Comparative Analysis of Long-Term Measles Immune Response After Natural Infection and Routine Vaccination in China
Abstract
1. Introduction
2. Materials and Methods
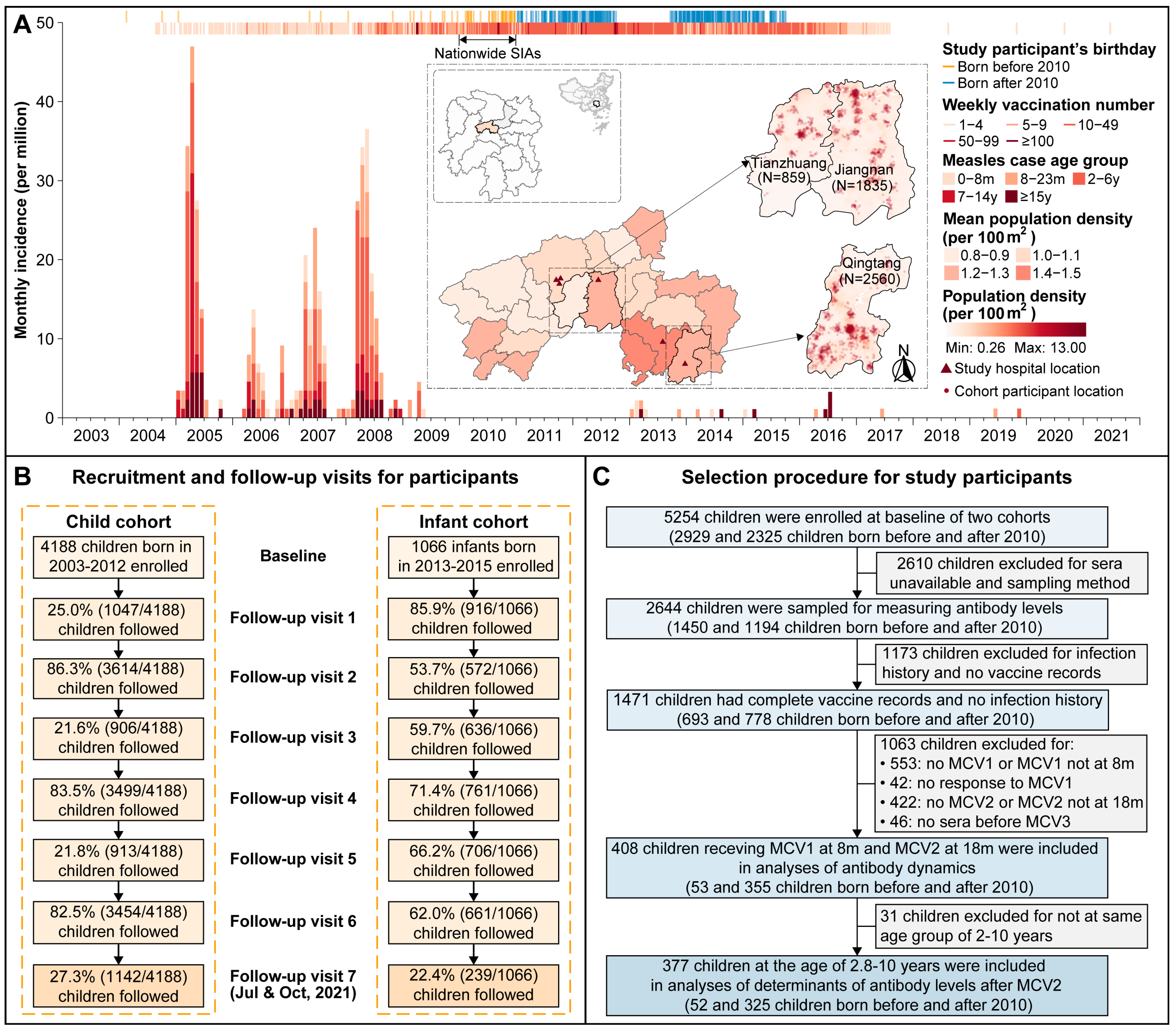
2.1. Study Population and Data Collection
2.2. Laboratory Procedures
2.3. Statistical Analyses
3. Results
3.1. Basic Characteristics of Study Participants
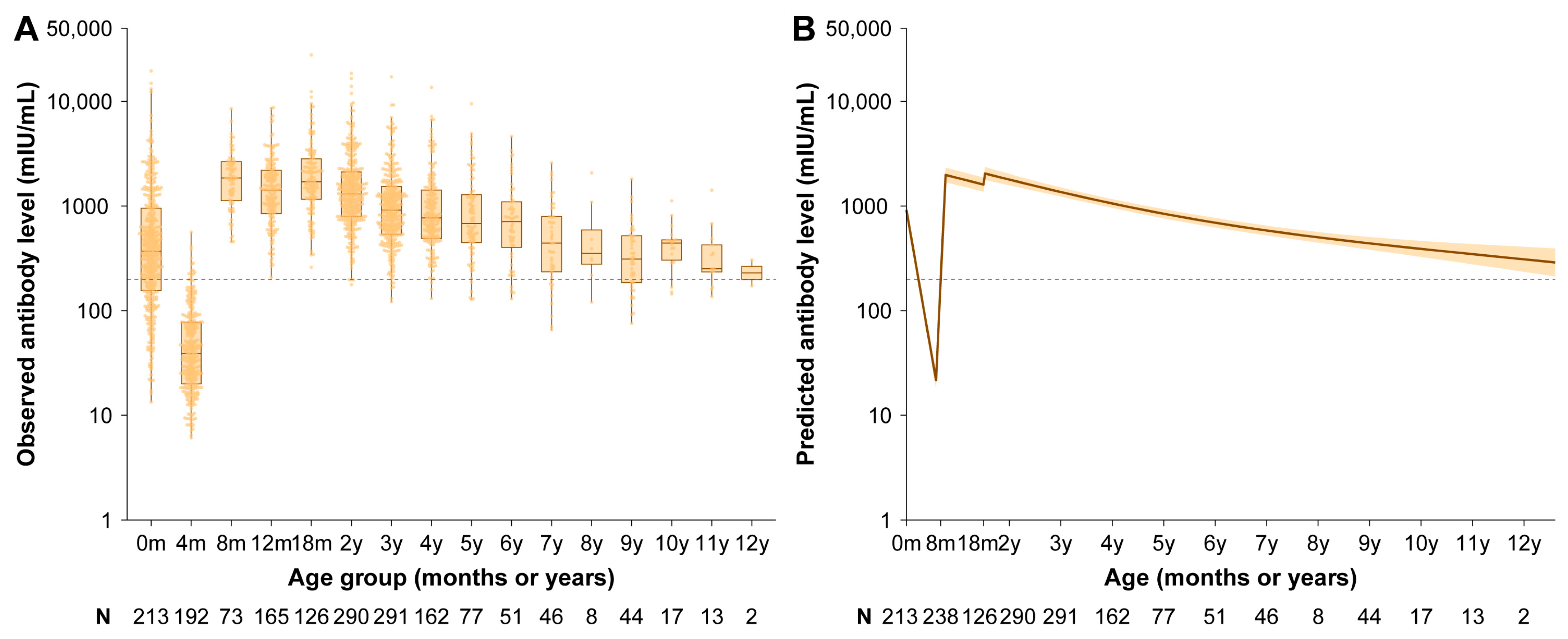
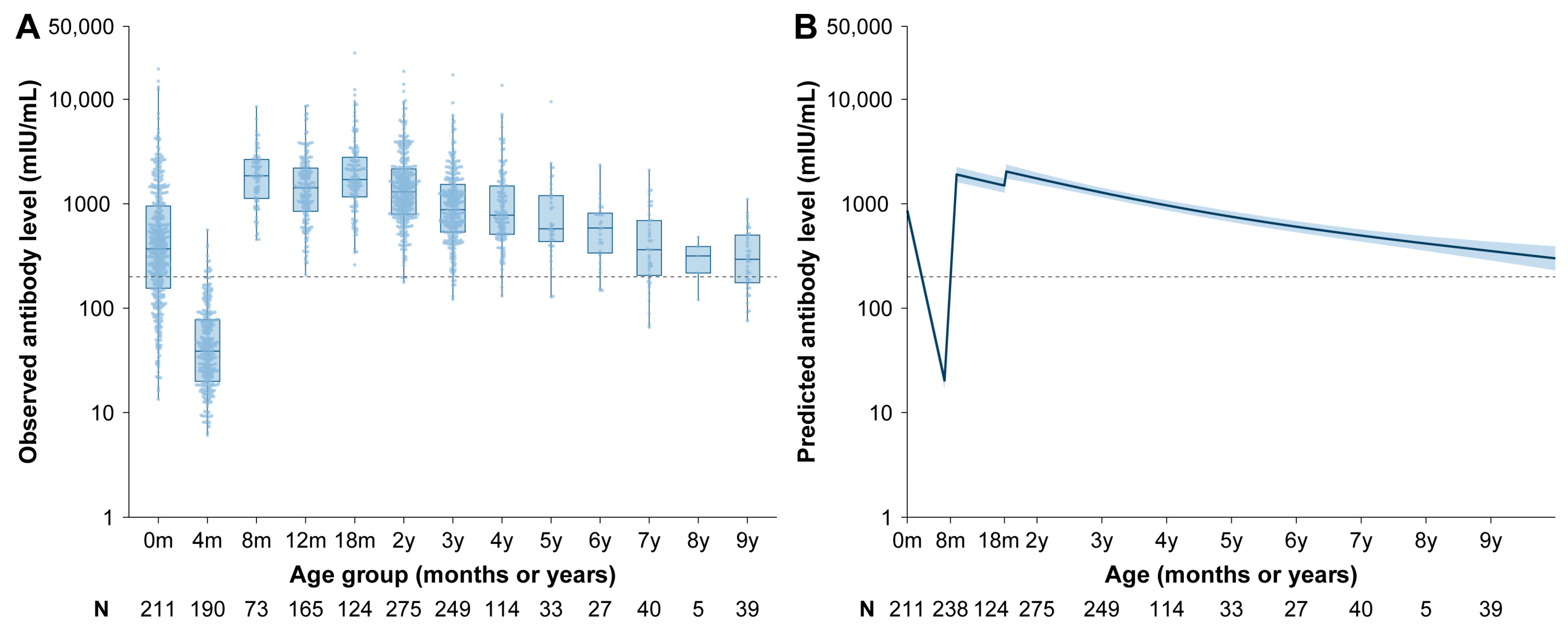
3.2. Vaccine-Induced Antibody
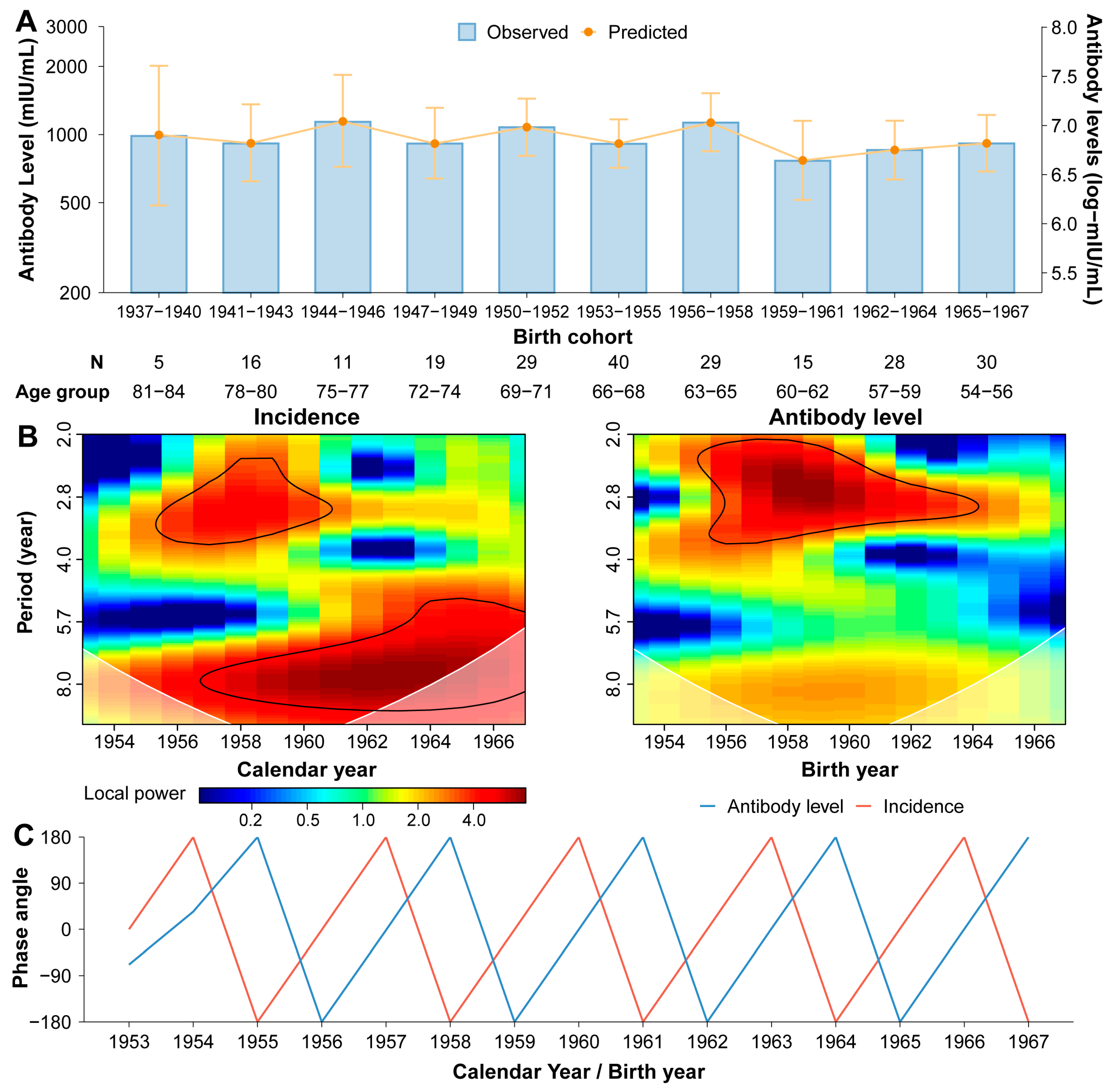
3.3. Naturally Acquired Antibody
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCV | measles-containing vaccine |
| MV | monovalent measles vaccine |
| MR | measles-rubella combined vaccine |
| MMR | measles-mumps-rubella combined vaccine |
| SIA | supplementary immunization activity |
| ELISA | enzyme-linked immunosorbent assay |
| GAMM | generalized additive mixed model |
| CI | confidence interval |
References
- Moss, W.J. Measles. Lancet 2017, 390, 2490–2502. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; An, Z.; Yin, Z. Achievements in prevention and control of seven infectious diseases targeted by the National Immunization Program in China across 70 years. Chin. J. Vaccines Immun. 2019, 25, 359–367. [Google Scholar]
- Trentini, F.; Poletti, P.; Merler, S.; Melegaro, A. Measles Immunity Gaps and the Progress towards Elimination: A Multi-Country Modelling Analysis. Lancet Infect. Dis. 2017, 17, 1089–1097. [Google Scholar] [CrossRef]
- Ma, C. Progress Toward Measles Elimination—China, January 2013–June 2019. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.; Munir, N.A.; Mathis, A.D.; Filardo, T.D.; Rota, P.A.; Sugerman, D.E.; Sowers, S.B.; Mercader, S.; Crooke, S.N.; Gastañaduy, P.A. The Effects of Vaccination Status and Age on Clinical Characteristics and Severity of Measles Cases in the United States in the Post-Elimination Era, 2001–2022. Clin. Infect. Dis. 2024, 80, 663–672. [Google Scholar] [CrossRef]
- World Health Organization. Measles Cases Surge Worldwide, Infecting 10.3 Million People in 2023. Available online: https://www.who.int/news/item/14-11-2024-measles-cases-surge-worldwide--infecting-10.3-million-people-in-2023 (accessed on 15 November 2024).
- Winter, A.K.; Martinez, M.E.; Cutts, F.T.; Moss, W.J.; Ferrari, M.J.; McKee, A.; Lessler, J.; Hayford, K.; Wallinga, J.; Metcalf, C.J.E. Benefits and Challenges in Using Seroprevalence Data to Inform Models for Measles and Rubella Elimination. J. Infect. Dis. 2018, 218, 355–364. [Google Scholar] [CrossRef]
- Amanna, I.J.; Carlson, N.E.; Slifka, M.K. Duration of Humoral Immunity to Common Viral and Vaccine Antigens. N. Engl. J. Med. 2007, 357, 1903–1915. [Google Scholar] [CrossRef]
- World Health Organization. The Immunological Basis for Immunization Series: Module 4: Pertussis; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. The Immunological Basis for Immunization Series: Module 23: Influenza Vaccines; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-151305-0. [Google Scholar]
- Wei, X.; Yang, J.; Gao, L.; Wang, L.; Liao, Q.; Qiu, Q.; Luo, K.; Yu, S.; Zhou, Y.; Liu, F.; et al. The Transfer and Decay of Maternal Antibodies against Enterovirus A71, and Dynamics of Antibodies Due to Later Natural Infections in Chinese Infants: A Longitudinal, Paired Mother–Neonate Cohort Study. Lancet Infect. Dis. 2021, 21, 418–426. [Google Scholar] [CrossRef]
- Wang, W.; O’Driscoll, M.; Wang, Q.; Zhao, S.; Salje, H.; Yu, H. Dynamics of Measles Immunity from Birth and Following Vaccination. Nat. Microbiol. 2024, 9, 1676–1685. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Winter, A.K.; Zhan, Z.; Ajelli, M.; Trentini, F.; Wang, L.; Li, F.; Yang, J.; Xiang, X.; et al. Long-Term Measles Antibody Profiles Following Different Vaccine Schedules in China, a Longitudinal Study. Nat. Commun. 2023, 14, 1746. [Google Scholar] [CrossRef]
- Chen, R.T.; Markowitz, L.E.; Albrecht, P.; Stewart, J.A.; Mofenson, L.M.; Preblud, S.R.; Orenstein, W.A. Measles Antibody: Reevaluation of Protective Titers. J. Infect. Dis. 1990, 162, 1036–1042. [Google Scholar] [CrossRef]
- Lauri, E.M.; Jaime, S.; Jose, L.D.-O.; Jose, L.V.; Paul, A.; Elizabeth, R.Z.; John, S.; Maria, L.Z.; Roger, H.B. Immunization of Six-Month-Old Infants with Different Doses of Edmonston–Zagreb and Schwarz Measles Vaccines. N. Engl. J. Med. 1990, 322, 580–587. [Google Scholar] [CrossRef]
- Lam, H.M.; Phuong, H.T.; Thao Vy, N.H.; Le Thanh, N.T.; Dung, P.N.; Ngoc Muon, T.T.; Van Vinh Chau, N.; Rodríguez-Barraquer, I.; Cummings, D.A.T.; Wills, B.A.; et al. Serological Inference of Past Primary and Secondary Dengue Infection: Implications for Vaccination. J. R. Soc. Interface 2019, 16, 20190207. [Google Scholar] [CrossRef]
- Grenfell, B.T.; Bjørnstad, O.N.; Kappey, J. Travelling Waves and Spatial Hierarchies in Measles Epidemics. Nature 2001, 414, 716–723. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Yang, L.; Grenfell, B.T.; Mina, M.J. Waning Immunity and Re-Emergence of Measles and Mumps in the Vaccine Era. Curr. Opin. Virol. 2020, 40, 48–54. [Google Scholar] [CrossRef]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex Differences in Vaccine-Induced Humoral Immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef]
- Bolotin, S.; Osman, S.; Hughes, S.L.; Ariyarajah, A.; Tricco, A.C.; Khan, S.; Li, L.; Johnson, C.; Friedman, L.; Gul, N.; et al. In Elimination Settings, Measles Antibodies Wane After Vaccination but Not After Infection: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2022, 226, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Mossong, J.; Nokes, D.J.; Edmunds, W.J.; Cox, M.J.; Ratnam, S.; Muller, C.P. Modeling the Impact of Subclinical Measles Transmission in Vaccinated Populations with Waning Immunity. Am. J. Epidemiol. 1999, 150, 1238–1249. [Google Scholar] [CrossRef]
- Ozanne, G.; d’Halewyn, M.A. Secondary Immune Response in a Vaccinated Population during a Large Measles Epidemic. J. Clin. Microbiol. 1992, 30, 1778–1782. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Chen, Z.H.; Liu, Q.C.; Wu, T.; Guo, C.Y.; Wang, X.Z.; Fang, H.H.; Xiang, Y.Z. Duration of Immunity Following Immunization with Live Measles Vaccine: 15 Years of Observation in Zhejiang Province, China. Bull. World Health Organ. 1991, 69, 415–423. [Google Scholar] [PubMed]
- Borremans, B.; Mummah, R.O.; Guglielmino, A.H.; Galloway, R.L.; Hens, N.; Prager, K.C.; Lloyd-Smith, J.O. Inferring Time of Infection from Field Data Using Dynamic Models of Antibody Decay. Methods Ecol. Evol. 2023, 14, 2654–2667. [Google Scholar] [CrossRef]
- Salje, H.; Cummings, D.A.T.; Rodriguez-Barraquer, I.; Katzelnick, L.C.; Lessler, J.; Klungthong, C.; Thaisomboonsuk, B.; Nisalak, A.; Weg, A.; Ellison, D.; et al. Reconstruction of Antibody Dynamics and Infection Histories to Evaluate Dengue Risk. Nature 2018, 557, 719–723. [Google Scholar] [CrossRef]
- Russell, T.W.; Townsley, H.; Hellewell, J.; Gahir, J.; Shawe-Taylor, M.; Greenwood, D.; Hodgson, D.; Hobbs, A.; Dowgier, G.; Penn, R.; et al. Real-Time Estimation of Immunological Responses against Emerging SARS-CoV-2 Variants in the UK: A Mathematical Modelling Study. Lancet Infect. Dis. 2024, 25, 80–93. [Google Scholar] [CrossRef]
- Gustafson, T.L.; Lievens, A.W.; Brunell, P.A.; Moellenberg, R.G.; Buttery, C.M.; Sehulster, L.M. Measles Outbreak in a Fully Immunized Secondary-School Population. N. Engl. J. Med. 1987, 316, 771–774. [Google Scholar] [CrossRef]
- Wintermeyer, L.; Myers, M.G. Measles in a Partially Immunized Community. Am. J. Public Health 1979, 69, 923–927. [Google Scholar] [CrossRef]
- Gans, H.A.; Yasukawa, L.L.; Sung, P.; Sullivan, B.; DeHovitz, R.; Audet, S.; Beeler, J.; Arvin, A.M. Measles Humoral and Cell-Mediated Immunity in Children Aged 5–10 Years after Primary Measles Immunization Administered at 6 or 9 Months of Age. J. Infect. Dis. 2013, 207, 574–582. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Chen, J.; Zhan, Z.; Xu, M.; Liu, N.; Ren, L.; You, L.; Zheng, W.; Shi, H.; et al. Transplacental Transfer Efficiency of Maternal Antibodies against Influenza A(H1N1)Pdm09 Virus and Dynamics of Naturally Acquired Antibodies in Chinese Children: A Longitudinal, Paired Mother–Neonate Cohort Study. Lancet Microbe 2023, 4, e893–e902. [Google Scholar] [CrossRef]
| Total (N = 408) | Born Before 2010 (N = 53) | Born After 2010 (N = 355) | p-Value | |
|---|---|---|---|---|
| Age at baseline, years | ||||
| Median (Interquartile range, IQR) | 0 (0−1.9) | 3.28 (3−3.9) | 0 (0−1.5) | 4.13 × 10−34 |
| Sex a | ||||
| Male | 199 (48.8) | 23 (43.4) | 176 (49.6) | 0.488 |
| Female | 209 (51.2) | 30 (56.6) | 179 (50.4) | |
| Socioeconomic status b | ||||
| Low | 64 (15.7) | 10 (18.9) | 54 (15.2) | 0.941 |
| Middle | 198 (48.5) | 28 (52.8) | 170 (47.9) | |
| High | 98 (24.0) | 15 (28.3) | 83 (23.4) | |
| Missing | 48 (11.8) | 0 (0) | 48 (13.5) | |
| Mode of delivery | ||||
| Vaginal delivery | 255 (62.5) | 35 (66.0) | 220 (62.0) | 0.676 |
| Caesarean section | 153 (37.5) | 18 (34.0) | 135 (38.0) | |
| Term of pregnancy | ||||
| Preterm birth | 17 (4.2) | 2 (3.8) | 15 (4.2) | 0.538 |
| Full-term birth | 379 (92.9) | 51 (96.2) | 328 (92.4) | |
| Post-term birth | 12 (2.9) | 0 (0.0) | 12 (3.4) | |
| Birth weight, grams | ||||
| Median (IQR) | 3300 (3000−3600) | 3250 (2925−3500) | 3300 (3000−3600) | 0.509 |
| Breastfeeding before 6 months | ||||
| No | 35 (8.6) | 6 (11.3) | 29 (8.2) | 0.432 |
| Yes | 373 (91.4) | 47 (88.7) | 326 (91.8) | |
| MCV doses received | ||||
| 2 doses | 403 (98.8) | 51 (96.2) | 352 (99.2) | 0.128 |
| 3 doses | 5 (1.2) | 2 (3.8) | 3 (0.8) | |
| Age at MCV1, months | ||||
| Median (IQR) | 8.2 (8.0−8.5) | 8.3 (8.1−8.5) | 8.2 (8.0−8.6) | 0.683 |
| Age at MCV2, months | ||||
| Median (IQR) | 18.2 (18.0−18.5) | 18.1 (18.0−18.5) | 18.2 (18.0−18.6) | 0.315 |
| Participants’ Characteristics | Mean | = 0.25) | = 0.50) | = 0.75) | |||||
|---|---|---|---|---|---|---|---|---|---|
| (95% CI) | p-Value | (95% CI) | p-Value | (95% CI) | p-Value | (95% CI) | p-Value | ||
| Intercept | 7.29 (7.05, 7.53) | 0.00 | 7.34 (7.03, 7.65) | 1.35 × 10−254 | 7.38 (7.07, 7.68) | 5.91 × 10−260 | 7.38 (7.08, 7.69) | 2.66 × 10−261 | |
| Time since MCV2, months b | |||||||||
| Median (IQR) | 31.2 (21.3, 47.7) | −0.01 (−0.02, −0.01) | 0.001 | −0.02 (−0.02, −0.01) | 4.20 × 10−9 | −0.01 (−0.02, −0.01) | 1.10 × 10−8 | −0.01 (−0.02, −0.01) | 4.48 × 10−10 |
| Sex a | |||||||||
| Male | 180 (47.7) | Reference | − | Reference | − | Reference | − | Reference | − |
| Female | 197 (52.3) | 0.24 (0.07, 0.41) | 0.006 | 0.12 (−0.07, 0.32) | 0.215 | 0.14 (−0.05, 0.34) | 0.146 | 0.16 (−0.03, 0.36) | 0.097 |
| Mode of delivery | |||||||||
| Vaginal delivery | 236 (62.6) | Reference | − | Reference | − | Reference | − | Reference | − |
| Caesarean section | 141 (37.4) | −0.06 (−0.23, 0.11) | 0.411 | −0.02 (−0.19, 0.16) | 0.864 | 0.00 (−0.18, 0.17) | 0.986 | −0.02 (−0.19, 0.15) | 0.828 |
| Birth cohort | |||||||||
| Before 2010 | 52 (13.8) | Reference | − | Reference | − | Reference | − | Reference | − |
| After 2010 | 325 (86.2) | −0.23 (−0.46, −0.01) | 0.046 | −0.29 (−0.55, −0.02) | 0.036 | −0.25 (−0.52, 0.01) | 0.061 | −0.23 (−0.50, 0.04) | 0.093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Wang, Q.; Yang, J.; Liao, Q.; Zhang, J.; Zhou, X.; Zhou, J.; Zhao, Z.; Liang, Y.; Luo, J.; et al. Comparative Analysis of Long-Term Measles Immune Response After Natural Infection and Routine Vaccination in China. Vaccines 2025, 13, 555. https://doi.org/10.3390/vaccines13060555
Zhao S, Wang Q, Yang J, Liao Q, Zhang J, Zhou X, Zhou J, Zhao Z, Liang Y, Luo J, et al. Comparative Analysis of Long-Term Measles Immune Response After Natural Infection and Routine Vaccination in China. Vaccines. 2025; 13(6):555. https://doi.org/10.3390/vaccines13060555
Chicago/Turabian StyleZhao, Sihong, Qianli Wang, Juan Yang, Qiaohong Liao, Juanjuan Zhang, Xiaoyu Zhou, Jiaxin Zhou, Zeyao Zhao, Yuxia Liang, Junteng Luo, and et al. 2025. "Comparative Analysis of Long-Term Measles Immune Response After Natural Infection and Routine Vaccination in China" Vaccines 13, no. 6: 555. https://doi.org/10.3390/vaccines13060555
APA StyleZhao, S., Wang, Q., Yang, J., Liao, Q., Zhang, J., Zhou, X., Zhou, J., Zhao, Z., Liang, Y., Luo, J., Cai, J., Wu, Y., Wang, W., & Yu, H. (2025). Comparative Analysis of Long-Term Measles Immune Response After Natural Infection and Routine Vaccination in China. Vaccines, 13(6), 555. https://doi.org/10.3390/vaccines13060555







