A Computationally Designed Prefusion Stabilized Human Metapneumovirus Fusion Protein Vaccine Antigen Elicited a Potent Neutralization Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Design
2.2. Protein Expression and Purification
2.3. Size Exclusion Chromatography (SEC) and SEC Coupled with Multi-Angle Light Scattering (SEC-MALS)
2.4. Nano Differential Scanning Fluorimetry
2.5. Linear and Conformational Antibody Binding Analysis by Biolayer Interferometry
2.6. Viruses and Cells
2.7. Mouse Immunogenicity Studies
2.8. Enzyme Linked Immunosorbent Assay (ELISA)
2.9. Competitive ELISA
2.10. Microneutralization Assays
2.11. Plaque Reduction Neutralization Test
2.12. Statistical Analysis
3. Results
3.1. Selection of the Base Sequence and Design of the Pre-F Constructs
3.1.1. Selection of the Base Sequence for the Pre-F Designs
3.1.2. Design of the Pre-F Constructs
3.2. Production and Biophysical Characterization of the Pre-F Constructs and Benchmark
3.2.1. Yields and Purity
3.2.2. Thermostability Assessments
3.2.3. Antigenicity Assessments
3.3. Immunogenicity in the Mouse Model
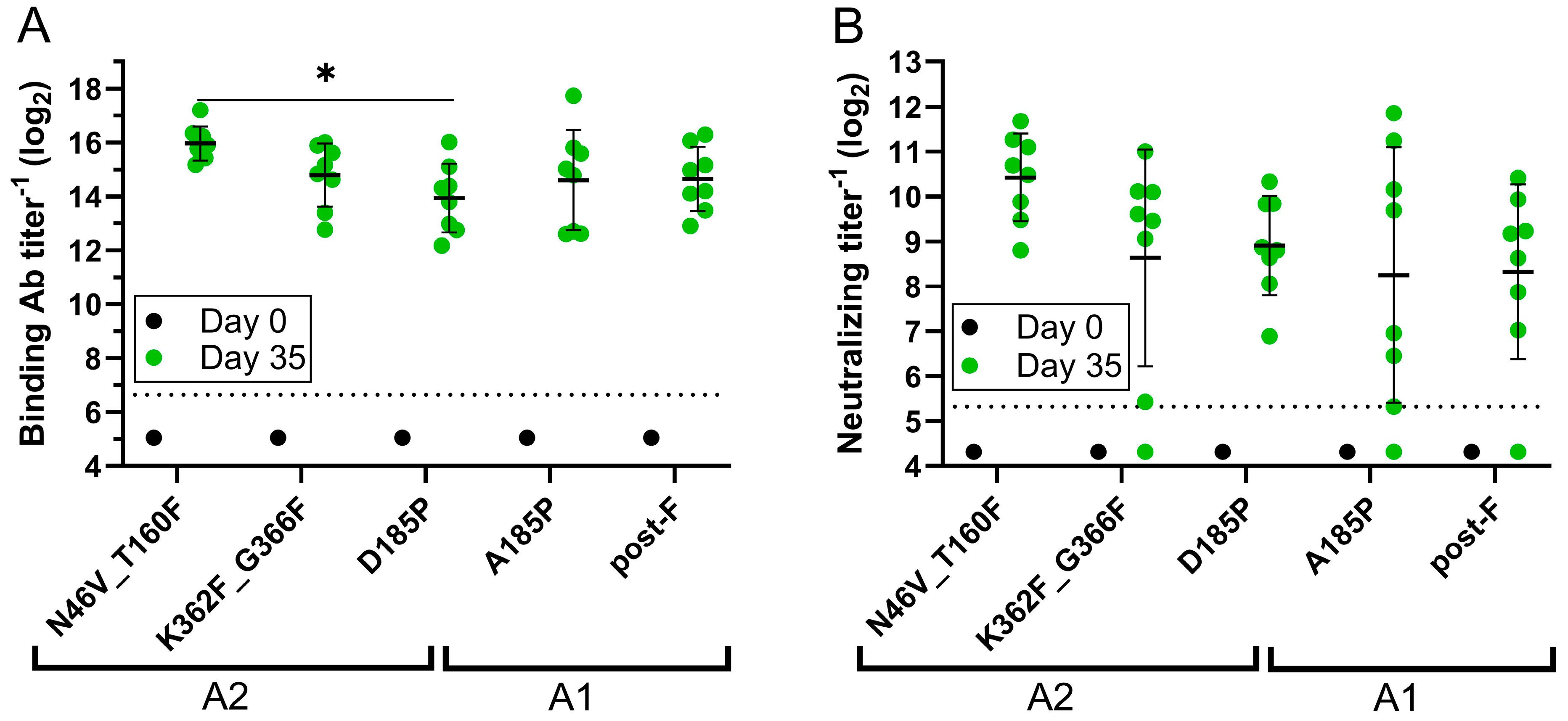
3.3.1. Assessments of Binding, Neutralizing, and Site-Specific Ab Titers
3.3.2. Cross-Subtype Neutralization Analysis
3.4. Comparison to Next Generation Structure-Based Designs
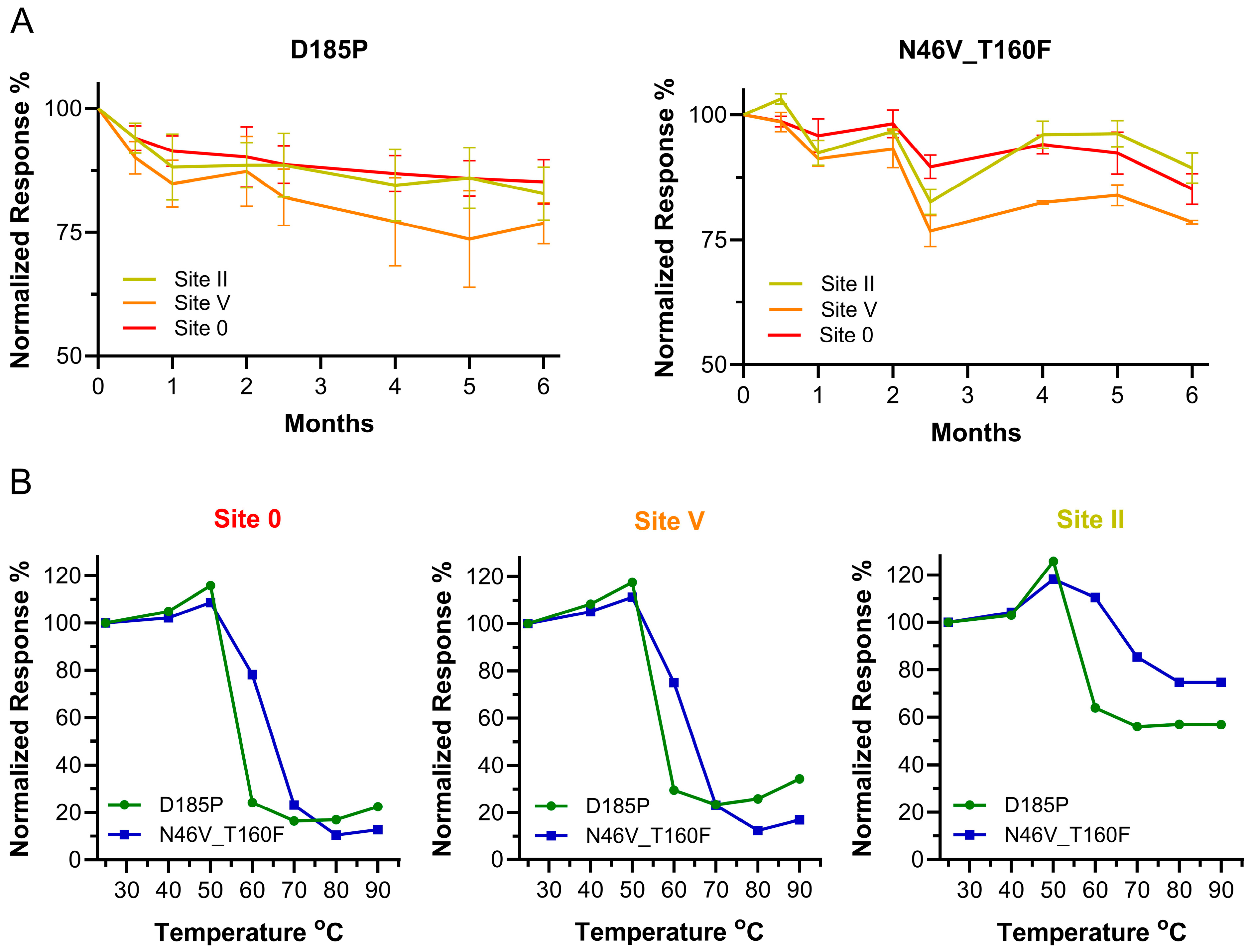
3.5. Long-Term Stability and Epitope Integrity at Higher Temperatures
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boivin, G.; Abed, Y.; Pelletier, G.; Ruel, L.; Moisan, D.; Côté, S.; Peret, T.C.T.; Erdman, D.D.; Anderson, L.J. Virological Features and Clinical Manifestations Associated with Human Metapneumovirus: A New Paramyxovirus Responsible for Acute Respiratory-Tract Infections in All Age Groups. J. Infect. Dis. 2002, 186, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Widmer, K.; Zhu, Y.; Williams, J.V.; Griffin, M.R.; Edwards, K.M.; Talbot, H.K. Rates of Hospitalizations for Respiratory Syncytial Virus, Human Metapneumovirus, and Influenza Virus in Older Adults. J. Infect. Dis. 2012, 206, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Criddle, M.M.; Biear, J.M.; Walsh, E.E. Humoral Immunity to Human Metapneumovirus Infection in Adults. Vaccine 2010, 28, 1477–1480. [Google Scholar] [CrossRef]
- Deffrasnes, C.; Hamelin, M.-E.; Boivin, G. Human Metapneumovirus. Semin. Respir. Crit. Care Med. 2007, 28, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, D.; Cong, B.; Ranjini, M.J.K.; Balchandani, G.; Chen, S.; Liang, J.; Gordon, L.G.; Meulen, A.S.-T.; Wang, X.; Li, Y.; et al. The Global Burden of Human Metapneumovirus-Associated Acute Respiratory Infections in Older Adults: A Systematic Review and Meta-Analysis. Lancet Healthy Longev. 2025, 6, 100679. [Google Scholar] [CrossRef]
- Dunn, S.R.; Ryder, A.B.; Tollefson, S.J.; Xu, M.; Saville, B.R.; Williams, J.V. Seroepidemiologies of Human Metapneumovirus and Respiratory Syncytial Virus in Young Children, Determined with a New Recombinant Fusion Protein Enzyme-Linked Immunosorbent Assay. Clin. Vaccine Immunol. 2013, 20, 1654–1656. [Google Scholar] [CrossRef]
- van den Hoogen, B.G.; de Jong, J.C.; Groen, J.; Kuiken, T.; de Groot, R.; Fouchier, R.A.; Osterhaus, A.D. A Newly Discovered Human Pneumovirus Isolated from Young Children with Respiratory Tract Disease. Nat. Med. 2001, 7, 719–724. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Deloria-Knoll, M.; Madhi, S.A.; Cohen, C.; Ali, A.; Basnet, S.; Bassat, Q.; Brooks, W.A.; Chittaganpitch, M.; et al. Global Burden of Acute Lower Respiratory Infection Associated with Human Metapneumovirus in Children under 5 Years in 2018: A Systematic Review and Modelling Study. Lancet Glob. Health 2021, 9, e33–e43. [Google Scholar] [CrossRef]
- Okamoto, M.; Sugawara, K.; Takashita, E.; Muraki, Y.; Hongo, S.; Nishimura, H.; Matsuzaki, Y. Longitudinal Course of Human Metapneumovirus Antibody Titers and Reinfection in Healthy Adults. J. Med. Virol. 2010, 82, 2092–2096. [Google Scholar] [CrossRef]
- Aguilar, H.C.; Henderson, B.A.; Zamora, J.L.; Johnston, G.P. Paramyxovirus Glycoproteins and the Membrane Fusion Process. Curr. Clin. Microbiol. Rep. 2016, 3, 142–154. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Rush, S.A.; Palomo, C.; Chou, C.-W.; Pickens, W.; Más, V.; McLellan, J.S. Structure-Based Design of Prefusion-Stabilized Human Metapneumovirus Fusion Proteins. Nat. Commun. 2022, 13, 1299. [Google Scholar] [CrossRef] [PubMed]
- Skiadopoulos, M.H.; Biacchesi, S.; Buchholz, U.J.; Amaro-Carambot, E.; Surman, S.R.; Collins, P.L.; Murphy, B.R. Individual Contributions of the Human Metapneumovirus F, G, and SH Surface Glycoproteins to the Induction of Neutralizing Antibodies and Protective Immunity. Virology 2006, 345, 492–501. [Google Scholar] [CrossRef] [PubMed]
- August, A.; Shaw, C.A.; Lee, H.; Knightly, C.; Kalidindia, S.; Chu, L.; Essink, B.J.; Seger, W.; Zaks, T.; Smolenov, I.; et al. Safety and Immunogenicity of an MRNA-Based Human Metapneumovirus and Parainfluenza Virus Type 3 Combined Vaccine in Healthy Adults. Open Forum. Infect. Dis. 2022, 9, ofac206. [Google Scholar] [CrossRef]
- Herfst, S.; de Graaf, M.; Schrauwen, E.J.A.; Ulbrandt, N.D.; Barnes, A.S.; Senthil, K.; Osterhaus, A.D.M.E.; Fouchier, R.A.M.; van den Hoogen, B.G. Immunization of Syrian Golden Hamsters with F Subunit Vaccine of Human Metapneumovirus Induces Protection against Challenge with Homologous or Heterologous Strains. J. Gen. Virol. 2007, 88, 2702–2709. [Google Scholar] [CrossRef]
- Cseke, G.; Wright, D.W.; Tollefson, S.J.; Johnson, J.E.; Crowe, J.E.J.; Williams, J.V. Human Metapneumovirus Fusion Protein Vaccines That Are Immunogenic and Protective in Cotton Rats. J. Virol. 2007, 81, 698–707. [Google Scholar] [CrossRef]
- Hammitt, L.L.; Dagan, R.; Yuan, Y.; Cots, M.B.; Bosheva, M.; Madhi, S.A.; Muller, W.J.; Zar, H.J.; Brooks, D.; Grenham, A.; et al. Nirsevimab for Prevention of RSV in Healthy Late-Preterm and Term Infants. N. Engl. J. Med. 2022, 386, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Piedra, P.A.; Jewell, A.M.; Cron, S.G.; Atmar, R.L.; Glezen, W.P. Correlates of Immunity to Respiratory Syncytial Virus (RSV) Associated-Hospitalization: Establishment of Minimum Protective Threshold Levels of Serum Neutralizing Antibodies. Vaccine 2003, 21, 3479–3482. [Google Scholar] [CrossRef]
- McLellan, J.S.; Chen, M.; Leung, S.; Graepel, K.W.; Du, X.; Yang, Y.; Zhou, T.; Baxa, U.; Yasuda, E.; Beaumont, T.; et al. Structure of RSV Fusion Glycoprotein Trimer Bound to a Prefusion-Specific Neutralizing Antibody. Science 2013, 340, 1113–1117. [Google Scholar] [CrossRef]
- McLellan, J.S.; Yang, Y.; Graham, B.S.; Kwong, P.D. Structure of Respiratory Syncytial Virus Fusion Glycoprotein in the Postfusion Conformation Reveals Preservation of Neutralizing Epitopes. J. Virol. 2011, 85, 7788–7796. [Google Scholar] [CrossRef]
- Liang, B.; Surman, S.; Amaro-Carambot, E.; Kabatova, B.; Mackow, N.; Lingemann, M.; Yang, L.; McLellan, J.S.; Graham, B.S.; Kwong, P.D.; et al. Enhanced Neutralizing Antibody Response Induced by Respiratory Syncytial Virus Prefusion F Protein Expressed by a Vaccine Candidate. J. Virol. 2015, 89, 9499–9510. [Google Scholar] [CrossRef]
- Ngwuta, J.O.; Chen, M.; Modjarrad, K.; Joyce, M.G.; Kanekiyo, M.; Kumar, A.; Yassine, H.M.; Moin, S.M.; Killikelly, A.M.; Chuang, G.-Y.; et al. Prefusion F-Specific Antibodies Determine the Magnitude of RSV Neutralizing Activity in Human Sera. Sci. Transl. Med. 2015, 7, 309ra162. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Marc, G.P.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an MRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- Battles, M.B.; Más, V.; Olmedillas, E.; Cano, O.; Vázquez, M.; Rodríguez, L.; Melero, J.A.; McLellan, J.S. Structure and Immunogenicity of Pre-Fusion-Stabilized Human Metapneumovirus F Glycoprotein. Nat. Commun. 2017, 8, 1528. [Google Scholar] [CrossRef]
- Más, V.; Rodriguez, L.; Olmedillas, E.; Cano, O.; Palomo, C.; Terrón, M.C.; Luque, D.; Melero, J.A.; McLellan, J.S. Engineering, Structure and Immunogenicity of the Human Metapneumovirus F Protein in the Postfusion Conformation. PLoS Pathog. 2016, 12, e1005859. [Google Scholar] [CrossRef]
- Ou, L.; Chen, S.J.; Teng, I.-T.; Yang, L.; Zhang, B.; Zhou, T.; Biju, A.; Cheng, C.; Kong, W.-P.; Morano, N.C.; et al. Structure-Based Design of a Single-Chain Triple-Disulfide-Stabilized Fusion-Glycoprotein Trimer That Elicits High-Titer Neutralizing Responses against Human Metapneumovirus. PLoS Pathog. 2023, 19, e1011584. [Google Scholar] [CrossRef]
- Lee, Y.-Z.; Han, J.; Zhang, Y.-N.; Ward, G.; Gomes, K.B.; Auclair, S.; Stanfield, R.L.; He, L.; Wilson, I.A.; Zhu, J. Rational Design of Uncleaved Prefusion-Closed Trimer Vaccines for Human Respiratory Syncytial Virus and Metapneumovirus. Nat. Commun. 2024, 15, 9939. [Google Scholar] [CrossRef]
- Groen, K.; van Nieuwkoop, S.; Meijer, A.; van der Veer, B.; van Kampen, J.J.A.; Fraaij, P.L.; Fouchier, R.A.M.; van den Hoogen, B.G. Emergence and Potential Extinction of Genetic Lineages of Human Metapneumovirus between 2005 and 2021. mBio 2023, 14, e0228022. [Google Scholar] [CrossRef]
- Otomaru, H.; Nguyen, H.A.T.; Vo, H.M.; Toizumi, M.; Le, M.N.; Mizuta, K.; Moriuchi, H.; Bui, M.X.; Dang, D.A.; Yoshida, L.-M. A Decade of Human Metapneumovirus in Hospitalized Children with Acute Respiratory Infection: Molecular Epidemiology in Central Vietnam, 2007–2020. Sci. Rep. 2023, 13, 15757. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, S.; Zhang, K.; Li, Y.; Chen, D.; Tan, Y.; Liang, L.; Liu, M.; Liang, J.; An, S.; et al. Epidemiology, Genetic Characteristics, and Association with Meteorological Factors of Human Metapneumovirus Infection in Children in Southern China: A 10-Year Retrospective Study. Int. J. Infect. Dis. 2023, 137, 40–47. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, B.G.; Herfst, S.; Sprong, L.; Cane, P.A.; Forleo-Neto, E.; de Swart, R.L.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Antigenic and Genetic Variability of Human Metapneumoviruses. Emerg. Infect. Dis. 2004, 10, 658–666. [Google Scholar] [CrossRef]
- Ryder, A.B.; Tollefson, S.J.; Podsiad, A.B.; Johnson, J.E.; Williams, J.V. Soluble Recombinant Human Metapneumovirus G Protein Is Immunogenic but Not Protective. Vaccine 2010, 28, 4145–4152. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-F.; Wang, C.K.; Tollefson, S.J.; Piyaratna, R.; Lintao, L.D.; Chu, M.; Liem, A.; Mark, M.; Spaete, R.R.; Crowe, J.E.J.; et al. Genetic Diversity and Evolution of Human Metapneumovirus Fusion Protein over Twenty Years. Virol. J. 2009, 6, 138. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, E.H.; Leaver-Fay, A.; Baker, D. Role of Conformational Sampling in Computing Mutation-Induced Changes in Protein Structure and Stability. Proteins 2011, 79, 830–838. [Google Scholar] [CrossRef]
- Park, H.; Bradley, P.; Greisen, P.J.; Liu, Y.; Mulligan, V.K.; Kim, D.E.; Baker, D.; DiMaio, F. Simultaneous Optimization of Biomolecular Energy Functions on Features from Small Molecules and Macromolecules. J. Chem. Theory Comput. 2016, 12, 6201–6212. [Google Scholar] [CrossRef]
- Frank, S.; Kammerer, R.A.; Mechling, D.; Schulthess, T.; Landwehr, R.; Bann, J.; Guo, Y.; Lustig, A.; Bächinger, H.P.; Engel, J. Stabilization of Short Collagen-like Triple Helices by Protein Engineering. J. Mol. Biol. 2001, 308, 1081–1089. [Google Scholar] [CrossRef]
- Kishko, M.; Catalan, J.; Swanson, K.; DiNapoli, J.; Wei, C.-J.; Delagrave, S.; Chivukula, S.; Zhang, L. Evaluation of the Respiratory Syncytial Virus G-Directed Neutralizing Antibody Response in the Human Airway Epithelial Cell Model. Virology 2020, 550, 21–26. [Google Scholar] [CrossRef]
- Rainho-Tomko, J.N.; Pavot, V.; Kishko, M.; Swanson, K.; Edwards, D.; Yoon, H.; Lanza, L.; Alamares-Sapuay, J.; Osei-Bonsu, R.; Mundle, S.T.; et al. Immunogenicity and Protective Efficacy of RSV G Central Conserved Domain Vaccine with a Prefusion Nanoparticle. NPJ Vaccines 2022, 7, 74. [Google Scholar] [CrossRef]
- Rush, S.A.; Brar, G.; Hsieh, C.-L.; Chautard, E.; Rainho-Tomko, J.N.; Slade, C.D.; Bricault, C.A.; Kume, A.; Kearns, J.; Groppo, R.; et al. Characterization of Prefusion-F-Specific Antibodies Elicited by Natural Infection with Human Metapneumovirus. Cell Rep. 2022, 40, 111399. [Google Scholar] [CrossRef]
- Wen, X.; Krause, J.C.; Leser, G.P.; Cox, R.G.; Lamb, R.A.; Williams, J.V.; Crowe, J.E.J.; Jardetzky, T.S. Structure of the Human Metapneumovirus Fusion Protein with Neutralizing Antibody Identifies a Pneumovirus Antigenic Site. Nat. Struct. Mol. Biol. 2012, 19, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Ulbrandt, N.D.; Ji, H.; Patel, N.K.; Riggs, J.M.; Brewah, Y.A.; Ready, S.; Donacki, N.E.; Folliot, K.; Barnes, A.S.; Senthil, K.; et al. Isolation and Characterization of Monoclonal Antibodies Which Neutralize Human Metapneumovirus in Vitro and in Vivo. J. Virol. 2006, 80, 7799–7806. [Google Scholar] [CrossRef] [PubMed]
- Rappazzo, C.G.; Hsieh, C.-L.; Rush, S.A.; Esterman, E.S.; Delgado, T.; Geoghegan, J.C.; Wec, A.Z.; Sakharkar, M.; Más, V.; McLellan, J.S.; et al. Potently Neutralizing and Protective Anti-Human Metapneumovirus Antibodies Target Diverse Sites on the Fusion Glycoprotein. Immunity 2022, 55, 1710–1724.e8. [Google Scholar] [CrossRef]
- Wen, X.; Mousa, J.J.; Bates, J.T.; Lamb, R.A.; Crowe, J.E.J.; Jardetzky, T.S. Structural Basis for Antibody Cross-Neutralization of Respiratory Syncytial Virus and Human Metapneumovirus. Nat. Microbiol. 2017, 2, 16272. [Google Scholar] [CrossRef]
- Mousa, J.J.; Binshtein, E.; Human, S.; Fong, R.H.; Alvarado, G.; Doranz, B.J.; Moore, M.L.; Ohi, M.D.; Crowe, J.E.J. Human Antibody Recognition of Antigenic Site IV on Pneumovirus Fusion Proteins. PLoS Pathog. 2018, 14, e1006837. [Google Scholar] [CrossRef]
- Boivin, G.; Serres, G.D.; Hamelin, M.-E.; Côté, S.; Argouin, M.; Tremblay, G.; Maranda-Aubut, R.; Sauvageau, C.; Ouakki, M.; Boulianne, N.; et al. An Outbreak of Severe Respiratory Tract Infection Due to Human Metapneumovirus in a Long-Term Care Facility. Clin. Infect. Dis. 2007, 44, 1152–1158. [Google Scholar] [CrossRef]
- Englund, J.A.; Boeckh, M.; Kuypers, J.; Nichols, W.G.; Hackman, R.C.; Morrow, R.A.; Fredricks, D.N.; Corey, L. Brief Communication: Fatal Human Metapneumovirus Infection in Stem-Cell Transplant Recipients. Ann. Intern. Med. 2006, 144, 344–349. [Google Scholar] [CrossRef]
- Liao, R.S.; Appelgate, D.M.; Pelz, R.K. An Outbreak of Severe Respiratory Tract Infection Due to Human Metapneumovirus in a Long-Term Care Facility for the Elderly in Oregon. J. Clin. Virol. 2012, 53, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Peterson, D.R.; Falsey, A.R. Human Metapneumovirus Infections in Adults: Another Piece of the Puzzle. Arch. Intern. Med. 2008, 168, 2489–2496. [Google Scholar] [CrossRef]
- Icosavax, Inc. Safety and Immunogenicity of IVX-A12 in Healthy Older Adults. 2022. Available online: https://clinicaltrials.gov/study/NCT05664334 (accessed on 3 January 2025).
- Swanson, K.A.; Rainho-Tomko, J.N.; Williams, Z.P.; Lanza, L.; Peredelchuk, M.; Kishko, M.; Pavot, V.; Alamares-Sapuay, J.; Adhikarla, H.; Gupta, S.; et al. A Respiratory Syncytial Virus (RSV) F Protein Nanoparticle Vaccine Focuses Antibody Responses to a Conserved Neutralization Domain. Sci. Immunol. 2020, 5, eaba6466. [Google Scholar] [CrossRef]




| Sample | Trimer (%) 1 | MW (kDa) 2 |
|---|---|---|
| D185P | 97.1 | 224.3 |
| N46V_T160F | 94.7 | 266.5 |
| K362F_G366F | 76.9 | 222.8 |
| K138F | 0 | 5860.0 |
| mAb | Site | A1 A185P | A1 Post-F | A2 D185P | A2 N46V_T160F | A2 K362F_G366F |
|---|---|---|---|---|---|---|
| San32-2 | Ø | + | − | + | + | + |
| San27-14 | V | + | − | + | + | + |
| 338 | II | + | + | + | + | + |
| MPE8 | III | + | − | + | + | + |
| 101F | IV | + | + | + | + | + |
| DS7 | I | − | + | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kishko, M.; Stuebler, A.; Sasmal, S.; Chan, Y.; Huang, D.; Reyes, C.; Lin, J.; Price, O.; Kume, A.; Zong, K.; et al. A Computationally Designed Prefusion Stabilized Human Metapneumovirus Fusion Protein Vaccine Antigen Elicited a Potent Neutralization Response. Vaccines 2025, 13, 523. https://doi.org/10.3390/vaccines13050523
Kishko M, Stuebler A, Sasmal S, Chan Y, Huang D, Reyes C, Lin J, Price O, Kume A, Zong K, et al. A Computationally Designed Prefusion Stabilized Human Metapneumovirus Fusion Protein Vaccine Antigen Elicited a Potent Neutralization Response. Vaccines. 2025; 13(5):523. https://doi.org/10.3390/vaccines13050523
Chicago/Turabian StyleKishko, Michael, Antonia Stuebler, Sukanya Sasmal, Yvonne Chan, Dean Huang, Christopher Reyes, Jasmine Lin, Owen Price, Ana Kume, Katie Zong, and et al. 2025. "A Computationally Designed Prefusion Stabilized Human Metapneumovirus Fusion Protein Vaccine Antigen Elicited a Potent Neutralization Response" Vaccines 13, no. 5: 523. https://doi.org/10.3390/vaccines13050523
APA StyleKishko, M., Stuebler, A., Sasmal, S., Chan, Y., Huang, D., Reyes, C., Lin, J., Price, O., Kume, A., Zong, K., Bricault, C., Alamares-Sapuay, J., & Zhang, L. (2025). A Computationally Designed Prefusion Stabilized Human Metapneumovirus Fusion Protein Vaccine Antigen Elicited a Potent Neutralization Response. Vaccines, 13(5), 523. https://doi.org/10.3390/vaccines13050523





