SARS-CoV-2 Variant-Specific Antibodies in Vaccinated Inflammatory Bowel Disease Patients
Abstract
1. Introduction
2. Materials and Methods
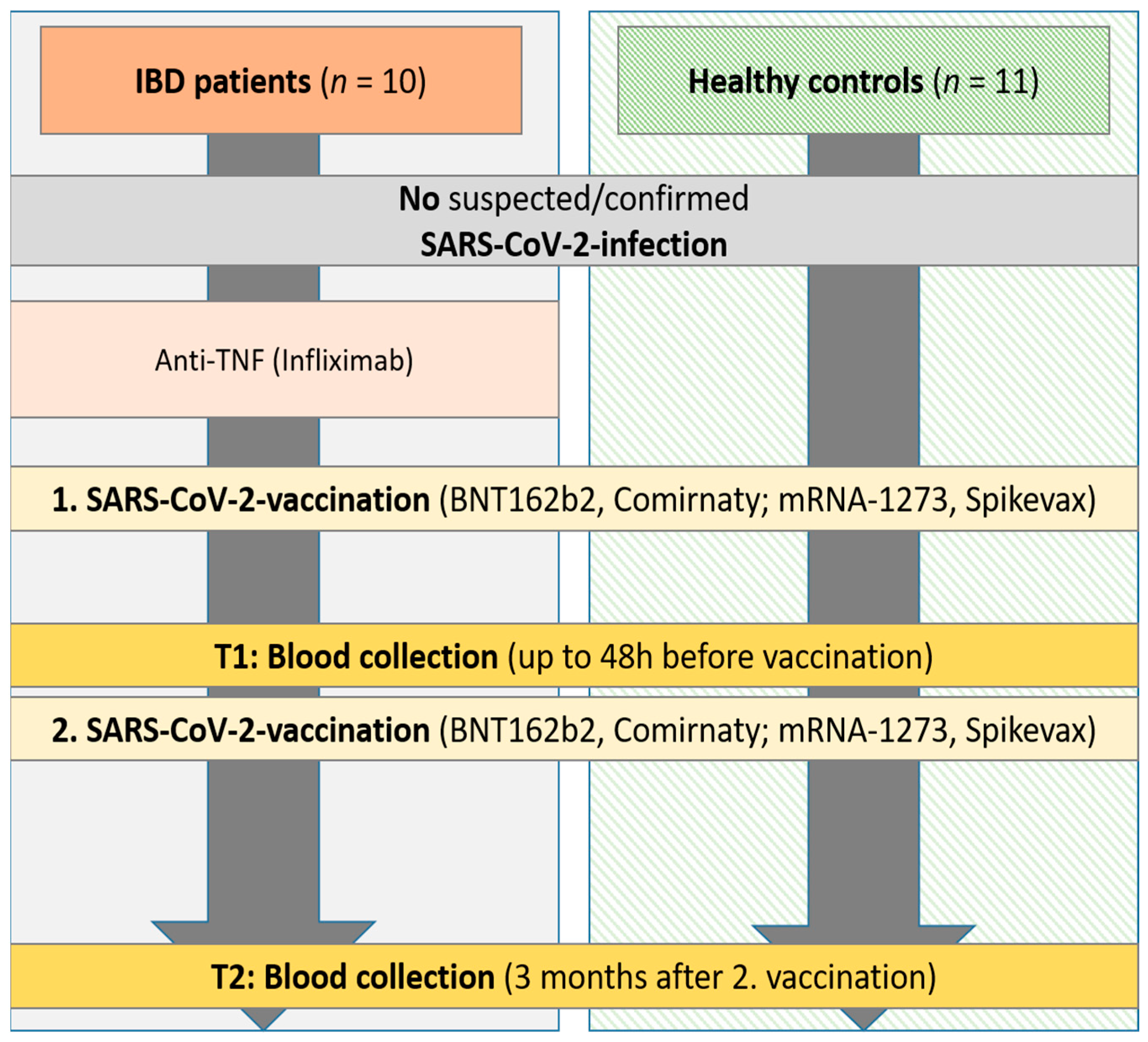
2.1. Study Subjects and Samples
2.2. Characterization of Sera with Commercial Assays
2.3. Characterization of the Neutralizing Capacity of Sera with In-House Assays
2.3.1. VSV-Pseudotyped Virus Neutralization Assay
2.3.2. Surrogate Virus Neutralization Assay
2.4. Statistical Analysis
3. Results
3.1. Cohort Characteristics
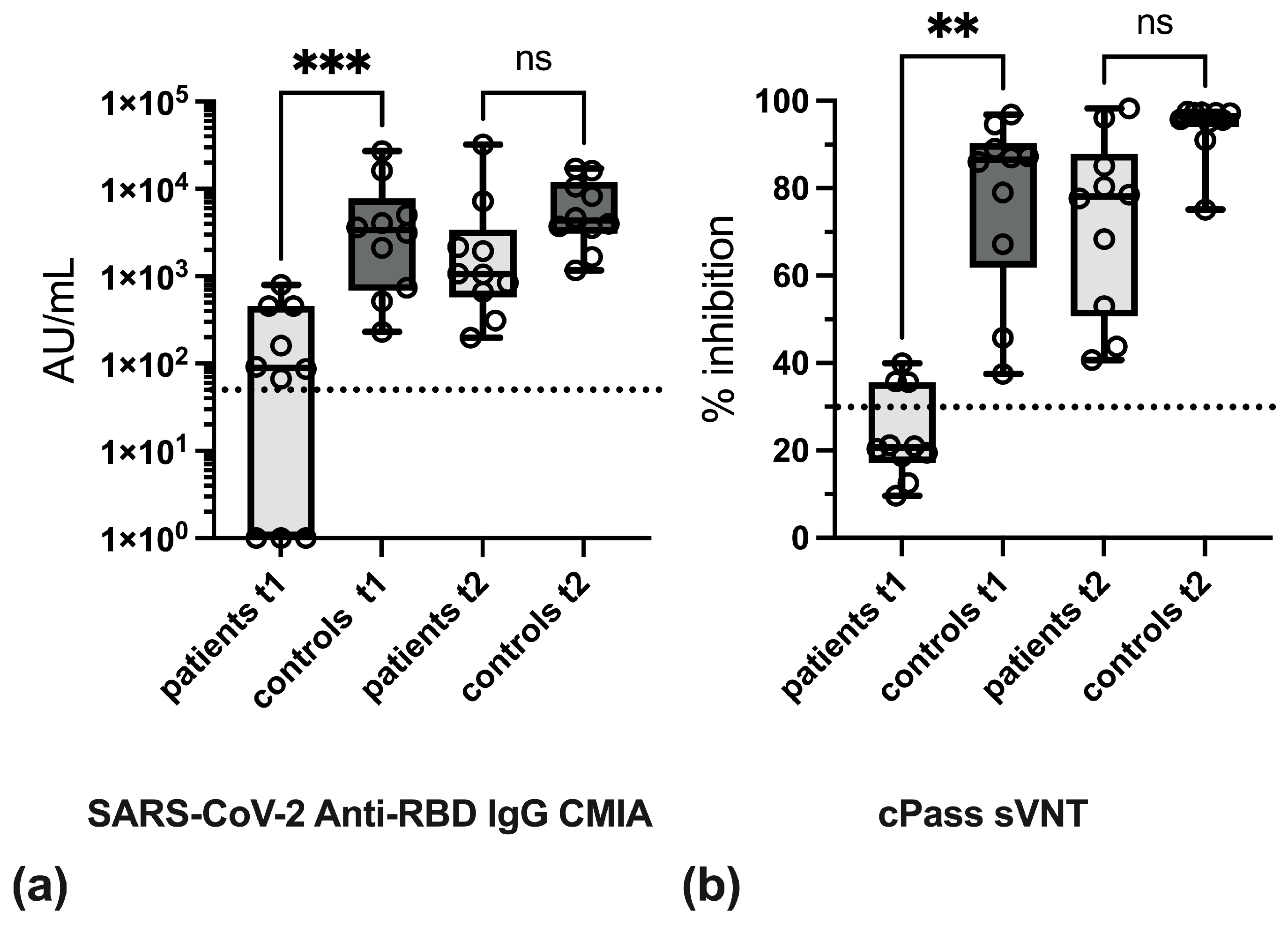
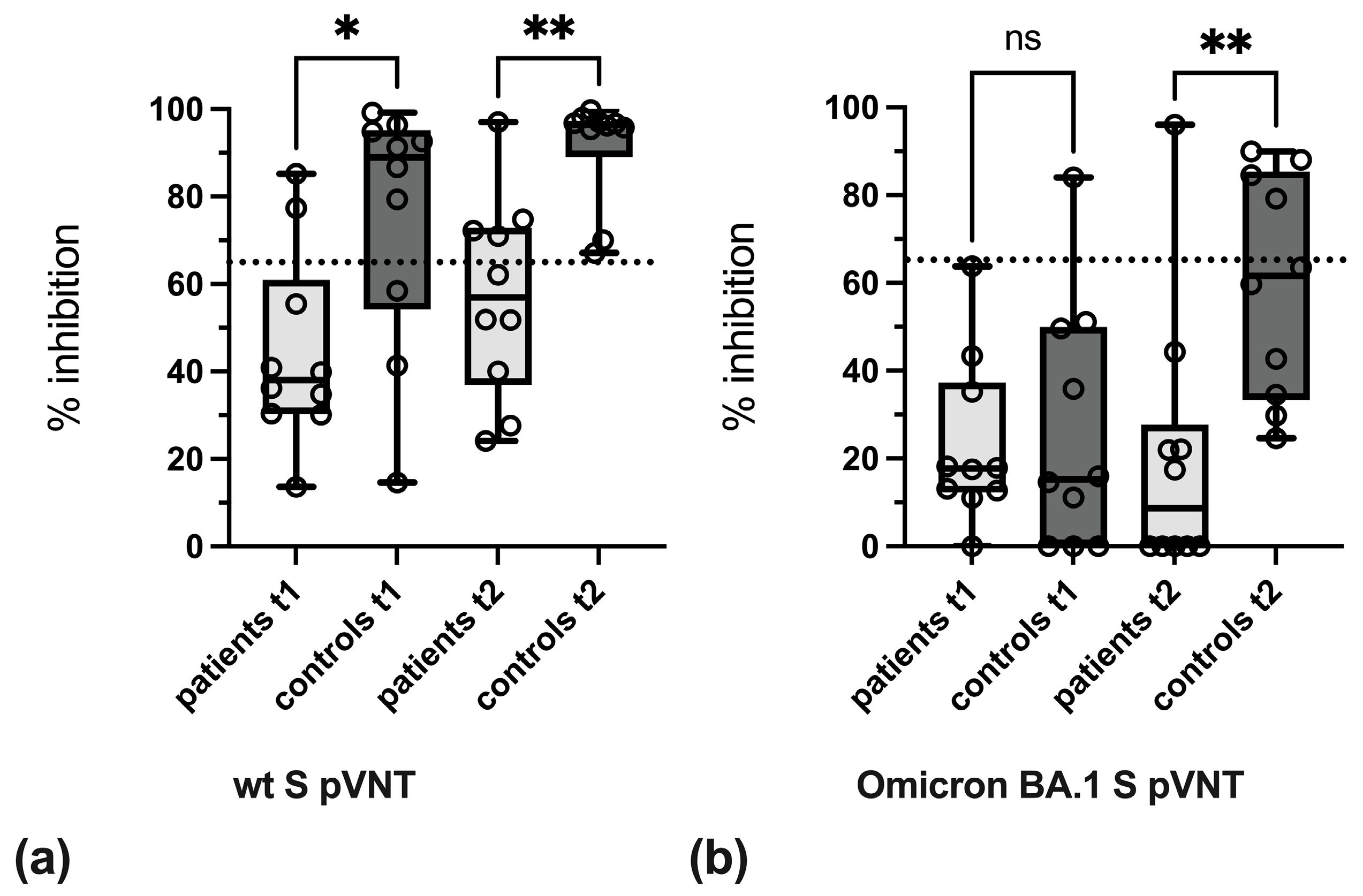
3.2. Effect of Anti-TNF Therapy on Antibody Levels
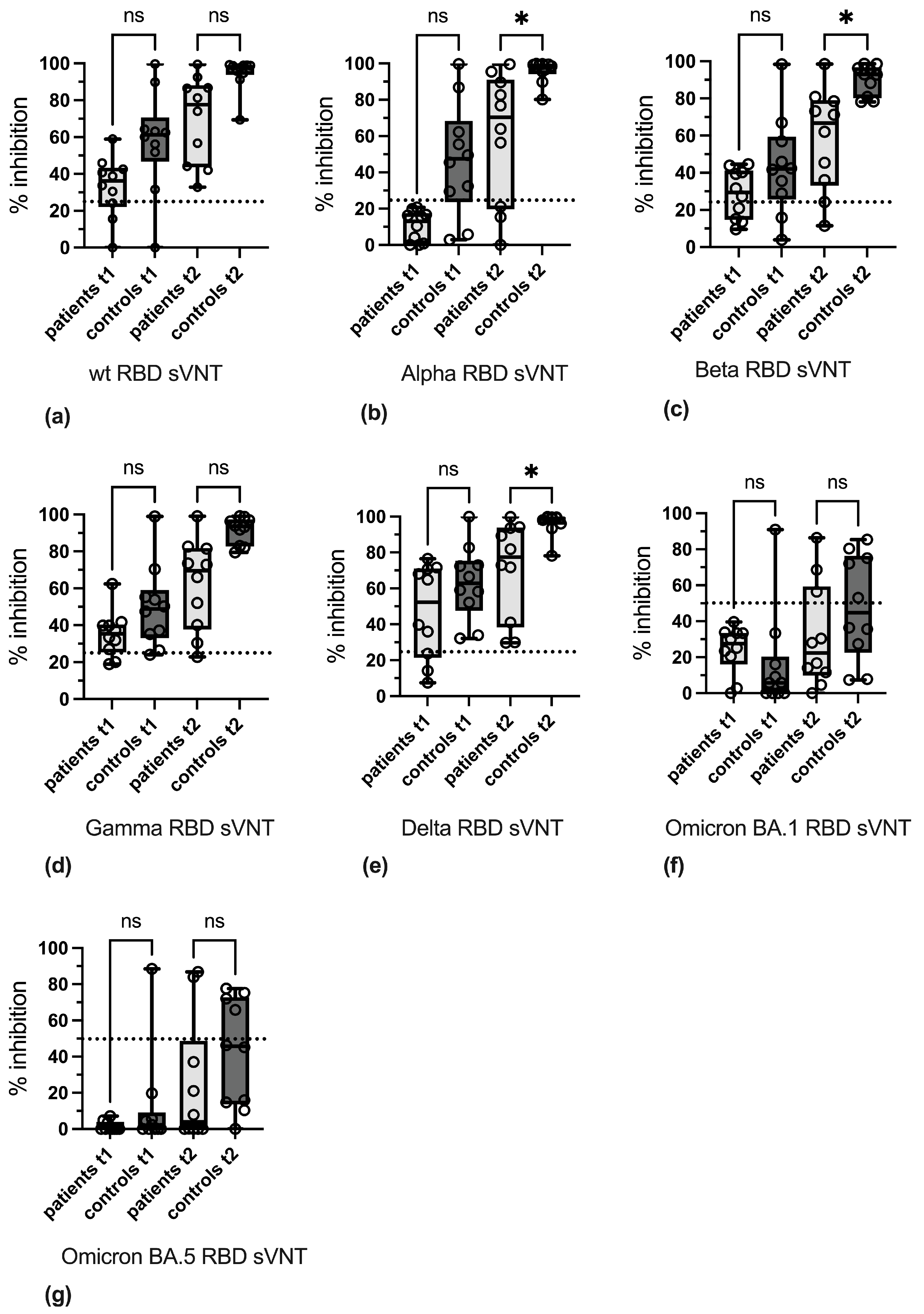
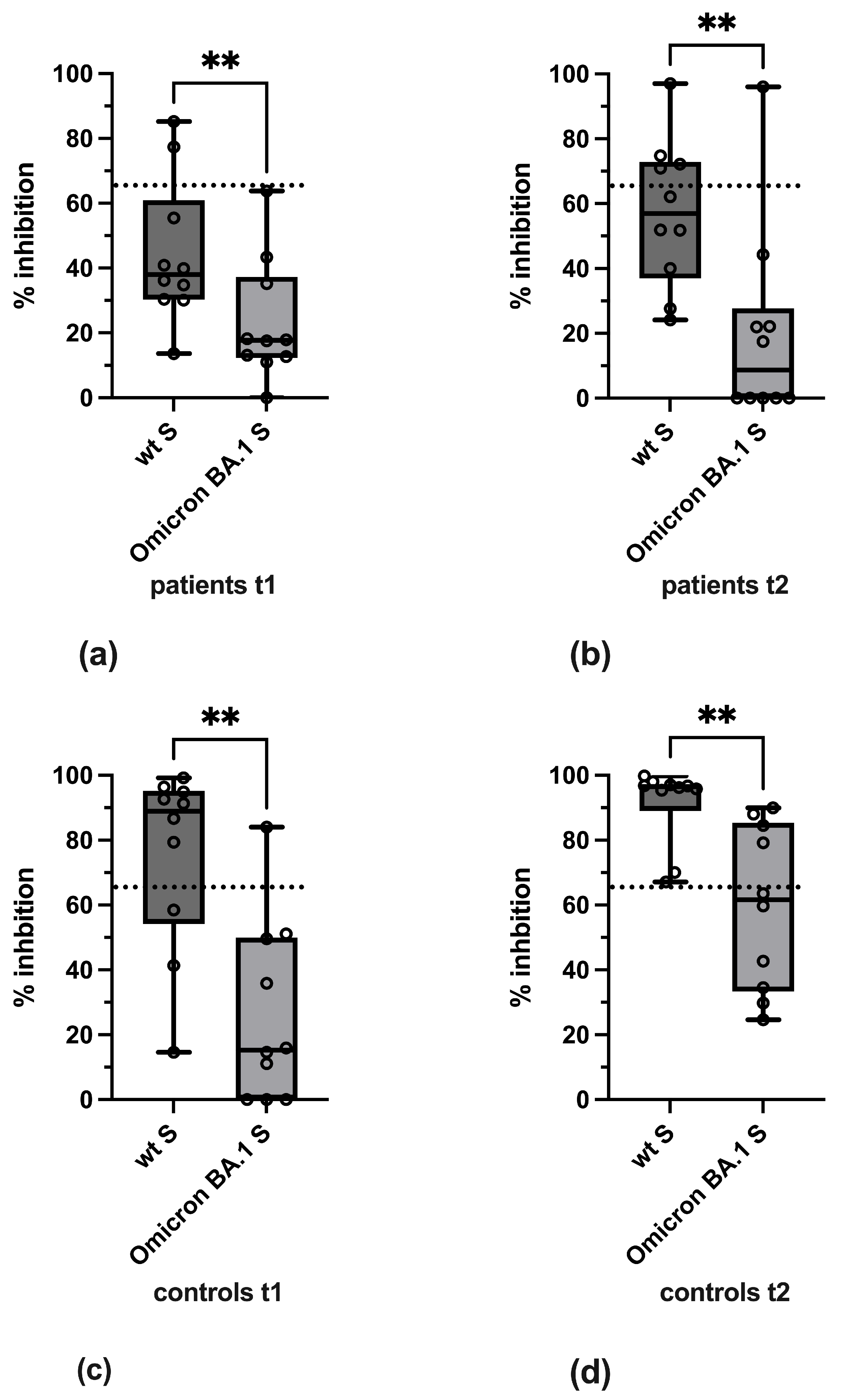
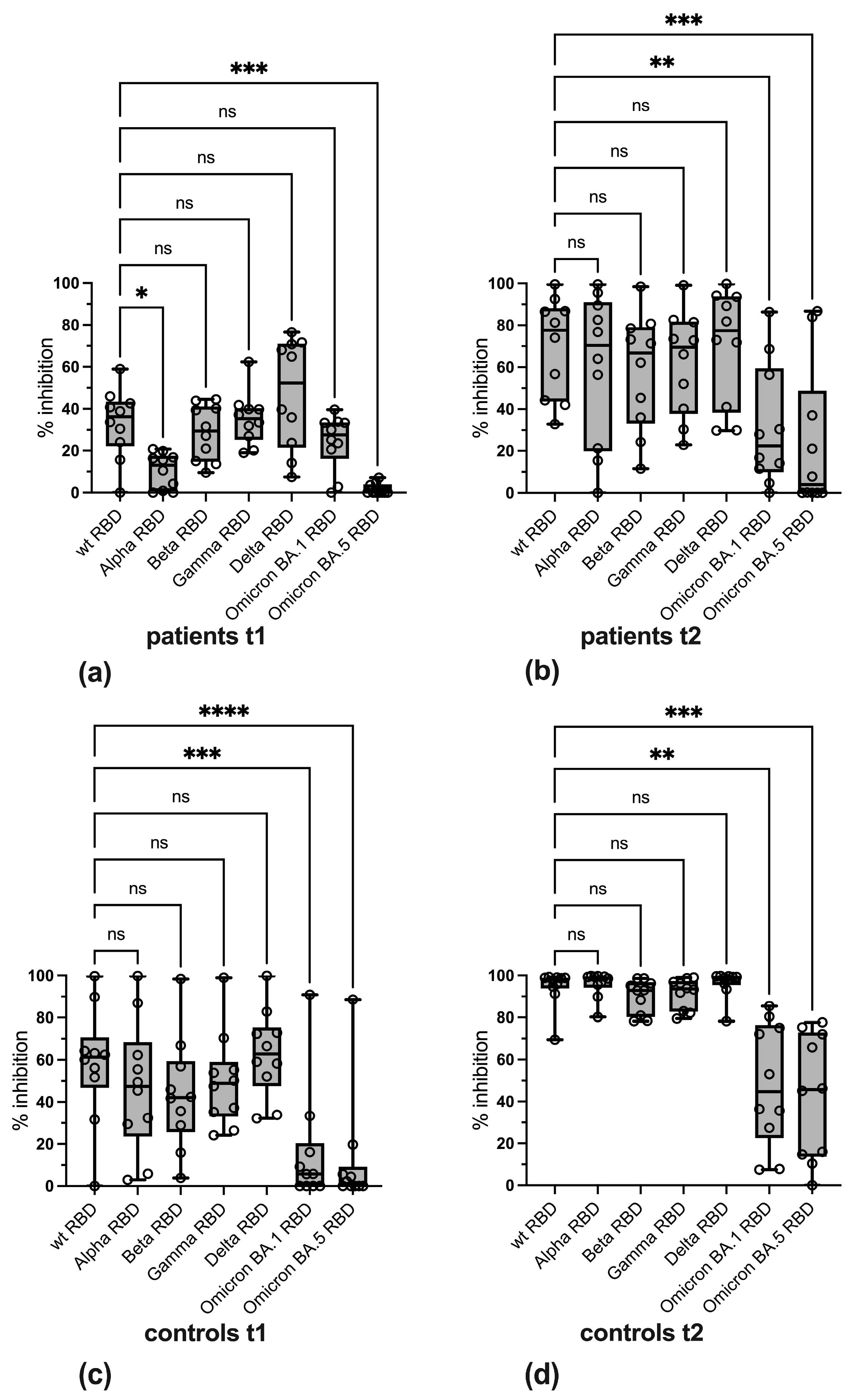
3.3. Variant-Specific Differences in Reactivity
3.4. Qualitative Evaluation of SARS-CoV-2-Specific Antibody Reactivity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| AU | arbitrary units |
| BAU | binding antibody units |
| CMIA | chemiluminescence microparticle assay |
| hACE2 | human angiotensin-converting enzyme 2 |
| NLuc | nanoluciferase |
| pVNT | VSV-pseudotyped virus neutralization assay |
| RBD | receptor-binding domain |
| ROC | receiver operating characteristic |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| sVNT | surrogate virus neutralization test |
| TNF | tumor necrosis factor |
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef]
- Vollenberg, R.; Matern, P.; Nowacki, T.; Fuhrmann, V.; Padberg, J.S.; Ochs, K.; Schutte-Nutgen, K.; Strauss, M.; Schmidt, H.; Tepasse, P.R. Prone Position in Mechanically Ventilated COVID-19 Patients: A Multicenter Study. J. Clin. Med. 2021, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Torres Acosta, M.A.; Singer, B.D. Pathogenesis of COVID-19-induced ARDS: Implications for an ageing population. Eur. Respir. J. 2020, 56, 2002049. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Kessel, C.; Vollenberg, R.; Masjosthusmann, K.; Hinze, C.; Wittkowski, H.; Debaugnies, F.; Nagant, C.; Corazza, F.; Vely, F.; Kaplanski, G.; et al. Discrimination of COVID-19 From Inflammation-Induced Cytokine Storm Syndromes Using Disease-Related Blood Biomarkers. Arthritis Rheumatol. 2021, 73, 1791–1799. [Google Scholar] [CrossRef]
- Jose, R.J.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms Among Patients with COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef]
- Deer, R.R.; Rock, M.A.; Vasilevsky, N.; Carmody, L.; Rando, H.; Anzalone, A.J.; Basson, M.D.; Bennett, T.D.; Bergquist, T.; Boudreau, E.A.; et al. Characterizing Long COVID: Deep Phenotype of a Complex Condition. EBioMedicine 2021, 74, 103722. [Google Scholar] [CrossRef]
- Varghese, J.; Sandmann, S.; Ochs, K.; Schrempf, I.M.; Frommel, C.; Dugas, M.; Schmidt, H.H.; Vollenberg, R.; Tepasse, P.R. Persistent symptoms and lab abnormalities in patients who recovered from COVID-19. Sci. Rep. 2021, 11, 12775. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Torres, J.; Mehandru, S.; Colombel, J.F.; Peyrin-Biroulet, L. Crohn’s disease. Lancet 2017, 389, 1741–1755. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kaitha, S.; Mahmood, S.; Ftesi, A.; Stone, J.; Bronze, M.S. Clinical use of anti-TNF therapy and increased risk of infections. Drug Health Patient Saf. 2013, 5, 79–99. [Google Scholar] [CrossRef]
- Vollenberg, R.; Lorentzen, E.U.; Kuhn, J.; Nowacki, T.M.; Meier, J.A.; Trebicka, J.; Tepasse, P.R. Humoral Immunity in Immunosuppressed IBD Patients after the Third SARS-CoV-2 Vaccination: A Comparison with Healthy Control Subjects. Vaccines 2023, 11, 1411. [Google Scholar] [CrossRef]
- Vollenberg, R.; Tepasse, P.R.; Kuhn, J.E.; Hennies, M.; Strauss, M.; Rennebaum, F.; Schomacher, T.; Boeckel, G.; Lorentzen, E.; Bokemeyer, A.; et al. Humoral Immune Response in IBD Patients Three and Six Months after Vaccination with the SARS-CoV-2 mRNA Vaccines mRNA-1273 and BNT162b2. Biomedicines 2022, 10, 171. [Google Scholar] [CrossRef]
- Vollenberg, R.; Tepasse, P.R.; Lorentzen, E.; Nowacki, T.M. Impaired Humoral Immunity with Concomitant Preserved T Cell Reactivity in IBD Patients on Treatment with Infliximab 6 Month after Vaccination with the SARS-CoV-2 mRNA Vaccine BNT162b2: A Pilot Study. J. Pers. Med. 2022, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Goodhand, J.R.; Bewshea, C.; Nice, R.; Chee, D.; Lin, S.; Chanchlani, N.; Butterworth, J.; Cooney, R.; Croft, N.M.; et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021, 70, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Le, K.; Zhou, X.; Alexander, J.L.; Lin, S.; Bewshea, C.; Chanchlani, N.; Nice, R.; McDonald, T.J.; Lamb, C.A.; et al. Neutralising antibody potency against SARS-CoV-2 wild-type and omicron BA.1 and BA.4/5 variants in patients with inflammatory bowel disease treated with infliximab and vedolizumab after three doses of COVID-19 vaccine (CLARITY IBD): An analysis of a prospective multicentre cohort study. Lancet Gastroenterol. Hepatol. 2023, 8, 145–156. [Google Scholar] [CrossRef]
- Lin, S.; Kennedy, N.A.; Saifuddin, A.; Sandoval, D.M.; Reynolds, C.J.; Seoane, R.C.; Kottoor, S.H.; Pieper, F.P.; Lin, K.M.; Butler, D.K.; et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat. Commun. 2022, 13, 1379. [Google Scholar] [CrossRef]
- Caldera, F.; Rolak, S.; Farraye, F.A.; Necela, B.M.; Cogen, D.; Zona, E.E.; Schell, T.L.; Ramirez, O.R.; Almasry, M.; Chun, K.; et al. Higher and Sustained Cell-Mediated Immune Responses After 3 Doses of mRNA COVID-19 Vaccine in Patients with Inflammatory Bowel Disease on Anti-Tumor Necrosis Factor Therapy. Clin. Transl. Gastroenterol. 2024, 15, e00688. [Google Scholar] [CrossRef]
- Martinez-Dominguez, S.J.; Garcia-Mateo, S.; Sainz-Arnal, P.; Martinez-Garcia, J.; Gallego-Llera, B.; Lozano-Limones, M.J.; Hidalgo, S.; Gargallo-Puyuelo, C.J.; Latre-Santos, M.; Nocito-Colon, M.M.L.; et al. Unravelling the cellular response to the SARS-COV-2 vaccine in inflammatory bowel disease patients on biologic drugs. Sci. Rep. 2023, 13, 23061. [Google Scholar] [CrossRef]
- Schell, T.L.; Caldera, F. A Practical Update on COVID-19 and Inflammatory Bowel Disease: COVID-19 Disease Risk and Vaccine Safety and Efficacy. Gastroenterol. Hepatol. 2024, 20, 88–97. [Google Scholar]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Viana, R.; Moyo, S.; Amoako, D.G.; Tegally, H.; Scheepers, C.; Althaus, C.L.; Anyaneji, U.J.; Bester, P.A.; Boni, M.F.; Chand, M.; et al. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature 2022, 603, 679–686. [Google Scholar] [CrossRef]
- Pulliam, J.R.C.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased risk of SARS-CoV-2 reinfection associated with emergence of Omicron in South Africa. Science 2022, 376, eabn4947. [Google Scholar] [CrossRef] [PubMed]
- Christensen, P.A.; Olsen, R.J.; Long, S.W.; Snehal, R.; Davis, J.J.; Ojeda Saavedra, M.; Reppond, K.; Shyer, M.N.; Cambric, J.; Gadd, R.; et al. Signals of Significantly Increased Vaccine Breakthrough, Decreased Hospitalization Rates, and Less Severe Disease in Patients with Coronavirus Disease 2019 Caused by the Omicron Variant of Severe Acute Respiratory Syndrome Coronavirus 2 in Houston, Texas. Am. J. Pathol. 2022, 192, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Martin-Arranz, M.D.; Garcia-Ramirez, L.; Hernandez-Perez, M.; Montero Vega, D.; Martin-Arranz, E.; Sanchez-Azofra, M.; Poza Cordon, J.; Rueda Garcia, J.L.; Noci Belda, J.; Verges Martinez-Meco, T.; et al. Seroprevalence of ANTI-SARS-CoV-2 antibodies in patients with inflammatory bowel disease. Sci. Rep. 2023, 13, 7044. [Google Scholar] [CrossRef]
- Schoefbaenker, M.; Neddermeyer, R.; Guenther, T.; Mueller, M.M.; Romberg, M.L.; Classen, N.; Hennies, M.T.; Hrincius, E.R.; Ludwig, S.; Kuehn, J.E.; et al. Surrogate Virus Neutralisation Test Based on Nanoluciferase-Tagged Antigens to Quantify Inhibitory Antibodies against SARS-CoV-2 and Characterise Omicron-Specific Reactivity in a Vaccination Cohort. Vaccines 2023, 11, 1832. [Google Scholar] [CrossRef]
- Springer, D.N.; Perkmann, T.; Jani, C.M.; Mucher, P.; Pruger, K.; Marculescu, R.; Reuberger, E.; Camp, J.V.; Graninger, M.; Borsodi, C.; et al. Reduced Sensitivity of Commercial Spike-Specific Antibody Assays after Primary Infection with the SARS-CoV-2 Omicron Variant. Microbiol. Spectr. 2022, 10, e0212922. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- von Rhein, C.; Scholz, T.; Henss, L.; Kronstein-Wiedemann, R.; Schwarz, T.; Rodionov, R.N.; Corman, V.M.; Tonn, T.; Schnierle, B.S. Comparison of potency assays to assess SARS-CoV-2 neutralizing antibody capacity in COVID-19 convalescent plasma. J. Virol. Methods 2021, 288, 114031. [Google Scholar] [CrossRef]
- Graninger, M.; Jani, C.M.; Reuberger, E.; Pruger, K.; Gaspar, P.; Springer, D.N.; Borsodi, C.; Weidner, L.; Rabady, S.; Puchhammer-Stockl, E.; et al. Comprehensive Comparison of Seven SARS-CoV-2-Specific Surrogate Virus Neutralization and Anti-Spike IgG Antibody Assays Using a Live-Virus Neutralization Assay as a Reference. Microbiol. Spectr. 2023, 11, e0231422. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.F.; Anhlan, D.; Schofbanker, M.; Schreiber, A.; Classen, N.; Hensel, A.; Hempel, G.; Scholz, W.; Kuhn, J.; Hrincius, E.R.; et al. Hypericum perforatum and Its Ingredients Hypericin and Pseudohypericin Demonstrate an Antiviral Activity against SARS-CoV-2. Pharmaceuticals 2022, 15, 530. [Google Scholar] [CrossRef]
- Schoefbaenker, M.; Gunther, T.; Lorentzen, E.U.; Romberg, M.L.; Hennies, M.T.; Neddermeyer, R.; Muller, M.M.; Mellmann, A.; Bojarzyn, C.R.; Lenz, G.; et al. Characterisation of the antibody-mediated selective pressure driving intra-host evolution of SARS-CoV-2 in prolonged infection. PLoS Pathog. 2024, 20, e1012624. [Google Scholar] [CrossRef]
- Glockner, S.; Hornung, F.; Baier, M.; Weis, S.; Pletz, M.W.; Deinhardt-Emmer, S.; Loffler, B.; The CoNAN Study Group. Robust Neutralizing Antibody Levels Detected after Either SARS-CoV-2 Vaccination or One Year after Infection. Viruses 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, A.; Wolf, J.; Fertey, J.; Kalbitz, S.; Schroth, S.; Lubbert, C.; Ulbert, S.; Borte, S. Correlation of humoral immune responses to different SARS-CoV-2 antigens with virus neutralizing antibodies and symptomatic severity in a German COVID-19 cohort. Emerg. Microbes Infect. 2021, 10, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Dolscheid-Pommerich, R.; Bartok, E.; Renn, M.; Kummerer, B.M.; Schulte, B.; Schmithausen, R.M.; Stoffel-Wagner, B.; Streeck, H.; Saschenbrecker, S.; Steinhagen, K.; et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J. Med. Virol. 2022, 94, 388–392. [Google Scholar] [CrossRef]
- Grunau, B.; Prusinkiewicz, M.; Asamoah-Boaheng, M.; Golding, L.; Lavoie, P.M.; Petric, M.; Levett, P.N.; Haig, S.; Barakauskas, V.; Karim, M.E.; et al. Correlation of SARS-CoV-2 Viral Neutralizing Antibody Titers with Anti-Spike Antibodies and ACE-2 Inhibition among Vaccinated Individuals. Microbiol. Spectr. 2022, 10, e0131522. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat. Med. 2021, 27, 1147–1148. [Google Scholar] [CrossRef]
- Martin Perez, C.; Aguilar, R.; Jimenez, A.; Salmeron, G.; Canyelles, M.; Rubio, R.; Vidal, M.; Cuamba, I.; Barrios, D.; Diaz, N.; et al. Correlates of protection and determinants of SARS-CoV-2 breakthrough infections 1 year after third dose vaccination. BMC Med. 2024, 22, 103. [Google Scholar] [CrossRef]
- Bubonja-Sonje, M.; Baticic, L.; Abram, M.; Cekinovic Grbesa, D. Diagnostic accuracy of three SARS-CoV2 antibody detection assays, neutralizing effect and longevity of serum antibodies. J. Virol. Methods 2021, 293, 114173. [Google Scholar] [CrossRef]
- Slotkin, R.; Kyriakides, T.C.; Kundu, A.; Stack, G.; Sutton, R.E.; Gupta, S. Correlation of a commercial platform’s results with post-vaccination SARS-CoV-2 neutralizing antibody response and clinical host factors. PLoS ONE 2023, 18, e0289713. [Google Scholar] [CrossRef]
- Infantino, M.; Pieri, M.; Nuccetelli, M.; Grossi, V.; Lari, B.; Tomassetti, F.; Calugi, G.; Pancani, S.; Benucci, M.; Casprini, P.; et al. The WHO International Standard for COVID-19 serological tests: Towards harmonization of anti-spike assays. Int. Immunopharmacol. 2021, 100, 108095. [Google Scholar] [CrossRef]
- Zhu, F.; Althaus, T.; Tan, C.W.; Costantini, A.; Chia, W.N.; Van Vinh Chau, N.; Van Tan, L.; Mattiuzzo, G.; Rose, N.J.; Voiglio, E.; et al. WHO international standard for SARS-CoV-2 antibodies to determine markers of protection. Lancet Microbe 2022, 3, e81–e82. [Google Scholar] [CrossRef] [PubMed]
- Muecksch, F.; Wise, H.; Templeton, K.; Batchelor, B.; Squires, M.; McCance, K.; Jarvis, L.; Malloy, K.; Furrie, E.; Richardson, C.; et al. Longitudinal variation in SARS-CoV-2 antibody levels and emergence of viral variants: A serological analysis. Lancet Microbe 2022, 3, e493–e502. [Google Scholar] [CrossRef] [PubMed]
- Giavarina, D.; Carta, M. Improvements and limits of anti SARS-CoV-2 antibodies assays by WHO (NIBSC 20/136) standardization. Diagnosis 2021, 9, 274–279. [Google Scholar] [CrossRef]
- Saker, K.; Escuret, V.; Pitiot, V.; Massardier-Pilonchery, A.; Paul, S.; Mokdad, B.; Langlois-Jacques, C.; Rabilloud, M.; Goncalves, D.; Fabien, N.; et al. Evaluation of Commercial Anti-SARS-CoV-2 Antibody Assays and Comparison of Standardized Titers in Vaccinated Health Care Workers. J. Clin. Microbiol. 2022, 60, e0174621. [Google Scholar] [CrossRef]
- Perkmann, T.; Perkmann-Nagele, N.; Koller, T.; Mucher, P.; Radakovics, A.; Marculescu, R.; Wolzt, M.; Wagner, O.F.; Binder, C.J.; Haslacher, H. Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: A Head-to-Head Comparison of Five Quantitative Assays. Microbiol. Spectr. 2021, 9, e0024721. [Google Scholar] [CrossRef] [PubMed]
- Karger, A.B.; Brien, J.D.; Christen, J.M.; Dhakal, S.; Kemp, T.J.; Klein, S.L.; Pinto, L.A.; Premkumar, L.; Roback, J.D.; Binder, R.A.; et al. The Serological Sciences Network (SeroNet) for COVID-19: Depth and Breadth of Serology Assays and Plans for Assay Harmonization. mSphere 2022, 7, e0019322. [Google Scholar] [CrossRef]
- Kroidl, I.; Winter, S.; Rubio-Acero, R.; Bakuli, A.; Geldmacher, C.; Eser, T.M.; Deak, F.; Horn, S.; Zielke, A.; Ahmed, M.I.M.; et al. Studying temporal titre evolution of commercial SARS-CoV-2 assays reveals significant shortcomings of using BAU standardization for comparison. Virol. J. 2023, 20, 200. [Google Scholar] [CrossRef]
- Walmsley, S.; Nabipoor, M.; Lovblom, L.E.; Ravindran, R.; Colwill, K.; McGeer, A.; Dayam, R.M.; Manase, D.; Gingras, A.C.; On behalf of the STOPCoV Team. Predictors of Breakthrough SARS-CoV-2 Infection after Vaccination. Vaccines 2023, 12, 36. [Google Scholar] [CrossRef]
- Zedan, H.T.; Yassine, H.M.; Al-Sadeq, D.W.; Liu, N.; Qotba, H.; Nicolai, E.; Pieri, M.; Bernardini, S.; Abu-Raddad, L.J.; Nasrallah, G.K. Evaluation of commercially available fully automated and ELISA-based assays for detecting anti-SARS-CoV-2 neutralizing antibodies. Sci. Rep. 2022, 12, 19020. [Google Scholar] [CrossRef]
- Gutlin, Y.; Albertos Torres, D.; Gensch, A.; Schlotterbeck, A.K.; Stoger, L.; Heller, S.; Infanti, L.; Barut, G.T.; Thiel, V.; Leuzinger, K.; et al. Anti-SARS-CoV-2 total immunoglobulin and neutralising antibody responses in healthy blood donors throughout the COVID-19 pandemic: A longitudinal observational study. Swiss Med. Wkly. 2024, 154, 3408. [Google Scholar] [CrossRef]
- Swadzba, J.; Anyszek, T.; Panek, A.; Chojeta, A.; Wyrzykowska, K.; Martin, E. Head-to-Head Comparison of 5 Anti-SARS-CoV-2 Assays Performance in One Hundred COVID-19 Vaccinees, over an 8-Month Course. Diagnostics 2022, 12, 1426. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Shroff, R.T.; Chalasani, P.; Wei, R.; Pennington, D.; Quirk, G.; Schoenle, M.V.; Peyton, K.L.; Uhrlaub, J.L.; Ripperger, T.J.; Jergovic, M.; et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021, 27, 2002–2011. [Google Scholar] [CrossRef]
- Springer, D.N.; Holtl, E.; Pruger, K.; Puchhammer-Stockl, E.; Aberle, J.H.; Stiasny, K.; Weseslindtner, L. Measuring Variant-Specific Neutralizing Antibody Profiles after Bivalent SARS-CoV-2 Vaccinations Using a Multivariant Surrogate Virus Neutralization Microarray. Vaccines 2024, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Springer, D.N.; Traugott, M.; Reuberger, E.; Kothbauer, K.B.; Borsodi, C.; Nageli, M.; Oelschlagel, T.; Kelani, H.; Lammel, O.; Deutsch, J.; et al. A Multivariant Surrogate Neutralization Assay Identifies Variant-Specific Neutralizing Antibody Profiles in Primary SARS-CoV-2 Omicron Infection. Diagnostics 2023, 13, 2278. [Google Scholar] [CrossRef]
- Santos da Silva, E.; Servais, J.Y.; Kohnen, M.; Arendt, V.; Staub, T.; The CON-VINCE Consortium; The CoVaLux Consortium; Kruger, R.; Fagherazzi, G.; Wilmes, P.; et al. Validation of a SARS-CoV-2 Surrogate Neutralization Test Detecting Neutralizing Antibodies against the Major Variants of Concern. Int. J. Mol. Sci. 2023, 24, 14965. [Google Scholar] [CrossRef]
- Ulrich, L.; Halwe, N.J.; Taddeo, A.; Ebert, N.; Schon, J.; Devisme, C.; Trueb, B.S.; Hoffmann, B.; Wider, M.; Fan, X.; et al. Enhanced fitness of SARS-CoV-2 variant of concern Alpha but not Beta. Nature 2022, 602, 307–313. [Google Scholar] [CrossRef]
- Han, P.; Li, L.; Liu, S.; Wang, Q.; Zhang, D.; Xu, Z.; Han, P.; Li, X.; Peng, Q.; Su, C.; et al. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell 2022, 185, 630–640.e10. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Plante, K.S.; Plante, J.A.; Xie, X.; Zhang, X.; Ku, Z.; An, Z.; Scharton, D.; Schindewolf, C.; et al. The N501Y spike substitution enhances SARS-CoV-2 infection and transmission. Nature 2022, 602, 294–299. [Google Scholar] [CrossRef]
- Geisen, U.M.; Rose, R.; Neumann, F.; Ciripoi, M.; Vullriede, L.; Reid, H.M.; Berner, D.K.; Bertoglio, F.; Hoff, P.; Hust, M.; et al. The long term vaccine-induced anti-SARS-CoV-2 immune response is impaired in quantity and quality under TNFalpha blockade. J. Med. Virol. 2022, 94, 5780–5789. [Google Scholar] [CrossRef] [PubMed]
- Kirsebom, F.C.M.; Andrews, N.; Stowe, J.; Ramsay, M.; Lopez Bernal, J. Duration of protection of ancestral-strain monovalent vaccines and effectiveness of bivalent BA.1 boosters against COVID-19 hospitalisation in England: A test-negative case-control study. Lancet Infect. Dis. 2023, 23, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Depery, L.; Bally, I.; Amen, A.; Nemoz, B.; Buisson, M.; Grossi, L.; Truffot, A.; Germi, R.; Guilligay, D.; Veloso, M.; et al. Anti-SARS-CoV-2 serology based on ancestral RBD antigens does not correlate with the presence of neutralizing antibodies against Omicron variants. Microbiol. Spectr. 2025, 13, e0156824. [Google Scholar] [CrossRef]
- Garcia, M.J.; Rodriguez-Duque, J.C.; Pascual, M.; Rivas, C.; Castro, B.; Raso, S.; Lopez-Hoyos, M.; Arias-Loste, M.T.; Rivero, M. Prevalence of antinuclear antibodies in inflammatory bowel disease and seroconversion after biological therapy. Ther. Adv. Gastroenterol. 2022, 15, 17562848221077837. [Google Scholar] [CrossRef]
- Muratori, P.; Lenzi, M.; Muratori, L.; Granito, A. Antinuclear antibodies in COVID 19. Clin. Transl. Sci. 2021, 14, 1627–1628. [Google Scholar] [CrossRef] [PubMed]
- Pascolini, S.; Granito, A.; Muratori, L.; Lenzi, M.; Muratori, P. Coronavirus disease associated immune thrombocytopenia: Causation or correlation? J. Microbiol. Immunol. Infect. 2021, 54, 531–533. [Google Scholar] [CrossRef]
- Hromic-Jahjefendic, A.; Lundstrom, K.; Adilovic, M.; Aljabali, A.A.A.; Tambuwala, M.M.; Serrano-Aroca, A.; Uversky, V.N. Autoimmune response after SARS-CoV-2 infection and SARS-CoV-2 vaccines. Autoimmun. Rev. 2024, 23, 103508. [Google Scholar] [CrossRef]
- Curman, P.; Kridin, K.; Zirpel, H.; Hernandez, G.; Akyuz, M.; Thaci, D.; Schmidt, E.; Ludwig, R.J. COVID-19 infection is associated with an elevated risk for autoimmune blistering diseases while COVID-19 vaccination decreases the risk: A large-scale population-based cohort study of 112 million individuals. J. Am. Acad. Dermatol. 2025, 92, 452–463. [Google Scholar] [CrossRef]
- Allocca, M.; Chaparro, M.; Gonzalez, H.A.; Bosca-Watts, M.M.; Palmela, C.; D’Amico, F.; Zacharopoulou, E.; Kopylov, U.; Ellul, P.; Bamias, G.; et al. Patients with Inflammatory Bowel Disease Are Not at Increased Risk of COVID-19: A Large Multinational Cohort Study. J. Clin. Med. 2020, 9, 3533. [Google Scholar] [CrossRef]
- Meyer, A.; Semenzato, L.; Zureik, M.; Weill, A.; Carbonnel, F.; Dray-Spira, R. Risk of severe COVID-19 in patients treated with IBD medications: A French nationwide study. Aliment. Pharmacol. Ther. 2021, 54, 160–166. [Google Scholar] [CrossRef]

| Patients | Infliximab (n = 10) | Healthy Controls (n = 11) | p-Value | |
|---|---|---|---|---|
| Patient characteristics | Age, years median (IQR) | 50 (31–65) | 35 (23–66) | 0.285 |
| Sex, male (%) | 4 (40) | 4 (36) | 0.863 | |
| SARS-CoV-2 vaccine | Comirnaty BNT162b2 (%) | 10 (100) | 4 (23) | |
| Spikevax mRNA-1273 (%) | 0 (0) | 7 (77) | ||
| IBD | Crohn’s disease (%) | 8 (80) | 0 (0) | |
| Ulcerative colitis (%) | 2 (20) | 0 (0) | ||
| Medication | Prednisolone p.o. (%) | 0 (0) | 0 (0) | |
| Budesonide p.o. (%) | 1 (10) | 0 (0) | ||
| Budesonide supp. (%) | 0 (0) | 0 (0) | ||
| Mesalazine p.o. (%) | 6 (60) | 0 (0) | ||
| Mesalazine supp. (%) | 0 (0) | 0 (0) | ||
| Pre-existing conditions | Cardiovascular disease (%) | 3 (30) | 0 (0) | 0.251 |
| Respiratory disease (%) | 0 (0) | 0 (0) | 1.000 | |
| Kidney insufficiency (%) | 0 (0) | 0 (0) | 1.000 | |
| Metastatic neoplasm (%) | 0 (0) | 0 (0) | 1.000 | |
| Diabetes (%) | 0 (0) | 0 (0) | 1.000 | |
| Hematologic malignancy (%) | 0 (0) | 0 (0) | 1.000 |
| Assay | Area 1 | p Value 1 | Adapted Cut-Off Value 1 | Sensitivity (%) at Adapted Cut-Off Value 1 | Specificity (%) at Adapted Cut-Off Value 1 |
|---|---|---|---|---|---|
| BA.1 S pVNT | 0.8670 | <0.0001 | ≥20.09% | 82.61 | 88.24 |
| Anti-RBD CMIA | 0.8619 | 0.0001 | ≥1126 AU/mL | 82.61 | 88.24 |
| cPass sVNT | 0.8542 | 0.0002 | ≥49.40% | 91.3 | 70.59 |
| wt RBD sVNT | 0.8338 | 0.0004 | ≥59.55% | 78.26 | 76.47 |
| Alpha RBD sVNT | 0.8414 | 0.0003 | ≥25.35% | 86.96 | 70.59 |
| Beta RBD sVNT | 0.8159 | 0.0007 | ≥72.27% | 60.87 | 94.12 |
| Gamma RBD sVNT | 0.8338 | 0.0004 | ≥54.60% | 73.91 | 82.35 |
| Delta RBD sVNT | 0.8235 | 0.0005 | ≥72.07% | 78.26 | 82.35 |
| BA.1 RBD sVNT | 0.6176 | 0.2082 | ≥34.73% | 43.48 | 88.24 |
| BA.5 RBD sVNT | 0.7187 | 0.0193 | ≥9.095% | 60.87 | 94.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorentzen, E.U.; Vollenberg, R.; Neddermeyer, R.; Schoefbaenker, M.; Hrincius, E.R.; Ludwig, S.; Tepasse, P.-R.; Kuehn, J.E. SARS-CoV-2 Variant-Specific Antibodies in Vaccinated Inflammatory Bowel Disease Patients. Vaccines 2025, 13, 595. https://doi.org/10.3390/vaccines13060595
Lorentzen EU, Vollenberg R, Neddermeyer R, Schoefbaenker M, Hrincius ER, Ludwig S, Tepasse P-R, Kuehn JE. SARS-CoV-2 Variant-Specific Antibodies in Vaccinated Inflammatory Bowel Disease Patients. Vaccines. 2025; 13(6):595. https://doi.org/10.3390/vaccines13060595
Chicago/Turabian StyleLorentzen, Eva Ulla, Richard Vollenberg, Rieke Neddermeyer, Michael Schoefbaenker, Eike R. Hrincius, Stephan Ludwig, Phil-Robin Tepasse, and Joachim Ewald Kuehn. 2025. "SARS-CoV-2 Variant-Specific Antibodies in Vaccinated Inflammatory Bowel Disease Patients" Vaccines 13, no. 6: 595. https://doi.org/10.3390/vaccines13060595
APA StyleLorentzen, E. U., Vollenberg, R., Neddermeyer, R., Schoefbaenker, M., Hrincius, E. R., Ludwig, S., Tepasse, P.-R., & Kuehn, J. E. (2025). SARS-CoV-2 Variant-Specific Antibodies in Vaccinated Inflammatory Bowel Disease Patients. Vaccines, 13(6), 595. https://doi.org/10.3390/vaccines13060595







