Past, Present, and Future of Viral Vector Vaccine Platforms: A Comprehensive Review
Abstract
1. Introduction
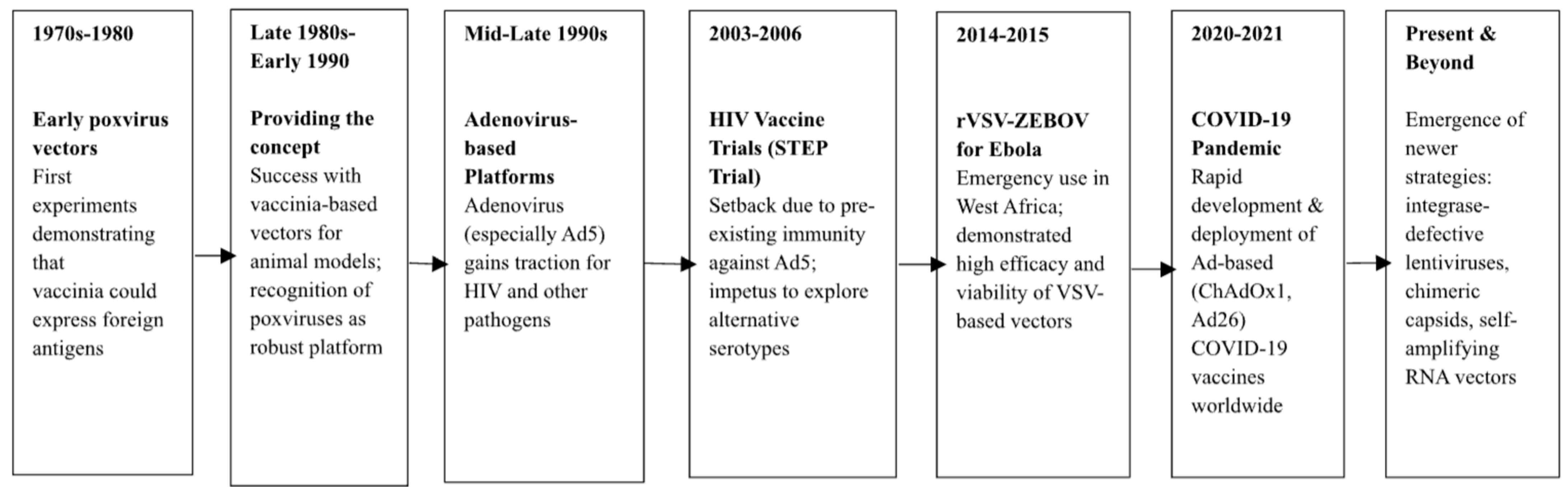
2. Historical Perspectives and Evolution of Viral Vector Vaccines
3. Classification and Characteristics of Common Viral Vector Platforms
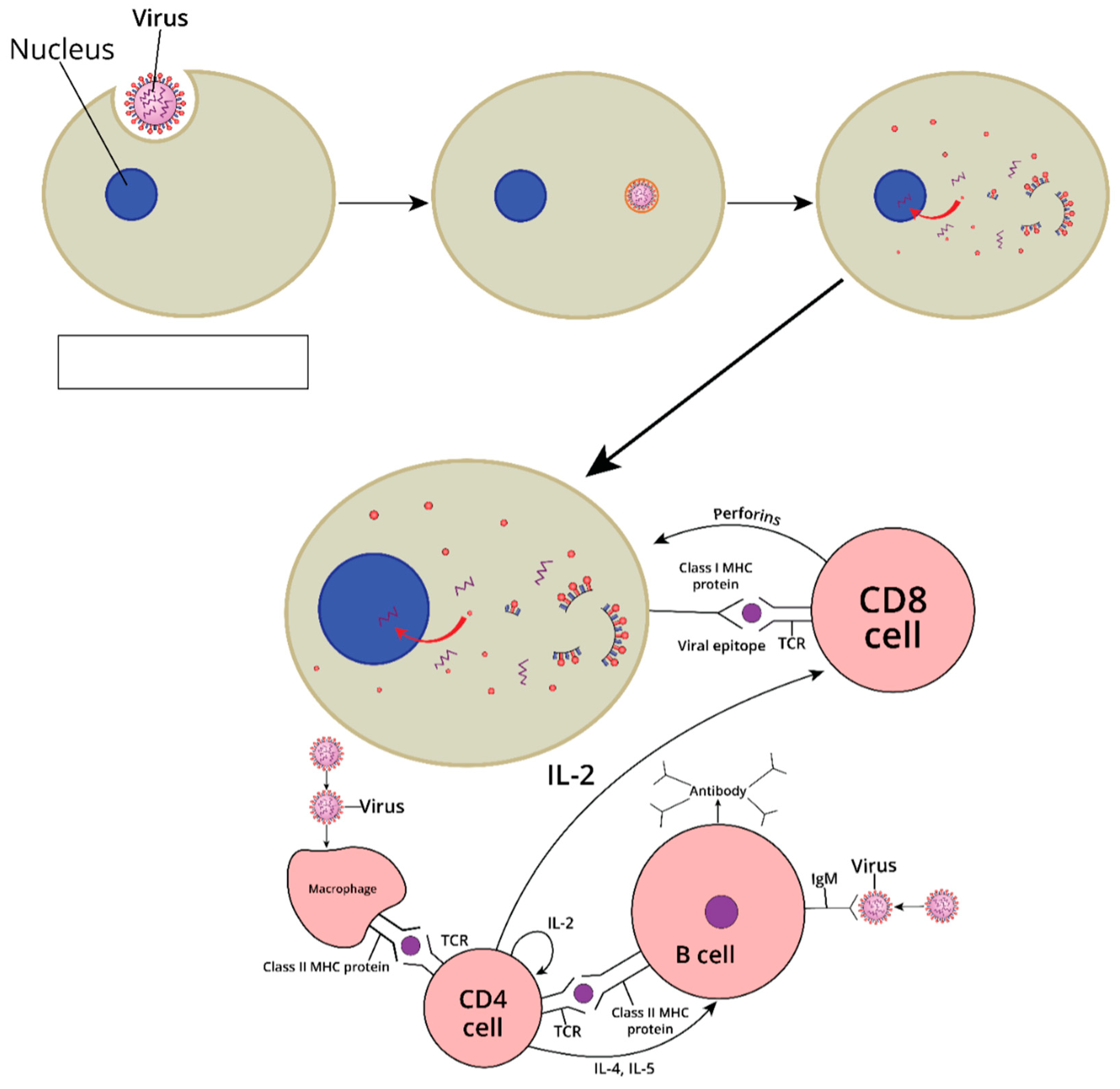
4. Mechanisms of Immunogenicity and Immune Response Induction
5. Current Trends in Preclinical and Clinical Development
6. Manufacturing, Scale-Up, and Quality Control
7. Safety and Efficacy Considerations
8. Innovations and Engineering Strategies in Vector Design
9. Lessons Learned from the COVID-19 Pandemic
10. Socioeconomic and Global Access Considerations
11. Future Perspectives: Next-Generation Viral Vector Platforms
12. Discussion and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Andrei, G. Vaccines and Antivirals: Grand Challenges and Great Opportunities. Specialty Grand Challenge article. Front. Virol. 2021, 23, 666548. [Google Scholar]
- Dogbey, D.M.; Torres, V.E.S.; Fajemisin, E.; Mpondo, L.; Ngwenya, T.; Akinrinmade, O.A.; Perriman, A.W.; Barth, S. Technological advances in the use of viral and non-viral vectors for delivering genetic and non-genetic cargos for cancer therapy. Drug Deliv. Transl. Res. 2023, 13, 2719–2738. [Google Scholar] [CrossRef]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Liang, H.; Chen, P.; Li, Y.; Li, Z.; Fan, S.; Wu, K.; Li, X.; Chen, W.; Qin, Y.; et al. Viral Vector Vaccine Development and Application during the COVID-19 Pandemic. Microorganisms 2022, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.P.M.; Piccini, A.; Lipinskas, B.R.; Mercer, S.R. A Modern Approach to Live Vaccines: Recombinant Poxviruses. In Biotechnology: Potentials and Limitations. Dahlem Workshop Reports; Silver, S., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; Volume 35. [Google Scholar]
- Kaynarcalidan, O.; Moreno Mascaraque, S.; Drexler, I. Vaccinia Virus: From Crude Smallpox Vaccines to Elaborate Viral Vector Vaccine Design. Biomedicines 2021, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Sumida, S.M.; Truitt, D.M.; Lemckert, A.A.; Vogels, R.; Custers, J.H.; Addo, M.M.; Lockman, S.; Peter, T.; Peyerl, F.W.; Kishko, M.G.; et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 2005, 174, 7179–7185. [Google Scholar] [CrossRef]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef]
- Mahesh, S.; Li, J.; Travieso, T.; Psaradelli, D.; Negri, D.; Klotman, M.; Cara, A.; Blasi, M. Integrase Defective Lentiviral Vector Promoter Impacts Transgene Expression in Target Cells and Magnitude of Vector-Induced Immune Responses. Viruses 2023, 15, 2255. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Interim Results of a Phase 1-2a Trial of Ad26.COV2.S Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1824–1835. [Google Scholar] [CrossRef]
- Salauddin, M.; Saha, S.; Hossain, M.G.; Okuda, K.; Shimada, M. Clinical Application of Adenovirus (AdV): A Comprehensive Review. Viruses 2024, 16, 1094. [Google Scholar] [CrossRef]
- Barouch, D.H.; Pau, M.G.; Custers, J.H.; Koudstaal, W.; Kostense, S.; Havenga, M.J.; Truitt, D.M.; Sumida, S.M.; Kishko, M.G.; Arthur, J.C.; et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 2004, 172, 6290–6297. [Google Scholar] [CrossRef] [PubMed]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccines Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef]
- Coughlan, L. Factors Which Contribute to the Immunogenicity of Non-replicating Adenoviral Vectored Vaccines. Front. Immunol. 2020, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- McCann, N.; O’Connor, D.; Lambe, T.; Pollard, A.J. Viral vector vaccines. Curr. Opin. Immunol. 2022, 77, 102210. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Sayedahmed, E.E.; Mittal, S.K. Significance of Preexisting Vector Immunity and Activation of Innate Responses for Adenoviral Vector-Based Therapy. Viruses 2022, 14, 2727. [Google Scholar] [CrossRef]
- Samulski, R.J.; Muzyczka, N. AAV-Mediated Gene Therapy for Research and Therapeutic Purposes. Annu. Rev. Virol. 2014, 1, 427–451. [Google Scholar] [CrossRef]
- Weber, T. Anti-AAV Antibodies in AAV Gene Therapy: Current Challenges and Possible Solutions. Front. Immunol. 2021, 12, 658399. [Google Scholar] [CrossRef]
- Calcedo, R.; Wilson, J.M. Humoral Immune Response to AAV. Front. Immunol. 2013, 4, 341. [Google Scholar] [CrossRef]
- Ail, D.; Dalkara, D. Preexisting Neutralizing Antibodies against Different Adeno-Associated Virus Serotypes in Humans and Large Animal Models for Gene Therapy. Adv. Exp. Med. Biol. 2023, 1415, 117–123. [Google Scholar]
- Gilbert, S.C. Clinical development of Modified Vaccinia virus Ankara vaccines. Vaccine 2013, 31, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Hannas, Z.T.J.; Zhang, Y.; Lhermitte, F.; Cleuziat, C.; Motes-Kreimeyer l Dhoms, P.; Bublot, M. Manufacturing and Control of Viral Vectored Vaccines: Challenges; Springer: Berlin/Heidelberg, Germany, 2020; pp. 183–199. [Google Scholar]
- Marzi, A.; Feldmann, H.; Geisbert, T.W.; Falzarano, D. Vesicular Stomatitis Virus-Based Vaccines for Prophylaxis and Treatment of Filovirus Infections. J. Bioterror. Biodef. 2011, S1, 2157–2526. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Alía, M.Á.; Nace, R.A.; Balakrishnan, B.; Zhang, L.; Packiriswamy, N.; Singh, G.; Warang, P.; Mena, I.; Narjari, R.; Vandergaast, R.; et al. Surface-modified measles vaccines encoding oligomeric, prefusion-stabilized SARS-CoV-2 spike glycoproteins boost neutralizing antibody responses to Omicron and historical variants, independent of measles seropositivity. mBio 2024, 15, e0292823. [Google Scholar] [CrossRef]
- Tcheou, J.; Raskin, A.; Singh, G.; Kawabata, H.; Bielak, D.; Sun, W.; González-Domínguez, I.; Sather, D.N.; García-Sastre, A.; Palese, P.; et al. Safety and Immunogenicity Analysis of a Newcastle Disease Virus (NDV-HXP-S) Expressing the Spike Protein of SARS-CoV-2 in Sprague Dawley Rats. Front. Immunol. 2021, 12, 791764. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Auguste, A.J.; Kaelber, J.T.; Luo, H.; Rossi, S.L.; Fenton, K.; Leal, G.; Kim, D.Y.; Chiu, W.; Wang, T.; et al. A chikungunya fever vaccine utilizing an insect-specific virus platform. Nat. Med. 2017, 23, 192–199. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, Y.; Kim, D.Y.; Li, R. Insect-Specific Chimeric Viruses Potentiated Antiviral Responses and Inhibited Pathogenic Alphavirus Growth in Mosquito Cells. Microbiol. Spectr. 2023, 11, e0361322. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, J.; Shen, A.; Lai, S.; Liu, Z.; He, T.S. The RNA-binding proteins regulate innate antiviral immune signaling by modulating pattern recognition receptors. Virol. J. 2024, 21, 225. [Google Scholar] [CrossRef]
- Panagioti, E.; Klenerman, P.; Lee, L.N.; van der Burg, S.H.; Arens, R. Features of Effective T Cell-Inducing Vaccines against Chronic Viral Infections. Front. Immunol. 2018, 9, 276. [Google Scholar] [CrossRef]
- Nemirov, K.; Bourgine, M.; Anna, F.; Wei, Y.; Charneau, P.; Majlessi, L. Lentiviral Vectors as a Vaccine Platform against Infectious Diseases. Pharmaceutics 2023, 15, 846. [Google Scholar] [CrossRef]
- Michelini, Z.; Negri, D.; Cara, A. Integrase defective, nonintegrating lentiviral vectors. Methods Mol Biol. 2010, 614, 101–110. [Google Scholar] [PubMed]
- Ravari, S.M.H.; Chakraborty, S.; Gandla, K.; Cherukuri, P. Transformative advances in lentiviral vector manufacturing: Unlocking efficiency and cost-effectiveness with Tet-Off PCL innovation. Cytotherapy 2024, 26, e20. [Google Scholar] [CrossRef]
- Porier, D.L.; Adam, A.; Kang, L.; Michalak, P.; Tupik, J.; Santos, M.A.; Tanelus, M.; López, K.; Auguste, D.I.; Lee, C.; et al. Humoral and T-cell-mediated responses to an insect-specific flavivirus-based Zika virus vaccine candidate. PLoS Pathog. 2024, 20, e1012566. [Google Scholar] [CrossRef] [PubMed]
- Belyakov, I.M.; Ahlers, J.D. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J. Immunol. 2009, 183, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A. Mucosal and transdermal vaccine delivery strategies against COVID-19. Drug Deliv. Transl. Res. 2022, 12, 968–972. [Google Scholar] [CrossRef]
- Marston, H.D.; Paules, C.I.; Fauci, A.S. The Critical Role of Biomedical Research in Pandemic Preparedness. JAMA 2017, 318, 1757–1758. [Google Scholar] [CrossRef]
- Modjarrad, K.; Roberts, C.C.; Mills, K.T.; Castellano, A.R.; Paolino, K.; Muthumani, K.; Reuschel, E.L.; Robb, M.L.; Racine, T.; Oh, M.D.; et al. Safety and immunogenicity of an anti-Middle East respiratory syndrome coronavirus DNA vaccine: A phase 1, open-label, single-arm, dose-escalation trial. Lancet Infect Dis. 2019, 19, 1013–1022. [Google Scholar] [CrossRef]
- Fitzgerald, D.W.; Janes, H.; Robertson, M.; Coombs, R.; Frank, I.; Gilbert, P.; Loufty, M.; Mehrotra, D.; Duerr, A. An Ad5-vectored HIV-1 vaccine elicits cell-mediated immunity but does not affect disease progression in HIV-1-infected male subjects: Results from a randomized placebo-controlled trial (the Step study). J. Infect Dis. 2011, 203, 765–772. [Google Scholar] [CrossRef]
- Michael, N.L. Rare serotype adenoviral vectors for HIV vaccine development. J. Clin. Investig. 2012, 122, 25–27. [Google Scholar] [CrossRef]
- Ondondo, B.O. The influence of delivery vectors on HIV vaccine efficacy. Front. Microbiol. 2014, 5, 439. [Google Scholar] [CrossRef]
- Shoukry, N.H. Hepatitis C Vaccines, Antibodies, and T Cells. Front. Immunol. 2018, 9, 1480. [Google Scholar] [CrossRef] [PubMed]
- Hamele, C.E.; Luo, Z.; Leonard, R.A.; Spurrier, M.A.; Burke, K.N.; Webb, S.R.; Rountree, W.; Li, Z.; Heaton, B.E.; Heaton, N.S. Headless hemagglutinin-containing influenza viral particles direct immune responses toward more conserved epitopes. J. Virol. 2024, 98, e0116624. [Google Scholar] [CrossRef]
- Misplon, J.A.; Lo, C.Y.; Crabbs, T.A.; Price, G.E.; Epstein, S.L. Adenoviral-vectored universal influenza vaccines administered intranasally reduce lung inflammatory responses upon viral challenge 15 months post-vaccination. J. Virol. 2023, 97, e0067423. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, A.; Sebastian, S.; Appelberg, S.; Cha, K.M.; Ulaszewska, M.; Purushotham, J.; Gilbride, C.; Sharpe, H.; Spencer, A.J.; Bibi, S.; et al. Potent immunogenicity and protective efficacy of a multi-pathogen vaccination targeting Ebola, Sudan, Marburg and Lassa viruse. PLoS Pathog. 2024, 20, e1012262. [Google Scholar] [CrossRef] [PubMed]
- D’Alise, A.M.; Leoni, G.; Cotugno, G.; Siani, L.; Vitale, R.; Ruzza, V.; Garzia, I.; Antonucci, L.; Micarelli, E.; Venafra, V.; et al. Phase I Trial of Viral Vector-Based Personalized Vaccination Elicits Robust Neoantigen-Specific Antitumor T-Cell Responses. Clin. Cancer Res. 2024, 30, 2412–2423. [Google Scholar] [CrossRef] [PubMed]
- Cappuccini, F.; Bryant, R.; Pollock, E.; Carter, L.; Verrill, C.; Hollidge, J.; Poulton, I.; Baker, M.; Mitton, C.; Baines, A.; et al. Safety and immunogenicity of novel 5T4 viral vectored vaccination regimens in early stage prostate cancer: A phase I clinical trial. J. Immunother. Cancer 2020, 8, e000928. [Google Scholar] [CrossRef]
- Kim, J.; Vasan, S.; Kim, J.H.; Ake, J.A. Current approaches to HIV vaccine development: A narrative review. J. Int. AIDS Soc. 2021, 24, e25793. [Google Scholar] [CrossRef]
- Colón, W.; Oriol-Mathieu, V.; Hural, J.; Hattingh, L.; Adungo, F.; Lagatie, O.; Lavreys, L.; Allen, M.; Anzala, O.; Espy, N.; et al. HIV Diagnostics and Vaccines: It Takes Two to Tango. J. Infect. Dis. 2024, 229, 1919–1925. [Google Scholar] [CrossRef]
- Nkolola, J.P.; Barouch, D.H. Prophylactic HIV-1 vaccine trials: Past, present, and future. Lancet HIV. 2024, 11, e117–e124. [Google Scholar] [CrossRef]
- Wu, D.W.; Jia, S.P.; Xing, S.J.; Ma, H.L.; Wang, X.; Tang, Q.Y.; Li, Z.W.; Wu, Q.; Bai, M.; Zhang, X.Y.; et al. Personalized neoantigen cancer vaccines: Current progression, challenges and a bright future. Clin. Exp. Med. 2024, 24, 229. [Google Scholar] [CrossRef]
- Wei Xie, P.G. From discovary to Production: Challenges and Novel Methodologies for Next Generation Biomanufacturing. In Proceedings of the 2022 Winter Simulation Conference (WSC), Singapore, 11–14 December 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 238–252. [Google Scholar]
- Yang, J.; Guertin, P.; Jia, G.; Lv, Z.; Yang, H.; Ju, D. Large-scale microcarrier culture of HEK293T cells and Vero cells in single-use bioreactors. AMB Express. 2019, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Li, Y.; Zhou, L.; Yao, W.; Zhang, H.; Hu, Z.; Han, J.; Wang, W.; Wu, J.; Xu, P.; et al. Lyophilized mRNA-lipid nanoparticle vaccines with long-term stability and high antigenicity against SARS-CoV-2. Cell Discov. 2023, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Björkman, A.; Benn, C.S.; Aaby, P.; Schapira, A. RTS,S/AS01 malaria vaccine-proven safe and effective? Lancet Infect. Dis. 2023, 23, e318–e322. [Google Scholar] [CrossRef]
- Stanisic, D.I. Good MF. Malaria Vaccines: Progress to Date. BioDrugs 2023, 37, 737–756. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.T.; Crozier, I.; Fischer, W.A.; Hewlett, A.; Kraft, C.S.; Vega, M.A.D.L.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Primers 2020, 6, 13. [Google Scholar] [CrossRef]
- Anderson, E.M.; Coller, B.A. Translational success of fundamental virology: A VSV-vectored Ebola vaccine. J. Virol. 2024, 98, e0162723. [Google Scholar] [CrossRef]
- Fortpied, J.; Collignon, S.; Moniotte, N.; Renaud, F.; Bayat, B.; Lemoine, D. The thermostability of the RTS,S/AS01 malaria vaccine can be increased by co-lyophilizing RTS,S and AS01. Malar. J. 2020, 19, 202. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Testing of Retroviral Vector-Based Human Gene Therapy Products for Replication Competent Retrovirus During Product Manufacture and Patient Follow-Up: Guidance for Industry; US Food and Drug Administration: Silver Spring, MD, USA, 2020. [Google Scholar]
- Tang, J. Using UV–Vis Titration to Elucidate Novel Epigallocatechin Gallate (EGCG)-Induced Binding of the c-MYC G-Quadruplex. Pharmaceuticals 2025, 18, 719. [Google Scholar] [CrossRef]
- US Food and Drug Administration. International Regulatory Harmonization FDA Guidance Documents; US Food and Drug Administration: Silver Spring, MD, USA, 1997. [Google Scholar]
- Tang, J.; Amin, M.A.; Campian, J.L. Glioblastoma Stem Cells at the Nexus of Tumor Heterogeneity, Immune Evasion, and Therapeutic Resistance. Cells 2025, 14, 562. [Google Scholar] [CrossRef]
- Coutant, F.; Frenkiel, M.P.; Despres, P.; Charneau, P. Protective antiviral immunity conferred by a nonintegrative lentiviral vector-based vaccine. PLoS ONE 2008, 3, e3973. [Google Scholar] [CrossRef]
- Tang, J.; Karbhari, N.; Campian, J.L. Therapeutic Targets in Glioblastoma: Molecular Pathways, Emerging Strategies, and Future Directions. Cells 2025, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liao, Q.; Chen, T.; Zhang, Y.; Yuan, W.; Xu, J.; Zhang, X. Freeze-Drying Formulations Increased the Adenovirus and Poxvirus Vaccine Storage Times and Antigen Stabilities. Virol. Sin. 2021, 36, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, B.; Baronti, C.; Gould, E.A.; Charrel, R.N.; de Lamballerie, X. Effect of chemical stabilizers on the thermostability and infectivity of a representative panel of freeze dried viruses. PLoS ONE 2015, 10, e0118963. [Google Scholar] [CrossRef]
- Arcidiacono, J. International Harmonization for Cell and Gene Therapy Products. Adv. Exp. Med. Biol. 2023, 1430, 235–240. [Google Scholar]
- Gabay, M.; Vora, N. RxLegal: Emergency Use Authorizations. Hosp. Pharm. 2023, 58, 16–17. [Google Scholar] [CrossRef]
- Gurumoorthy, N.; Nordin, F.; Tye, G.J.; Wan Kamarul Zaman, W.S.; Ng, M.H. Non-Integrating Lentiviral Vectors in Clinical Applications: A Glance Through. Biomedicines 2022, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Dicks, M.D.; Spencer, A.J.; Edwards, N.J.; Wadell, G.; Bojang, K.; Gilbert, S.C.; Hill, A.V.; Cottingham, M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: Improved systems for vector derivation and comparative immunogenicity. PLoS ONE 2012, 7, e40385. [Google Scholar] [CrossRef]
- Flickinger Jr, J.C.; Singh, J.; Carlson, R.; Leong, E.; Baybutt, T.R.; Barton, J.; Caparosa, E.; Pattison, A.; Rappaport, J.A.; Roh, J.; et al. Chimeric Ad5.F35 vector evades anti-adenovirus serotype 5 neutralization opposing GUCY2C-targeted antitumor immunity. J. Immunother. Cancer 2020, 8, e001046. [Google Scholar] [CrossRef]
- Greinacher, A.; Selleng, K.; Palankar, R.; Wesche, J.; Handtke, S.; Wolff, M.; Aurich, K.; Lalk, M.; Methling, K.; Völker, U.; et al. Insights in ChAdOx1 nCoV-19 vaccine-induced immune thrombotic thrombocytopenia. Blood 2021, 138, 2256–2268. [Google Scholar] [CrossRef]
- Cines, D.B.; Greinacher, A. Vaccine-induced immune thrombotic thrombocytopenia. Blood 2023, 141, 1659–1665. [Google Scholar] [CrossRef]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.; Blasi, M. The use of viral vectors in vaccine development. npj Vaccines 2022, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Pai, M.; Huisman, M.V.; Makris, M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022, 9, e73–e80. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Garg, I.; Sheikh, A.B.; Pal, S.; Shekhar, R. Mix-and-Match COVID-19 Vaccinations (Heterologous Boost): A Review. Infect Dis. Rep. 2022, 14, 537–546. [Google Scholar] [CrossRef]
- Stevens, C.S.; Carmichael, J.C.; Watkinson, R.; Kowdle, S.; Reis, R.A.; Hamane, K.; Jang, J.; Park, A.; Pernet, O.; Khamaikawin, W.; et al. A temperature-sensitive and less immunogenic Sendai virus for efficient gene editing. J. Virol. 2024, 98, e0083224. [Google Scholar] [CrossRef]
- Fu, H.; Liang, Y.; Zhong, X.; Pan, Z.; Huang, L.; Zhang, H.; Xu, Y.; Zhou, W.; Liu, Z. Codon optimization with deep learning to enhance protein expression. Sci. Rep. 2020, 10, 17617. [Google Scholar] [CrossRef] [PubMed]
- Tenbusch, M.; Kuate, S.; Tippler, B.; Gerlach, N.; Schimmer, S.; Dittmer, U.; Überla, K. Coexpression of GM-CSF and antigen in DNA prime-adenoviral vector boost immunization enhances polyfunctional CD8+ T cell responses, whereas expression of GM-CSF antigen fusion protein induces autoimmunity. BMC Immunol. 2008, 9, 13. [Google Scholar] [CrossRef]
- Ho, N.T.; Hughes, S.G.; Sekulovich, R.; Ta, V.T.; Nguyen, T.V.; Van Pham, A.T.; Luong, Q.C.; Le Tran, L.T.; Van Luu, A.T.; Nguyen, A.N.; et al. A randomized trial comparing safety, immunogenicity and efficacy of self-amplifying mRNA and adenovirus-vector COVID-19 vaccines. npj Vaccines 2024, 9, 233. [Google Scholar] [CrossRef]
- Graham, B.S. Rapid COVID-19 vaccine development. Science 2020, 368, 945–946. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Park, S.P.; Lee, H.J.; Yu, Y.; Lee, E.Y.J.; Park, Y.S. Designing the global vaccine supply chain: Balancing intellectual property rights with post COVID-19 vaccine equity. BMJ Glob. Health 2023, 8, e013669. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Lienert, F.; Lohmueller, J.J.; Garg, A.; Silver, P.A. Synthetic biology in mammalian cells: Next generation research tools and therapeutics. Nat. Rev. Mol. Cell Biol. 2014, 15, 95–107. [Google Scholar] [CrossRef]
- Silva-Pilipich, N.; Beloki, U.; Salaberry, L.; Smerdou, C. Self-Amplifying RNA: A Second Revolution of mRNA Vaccines against COVID-19. Vaccines 2024, 12, 318. [Google Scholar] [CrossRef]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Golan, M.S.T.B.; Cegan, J.C.; Linkov, I. The vaccine supply chain: A call for resilience analytics to support COVID-19 vaccine production and distribution. Syst. Risk Resil. 2020, 2011, 389–437. [Google Scholar]
- Alizadeh, M.; Amini-Khoei, H.; Tahmasebian, S.; Ghatrehsamani, M.; Ghatreh Samani, K.; Edalatpanah, Y.; Rostampur, S.; Salehi, M.; Ghasemi-Dehnoo, M.; Azadegan-Dehkordi, F.; et al. Designing a novel multi–epitope vaccine against Ebola virus using reverse vaccinology approach. Sci. Rep. 2022, 12, 7757. [Google Scholar] [CrossRef]
| Viral Vector | Genome Type | Key Advantages | Key Limitations | Notable Examples/Uses |
|---|---|---|---|---|
| Adenovirus (Ad) | Non-enveloped, dsDNA |
|
|
|
| Adeno-Associated Virus (AAV) | Non-enveloped, ssDNA |
|
|
|
| Poxviruses (MVA, NYVAC) | Enveloped, dsDNA (large genome) |
|
|
|
| Vesicular Stomatitis Virus (VSV) | Enveloped, negative-sense RNA |
|
|
|
| Measles Virus (MV) | Enveloped, negative-sense RNA |
|
|
|
| Alphavirus Vectors (e.g., Sindbis, Semliki Forest) | Enveloped, positive-sense RNA |
|
|
|
| Lentiviruses (LV) | Enveloped, ssRNA (retrovirus) |
|
|
|
| Insect-Specific Flaviviruses | Enveloped, positive-sense RNA |
|
|
|
| Challenge | Potential Solutions/Mitigation Strategies |
|---|---|
| Pre-Existing Immunity (e.g., to Ad5) |
|
| |
| |
| Manufacturing Bottlenecks (Scale-up, QC) |
|
| |
| |
| Rare Adverse Events (e.g., VITT in Ad vectors) |
|
| |
| |
| Equitable Distribution (Global access) |
|
| |
| |
| Maintaining Efficacy and Durability |
|
| |
| |
| Public Trust & Vaccine Hesitancy |
|
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Amin, M.A.; Campian, J.L. Past, Present, and Future of Viral Vector Vaccine Platforms: A Comprehensive Review. Vaccines 2025, 13, 524. https://doi.org/10.3390/vaccines13050524
Tang J, Amin MA, Campian JL. Past, Present, and Future of Viral Vector Vaccine Platforms: A Comprehensive Review. Vaccines. 2025; 13(5):524. https://doi.org/10.3390/vaccines13050524
Chicago/Turabian StyleTang, Justin, Md Al Amin, and Jian L. Campian. 2025. "Past, Present, and Future of Viral Vector Vaccine Platforms: A Comprehensive Review" Vaccines 13, no. 5: 524. https://doi.org/10.3390/vaccines13050524
APA StyleTang, J., Amin, M. A., & Campian, J. L. (2025). Past, Present, and Future of Viral Vector Vaccine Platforms: A Comprehensive Review. Vaccines, 13(5), 524. https://doi.org/10.3390/vaccines13050524





