unvaccinated n = 514 | SMD | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), median [IQR] | 34 [28, 40] | 34 [28, 40] | <0.001 |
| Born ≤ 1974, n (%) | 36 (7.0%) | 36 (7.0%) | 0.003 |
| Gender, n (%) | |||
| Cisgender men | 456 (88.7) | 437 (85.0) | 0.148 |
| Transgender men | 5 (1.0) | 5 (1.0) | |
| Cisgender women | 5 (1.0) | 13 (2.5) | |
| Transgender women | 12 (2.3) | 15 (2.9) | |
| Non-binary individuals | 8 (1.6) | 12 (2.3) | |
| Other 1 | 18 (3.5) | 19 (3.7) | |
| Prefer not to answer | 10 (1.9) | 13 (2.5) | |
| Ethnicity, n (%) | |||
| Asian | 6 (1.2) | 2 (0.4) | 0.117 |
| Black | 10 (1.9) | 10 (1.9) | |
| Latin American | 217 (42.2) | 220 (42.8) | |
| Middle East | 2 (0.4) | 4 (0.8) | |
| White | 265 (51.6) | 259 (50.4) | |
| Other | 14 (2.7) | 19 (3.7) | |
| Sexual partner, n (%) | |||
| Men | 480 (94.1) | 473 (92.9) | 0.076 |
| Women | 6 (1.2) | 11 (2.2) | |
| Both | 24 (4.7) | 25 (4.9) | |
| Country of recruitment, n (%) | |||
| Spain | 294 (57.2) | 294 (57.2) | <0.001 |
| Peru | 170 (33.1) | 170 (33.1) | |
| Panama | 48 (9.3) | 48 (9.3) | |
| Chile | 2 (0.4) | 2 (0.4) | |
| Site of recruitment, n (%) | |||
| Health center 2 | 379 (74.9) | 244 (48.4) | 0.576 |
| Social media | 40 (7.9) | 106 (21.0) | |
| Events | 51 (10.1) | 85 (16.9) | |
| Other | 36 (7.1) | 69 (13.7) | |
| Clinical characteristics | |||
| Smallpox vaccination before 2022, n (%) | 87 (16.9) | 97 (18.9) | 0.053 |
| People living with HIV, n (%) | 183 (35.6) | 183 (35.6) | <0.001 |
| Currently undergoing ART, n (%) | 176 (98.3) | 178 (98.3) | <0.001 |
| CD4 count (cells per mm3), median [IQR] | 601 [324, 943] | 636 [445, 878] | 0.101 |
| CD4 count groups, n (%) | |||
| <350 cells per mm3 | 36 (29.5) | 21 (17.5) | 0.284 |
| ≥350 cells per mm3 | 86 (70.5) | 99 (82.5) | |
| Immunosuppressive disease, n (%) | 61 (12.0) | 55 (10.8) | 0.031 |
| Immunosuppressive treatment, n (%) | 3 (0.6) | 2 (0.4) | 0.029 |
| Use of preexposure prophylaxis against HIV, n (%) | 138 (41.7) | 138 (41.7) | 0.005 |
| STI in the past 12 months, n (%) | 199 (38.7) | 201 (39.3) | 0.019 |
| Anogenital Molluscum contagiosum | 0 (0.0) | 4 (0.8) | 0.125 |
| Chlamydia | 65 (12.6) | 66 (12.8) | 0.006 |
| Gonorrhoea | 86 (16.7) | 85 (16.5) | 0.005 |
| Genital warts | 18 (3.5) | 13 (2.5) | 0.057 |
| Herpes simplex virus | 18 (3.5) | 15 (2.9) | 0.033 |
| Lymphogranuloma venereum (LGV) | 2 (0.4) | 3 (0.6) | 0.028 |
| Scabies/Pthirus pubis | 11 (2.1) | 14 (2.7) | 0.038 |
| Syphilis | 78 (15.2) | 77 (15.0) | 0.005 |
| Does not know/Does not remember which | 3 (0.6) | 8 (1.6) | 0.095 |
| Sexual behavior | |||
| Number of sexual partners in the past 12 months, n (%) | |||
| None | 5 (1.0) | 16 (3.1) | 0.159 |
| 1 | 48 (9.3) | 46 (8.9) | |
| 2–9 | 202 (39.3) | 187 (36.4) | |
| >10 | 259 (50.4) | 265 (51.6) | |
| Number of sexual partners in the past 3 months, median [IQR] | 5 [2, 10] | 5 [2, 10] | 0.041 |
| Chemsex in the past 6 months, n (%) | 91 (17.7) | 117 (22.8) | 0.131 |
| Attendance to SOPV in the past 6 months, n (%) | 148 (28.8) | 161 (31.3) | 0.056 |
| Sex with a person with mpox in the past 3 months, n (%) | |||
| Yes, a confirmed mpox | 18 (3.5) | 29 (5.7) | 0.115 |
| Yes, a suspected mpox | 27 (5.3) | 30 (5.9) |
| Vaccinated n = 514 | Unvaccinated n = 514 | SMD | |
|---|---|---|---|
| Diagnosis of STIs during follow-up | |||
| Participants who answered/Number of answers, n (%)/n | 495 (96.3)/3027 | 499 (97.1)/2876 | 0.085 |
| Answers per participant, median [IQR] | 6 [3, 9] | 5 [3, 9] | |
| STIs reported, n (%) | |||
| Any STI | 220 (42.9) | 245 (47.8) | 0.098 |
| Anogenital Molluscum contagiosum | 2 (0.4) | 3 (0.6) | 0.028 |
| Chlamydia | 65 (12.6) | 61 (11.9) | 0.024 |
| Genital warts | 17 (3.3) | 21 (4.1) | 0.041 |
| Gonorrhoea | 86 (16.7) | 105 (20.4) | 0.095 |
| Herpes simplex virus | 13 (2.5) | 23 (4.5) | 0.106 |
| Lymphogranuloma venereum (LGV) | 5 (1.0) | 4 (0.8) | 0.021 |
| Scabies/Pthirus pubis | 10 (1.9) | 19 (3.7) | 0.106 |
| Syphilis | 77 (15.0) | 94 (18.3) | 0.089 |
| New HIV (% of People living without HIV at baseline) | 5 (1.5) | 5 (1.5) | <0.001 |
| Does not know/Does not remember which | 8 (1.6) | 18 (3.5) | 0.124 |
| Number of STIs reported per participant during follow-up, n (%) | |||
| 0 | 276 (53.7) | 261 (50.8) | 0.134 |
| 1 | 78 (15.2) | 83 (16.4) | |
| 2 | 65 (12.7) | 57 (11.3) | |
| 3 or more | 76 (14.8) | 98 (19.4) | |
| Number of STIs diagnosed during follow-up/subject, median [IQR] | 0 [0, 2] | 0 [0, 2] | 0.119 |
| Immunosuppressive disease or treatment | |||
| New immunosuppressive disease or treatment, n (%) | 4 (0.8) | 3 (0.6) | 0.027 |
| Sexual partners in the past month | |||
| Participants who answered/Number of answers, n (%)/n | 492 (95.7)/2942 | 495 (96.3)/2787 | 0.087 |
| Answers per participant, median [IQR] | 6 [3, 8] | 5 [2, 9] | |
| Number of sexual partners in the past month, median [IQR] | 3 [1, 8] | 4 [2, 8] | 0.037 |
| Chemsex in the past month | |||
| Participants who answered/Number of answers, n (%)/n | 494 (96.1)/3021 | 500 (97.3)/2879 | 0.080 |
| Answers per participant, median [IQR] | 6 [3, 9] | 5 [2, 9] | |
| Chemsex, n (%) | 127 (24.8) | 151 (29.4) | 0.105 |
| Regular chemsex, n (%) | 58 (11.3) | 85 (16.6) | 0.153 |
| Sex in social venues in the past month | |||
| Participants who answered/Number of answers, n (%)/n | 495 (96.3)/3005 | 500 (97.3)/2853 | 0.086 |
| Answers per participant, median [IQR] | 6 [3, 9] | 5 [2, 9] | |
| Attendance to SOPV, n (%) | 178 (34.7) | 207 (40.4) | 0.117 |
| Regular attendance to SOPV, n (%) | 72 (14.0) | 115 (22.4) | 0.219 |
| Sex with a person with mpox in the past month | |||
| Participants who answered/Number of answers, n (%)/n | 494 (96.1)/3021 | 500 (97.3)/2867 | 0.087 |
| Answers per participant, median [IQR] | 6 [3, 9] | 5 [2, 9] | |
| Sex with a person with mpox, n (%) | 72 (14.0) | 73 (14.2) | 0.006 |
| Yes, confirmed mpox, n (%) | 21 (29.2) | 35 (47.9) | 0.393 |
| Yes, suspected mpox, n (%) | 51 (70.8) | 38 (52.1) | |
| Number of times of reported sex with a person with mpox, median [IQR] | 1 [1, 2] | 1 [1, 2] | 0.076 |
| Skin Adverse Reactions | No. (%) |
|---|---|
| Any skin adverse reaction 1 | |
| Yes | 703 (47.7) |
| No | 731 (49.6) |
| Number of skin adverse reactions/participant 1 | |
| 1 | 101 (14.4) |
| 2 | 105 (14.9) |
| 3 or more | 497 (70.7) |
| Skin adverse reactions 1 | |
| Itch | 455 (30.8) |
| Pain | 106 (15.1) |
| Rash at the injection site | 160 (10.8) |
| Rash distant to the injection site | 15 (1) |
| Rash surrounding the injection site | 120 (8.1) |
| Erythema at the injection site | 541 (36.7) |
| Swelling | 463 (31.4) |
| Wound/Ulcer/Pus | 13 (0.9) |
| Others | 541 (36.7) |
| Skin adverse reaction within 2 h after the injection 2 | 499 (73.9) |
| Systemic adverse reactions | |
| Any systemic adverse reactions 1 | |
| Yes | 107 (7.3) |
| No | 1368 (92.7) |
| Number of systemic adverse reactions/participant 1 | |
| 1 | 38 (37.3) |
| 2 | 27 (26.5) |
| 3 or more | 37 (36.3) |
| Systemic adverse reactions 1 | |
| Fever ≥ 38 °C | 27 (1.8) |
| Headache | 41 (2.8) |
| Malaise/Fatigue | 60 (4.1) |
| Muscle pain | 47 (3.2) |
| Nausea/Vomit/Diarrhea | 11 (0.7) |
| Others | 33 (2.2) |
| Systemic adverse reaction within 2 h after the injection 2 | 48 (48.0) |
| Any adverse reaction (skin or systemic) | |
| Any adversereaction (skin or systemic) 1 | |
| Yes | 731 (49.6) |
| No | 744 (50.4) |
| Consequences derived from adverse reactions 3 | |
| Medical treatment required | 36 (4.9) |
| Interfered with daily life activities | 35 (4.8) |
| Hospitalization | 1 (0.1) |
| Death | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suñer, C.; Escrig-Sarreta, R.; Galván-Casas, C.; Matos, E.; Gabster, A.; Wolff, M.; Ouchi, D.; Alemany, A.; Sánchez, H.; Huaman, S.; et al. Target Trial Emulation of the Modified Vaccinia Ankara-Bavarian Nordic Vaccine for Pre-Exposure Mpox Prevention in At-Risk Populations. Vaccines 2025, 13, 594. https://doi.org/10.3390/vaccines13060594
Suñer C, Escrig-Sarreta R, Galván-Casas C, Matos E, Gabster A, Wolff M, Ouchi D, Alemany A, Sánchez H, Huaman S, et al. Target Trial Emulation of the Modified Vaccinia Ankara-Bavarian Nordic Vaccine for Pre-Exposure Mpox Prevention in At-Risk Populations. Vaccines. 2025; 13(6):594. https://doi.org/10.3390/vaccines13060594
Chicago/Turabian StyleSuñer, Clara, Roser Escrig-Sarreta, Cristina Galván-Casas, Eduardo Matos, Amanda Gabster, Marcelo Wolff, Dan Ouchi, Andrea Alemany, Hugo Sánchez, Sandra Huaman, and et al. 2025. "Target Trial Emulation of the Modified Vaccinia Ankara-Bavarian Nordic Vaccine for Pre-Exposure Mpox Prevention in At-Risk Populations" Vaccines 13, no. 6: 594. https://doi.org/10.3390/vaccines13060594
APA StyleSuñer, C., Escrig-Sarreta, R., Galván-Casas, C., Matos, E., Gabster, A., Wolff, M., Ouchi, D., Alemany, A., Sánchez, H., Huaman, S., Bejarano, D., Carrés-Esteve, L., Santiago-Fernández, C., Corral-Rubio, J., Mendoza, A., Rivero, À., Descalzo, V., Orviz, E., Martínez-Riveros, H., ... REMAIN Study Group. (2025). Target Trial Emulation of the Modified Vaccinia Ankara-Bavarian Nordic Vaccine for Pre-Exposure Mpox Prevention in At-Risk Populations. Vaccines, 13(6), 594. https://doi.org/10.3390/vaccines13060594







