Potential for a Combined Oral Inactivated Whole-Cell Vaccine Against ETEC and Shigella: Preclinical Studies Supporting Feasibility
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccines and Adjuvant
2.2. Antigens
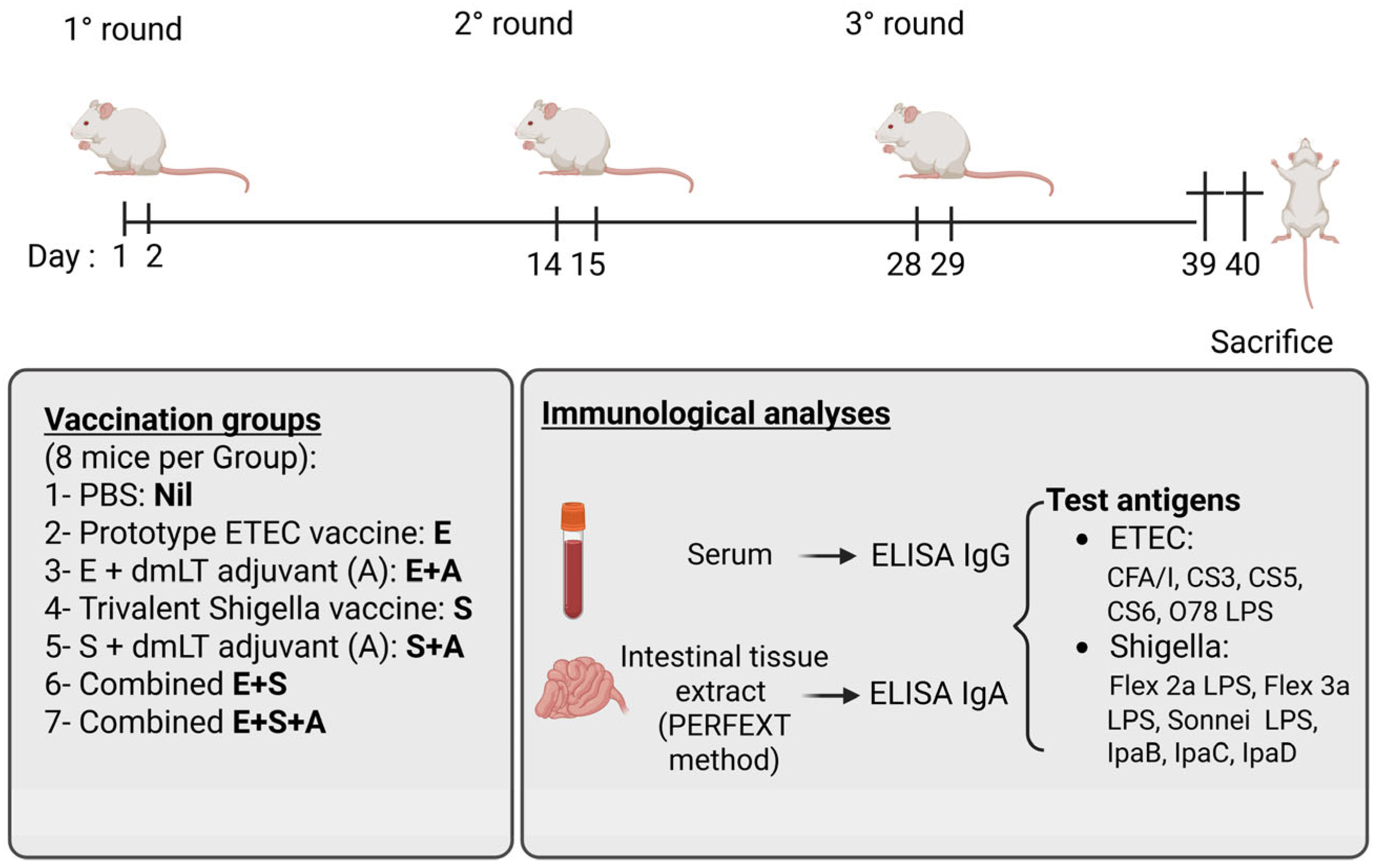
2.3. Immunizations and Collection of Samples
Mice
2.4. Immunizations and Collections of Specimens
2.5. Immunological Analyses
2.6. Statistical Analysis
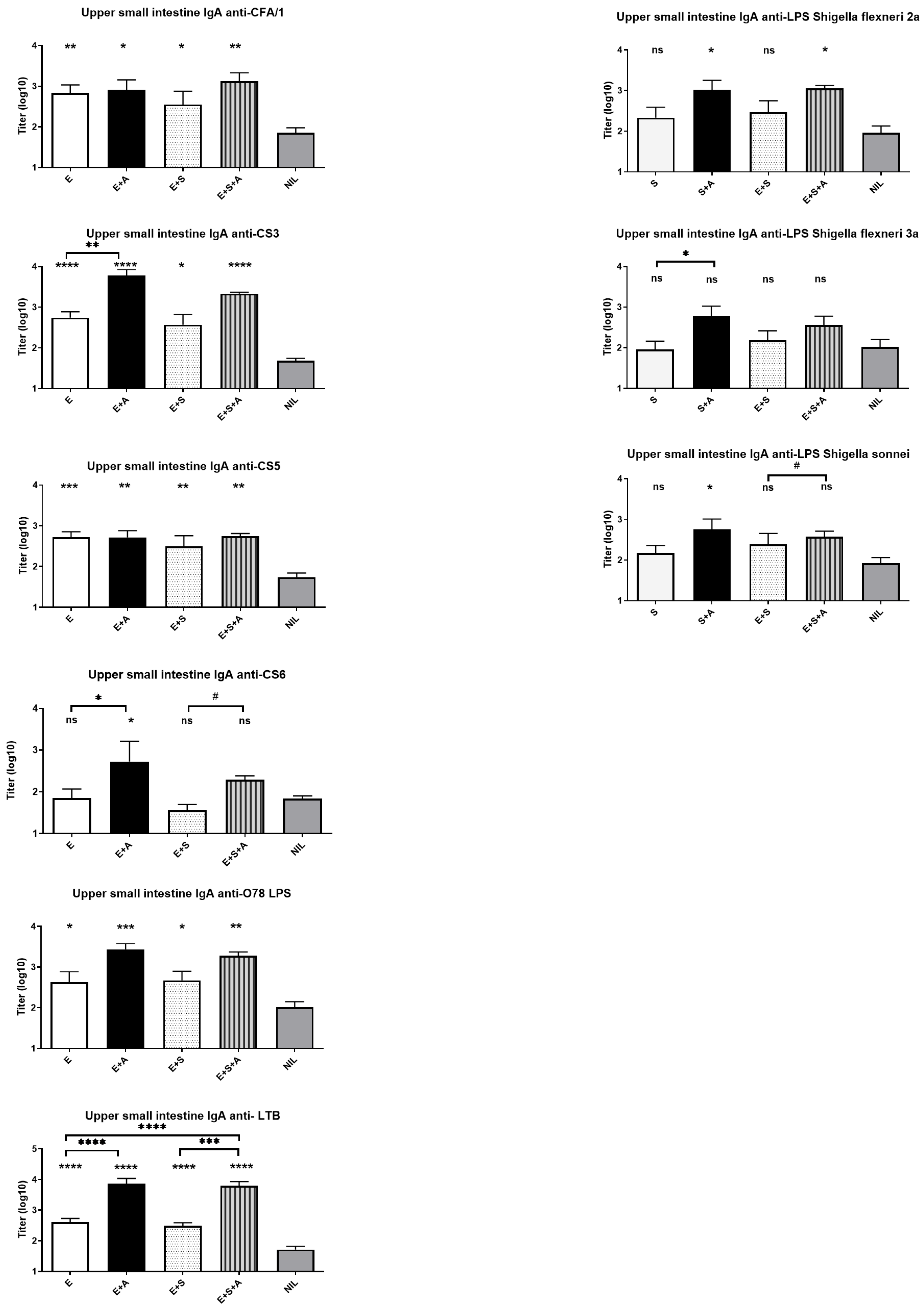
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalil, I.A. (Institute for Health Metrics and Evaluation, Seattle, WA, USA). Personal communication, 2021. [Google Scholar]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Troeger, C.; Blacker, B.F.; Khalil, I.A.; Rao, P.C.; Cao, S.; Zimsen, S.R.; Albertson, S.B.; Stanaway, J.D.; Deshpande, A.; Abebe, Z.; et al. Diarrhoeal Disease Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Anderson, J.D.; Bagamian, K.H.; Baqar, S.; Giersing, B.; Hausdorff, W.P.; Marshall, C.; Porter, C.K.; Walker, R.I.; Bourgeois, A.L. Vaccine value profile for enterotoxigenic Escherichia coli (ETEC). Vaccine 2023, 41 (Suppl. 2), S95–S113. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, W.P.; Anderson, J.D.t.; Bagamian, K.H.; Bourgeois, A.L.; Mills, M.; Sawe, F.; Scheele, S.; Talaat, K.; Giersing, B.K. Vaccine value profile for Shigella. Vaccine 2023, 41 (Suppl. 2), S76–S94. [Google Scholar] [CrossRef]
- Riddle, T.D.; Cachafiero, S.P.; Putnam, S.D.; Hooper, T.I. Development of a travelers’ diarrhea vaccine for the military: How much is an once of prevention really worth? Vaccine 2008, 26, 2490–2502. [Google Scholar] [CrossRef]
- Olson, S.; Hall, A.; Riddle, M.S.; Porter, C.K. Travelers’ diarrhea: Update on the incidence, etiology and risk in military and similar populations—1990–2005 versus 2005–2015, does a decade make a difference? Trop. Dis. Travel Med. Vaccines 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diarrhoeal Disease Fact Sheet; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 7 March 2024).
- PATH. DefeatDD Report; PATH: Sri Lanka, The Netherlands, 2023; Available online: https://report.defeatdd.org/challenge/ (accessed on 7 March 2024).
- Dhimal, M.; Bhandari, D.; Karki, K.B.; Shrestha, S.L.; Khanal, M.; Shrestha, R.R.P.; Dahal, S.; Bista, B.; Ebi, K.L.; Cisse, G.; et al. Effects of Climatic Factors on Diarrheal Diseases among Children below 5 Years of Age at National and Subnational Levels in Nepal: An Ecological Study. Int. J. Environ. Res. Public Health 2022, 19, 6138. [Google Scholar] [CrossRef] [PubMed]
- Dhimal, M.; Bhandari, D. Climate change and imperatives to ascertain causes of infectious diarrhoea in low-income and middle-income countries. Lancet Glob. Health 2023, 11, e308–e309. [Google Scholar] [CrossRef]
- Colston, J.M.; Zaitchik, B.F.; Badr, H.S.; Burnett, E.; Ali, S.A.; Rayamajhi, A.; Satter, S.M.; Eibach, D.; Krumkamp, R.; May, J.; et al. Associations Between Eight Earth Observation-Derived Climate Variables and Enteropathogen Infection: An Independent Participant Data Meta-Analysis of Surveillance Studies With Broad Spectrum Nucleic Acid Diagnostics. Geohealth 2022, 6, e2021GH000452. [Google Scholar] [CrossRef]
- Puett, C.; Anderson, J.D.t.; Bagamian, K.H.; Muhib, F.; Scheele, S.; Hausdorff, W.P.; Pecenka, C. Projecting the long-term economic benefits of reducing Shigella-attributable linear growth faltering with a potential vaccine: A modelling study. Lancet Glob. Health 2023, 11, e892–e902. [Google Scholar] [CrossRef]
- Guerrant, R.L.; Bolick, D.T.; Swann, J.R. Modeling Enteropathy or Diarrhea with the Top Bacterial and Protozoal Pathogens: Differential Determinants of Outcomes. ACS Infect. Dis. 2021, 7, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, W.P.; Anderson, J.D.t.; Bourgeois, A.L.; Clifford, A.; Fleming, J.A.; Muhib, F.; Pecenka, C.; Puett, C.; Riddle, M.S.; Scheele, S.; et al. Reassessing potential economic value and health impact of effective Shigella vaccines. Bull. World Health Organ. 2024, 102, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Hasso-Agopsowicz, M.; Sparrow, E.; Cameron, A.M.; Sati, H.; Srikantiah, P.; Gottlieb, S.; Bentsi-Enchill, A.; Le Doare, K.; Hamel, M.; Giersing, B.K.; et al. The role of vaccines in reducing antimicrobial resistance: A review of potential impact of vaccines on AMR and insights across 16 vaccines and pathogens. Vaccine 2024, 42 (Suppl. S1), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- UN AMR Declaration of Sept. 2024. Available online: https://www.who.int/news-room/events/detail/2024/09/26/default-calendar/un-general-assembly-high-level-meeting-on-antimicrobial-resistance-2024 (accessed on 28 September 2024).
- Hausdorff, W.P.; Madhi, S.A.; Kang, G.; Kabore, L.; Tufet Bayona, M.; Giersing, B.K. Facilitating the development of urgently required combination vaccines. Lancet Glob. Health 2024, 12, e1059–e1067. [Google Scholar] [CrossRef]
- Walker, R.I.; Bourgeois, A.L. Oral inactivated whole cell vaccine for mucosal immunization: ETVAX case study. Front. Immunol. 2023, 14, 1125102. [Google Scholar] [CrossRef]
- Walker, R.I.; Clifford, A. Recommendations regarding the development of combined enterotoxigenic Eschericha coli and Shigella vaccines for infants. Vaccine 2015, 33, 946–953. [Google Scholar] [CrossRef]
- Riddle, M.S.; Louis Bourgeois, A.; Clifford, A.; Jeon, S.; Giersing, B.K.; Jit, M.; Tufet Bayona, M.; Ovitt, J.; Hausdorff, W.P. Challenges and opportunities in developing a Shigella-containing combination vaccine for children in low- and middle-income countries: Report of an expert convening. Vaccine 2023, 41, 2634–2644. [Google Scholar] [CrossRef]
- Walker, R.I. Conserved antigens for enteric vaccines. Vaccine 2025, 50, 126828. [Google Scholar] [CrossRef]
- Large, K.; Ruiz, A.A.; Slovenski, I.; Vieira, M.; Moon, S. The 30-year evolution of oral cholera vaccines: A case study of a collaborative network alternative innovation model. PLoS Glob. Public Health 2025, 5, e0003599. [Google Scholar] [CrossRef]
- Clements, J.D.; Norton, E.B. The Mucosal Vaccine Adjuvant LT(R192G/L211A) or dmLT. mSphere 2018, 3, 4. [Google Scholar] [CrossRef]
- Norton, E.B.; Lawson, L.B.; Freytag, L.C.; Clements, J.D. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 2011, 18, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Akhtar, M.; Bhuiyan, T.R.; Chowdhury, M.I.; Ahmed, T.; Rafique, T.A.; Khan, A.; Rahman, S.I.A.; Khanam, F.; Lundgren, A.; et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: A double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2020, 20, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Sukwa, N.; Mubanga, C.; Hatyoka, L.M.; Chilyabanyama, O.N.; Chibuye, M.; Mundia, S.; Munyinda, M.; Kamuti, E.; Siyambango, M.; Badiozzaman, S.; et al. Safety, tolerability, and immunogenicity of an oral inactivated ETEC vaccine (ETVAX(R)) with dmLT adjuvant in healthy adults and children in Zambia: An age descending randomised, placebo-controlled trial. Vaccine 2023, 41, 6884–6894. [Google Scholar] [CrossRef]
- Hossain, M.J.; Svennerholm, A.M.; Carlin, N.; D’Alessandro, U.; Wierzba, T.F. A Perspective on the Strategy for Advancing ETVAX((R)), An Anti-ETEC Diarrheal Disease Vaccine, into a Field Efficacy Trial in Gambian Children: Rationale, Challenges, Lessons Learned, and Future Directions. Microorganisms 2023, 12, 90. [Google Scholar] [CrossRef]
- Mubanga, C.; Simuyandi, M.; Mwape, K.; Chibesa, K.; Chisenga, C.; Chilyabanyama, O.N.; Randall, A.; Liang, X.; Glashoff, R.H.; Chilengi, R. Use of an ETEC Proteome Microarray to Evaluate Cross-Reactivity of ETVAX((R)) Vaccine-Induced IgG Antibodies in Zambian Children. Vaccines 2023, 11, 939. [Google Scholar] [CrossRef]
- Kantele, A.; Riekkinen, M.; Jokiranta, T.S.; Pakkanen, S.H.; Pietila, J.P.; Patjas, A.; Eriksson, M.; Khawaja, T.; Klemets, P.; Marttinen, K.; et al. Safety and immunogenicity of ETVAX(R), an oral inactivated vaccine against enterotoxigenic Escherichia coli diarrhoea: A double-blinded, randomized, placebo-controlled trial amongst Finnish travellers to Benin, West Africa. J. Travel Med. 2023, 30, taad045. [Google Scholar] [CrossRef] [PubMed]
- Kantele, A.; Carlin, N.; Jokiranta, S.; Svennerholm, A.M. A phase 2b placebo controlled clinical trial of the oral vaccine ETVAX® to examine safety, immunogenicity, diagnostic methods and protective efficacy against travelers’ diarrhea among Finnish travelers to Benin, West Africa. In Proceedings of the 2022 VASE Virtual Symposium, Washington, DC, USA, 28–30 September 2021. [Google Scholar]
- Scandinavian Biopharma Announces Successful Completion of Pediatric Phase IIb Trial for ETVAX® Vaccine in The Gambia. 2024. Available online: https://scandinavianbiopharma.se/scandinavian-biopharma-announces-successful-completion-of-pediatric-phase-iib-trial-for-etvax-vaccine-in-the-gambia/ (accessed on 2 October 2024).
- Akhtar, M.; Nizam, N.N.; Basher, S.R.; Hossain, L.; Akter, S.; Bhuiyan, T.R.; Qadri, F.; Lundgren, A. dmLT Adjuvant Enhances Cytokine Responses to T Cell Stimuli, WholeCell Vaccine Antigens and Lipopolysaccharide in Both Adults and Infants. Front. Immunol. 2021, 12, 654872. [Google Scholar] [CrossRef]
- Svennerholm, A.M.; Lundgren, A.; Leach, S.; Akhtar, M.; Qadri, F. Mucosal Immune Responses Against an Oral Enterotoxigenic Escherichia coli Vaccine Evaluated in Clinical Trials. J. Infect. Dis. 2021, 224 (Suppl. 2), S821–S828. [Google Scholar] [CrossRef]
- Albert, M.J.; Haridas, S.; Ebenezer, M.; Raghupathy, R.; Khan, I. Immunization with a Double-Mutant (R192G/L211A) of the Heat-Labile Enterotoxin of Escherichia coli Offers Partial Protection against Campylobacter jejuni in an Adult Mouse Intestinal Colonization Model. PLoS ONE 2015, 10, e0142090. [Google Scholar] [CrossRef]
- Albert, M.J.; Mustafa, A.S.; Islam, A.; Haridas, S. Oral immunization with cholera toxin provides protection against Campylobacter jejuni in an adult mouse intestinal colonization model. mBio 2013, 4, e00246-13. [Google Scholar] [CrossRef]
- Sandefur, P.D.; Peterson, J.W. Neutralization of Salmonella toxin-induced elongation of Chinese hamster ovary cells by cholera antitoxin. Infect. Immun. 1977, 15, 988–992. [Google Scholar] [CrossRef] [PubMed]
- Maier, N.; Grahek, S.L.; Halpern, J.; Restrepo, S.; Troncoso, F.; Shimko, J.; Torres, O.; Belkind-Gerson, J.; Sack, D.A.; Svennerholm, A.M.; et al. Efficacy of an Enterotoxigenic Escherichia coli (ETEC) Vaccine on the Incidence and Severity of Traveler’s Diarrhea (TD): Evaluation of Alternative Endpoints and a TD Severity Score. Microorganisms 2023, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, R.W.; Oaks, E.V. Inactivated and subunit vaccines to prevent shigellosis. Expert Rev. Vaccines 2009, 8, 1693–1704. [Google Scholar] [CrossRef]
- McKenzie, R.; Walker, R.I.; Nabors, G.S.; Van De Verg, L.L.; Carpenter, C.; Gomes, G.; Forbes, E.; Tian, J.H.; Yang, H.H.; Pace, J.L.; et al. Safety and immunogenicity of an oral, inactivated, whole-cell vaccine for Shigella sonnei: Preclinical studies and a Phase I trial. Vaccine 2006, 24, 3735–3745. [Google Scholar] [CrossRef]
- Chakraborty, S.; Harro, C.; DeNearing, B.; Bream, J.; Bauers, N.; Dally, L.; Flores, J.; Van de Verg, L.; Sack, D.A.; Walker, R. Evaluation of the Safety, Tolerability, and Immunogenicity of an Oral, Inactivated Whole-Cell Shigella flexneri 2a Vaccine in Healthy Adult Subjects. Clin. Vaccine Immunol. 2016, 23, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, J.; Bourgeois, L.; Carlin, N.; Clements, J.; Gustafsson, B.; Lundgren, A.; Nygren, E.; Tobias, J.; Walker, R.; Svennerholm, A.M. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 2013, 31, 2457–2464. [Google Scholar] [CrossRef]
- Kaminski, R.W.; Wu, M.; Turbyfill, K.R.; Clarkson, K.; Tai, B.; Bourgeois, A.L.; Van De Verg, L.L.; Walker, R.I.; Oaks, E.V. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin. Vaccine Immunol. 2014, 21, 366–382. [Google Scholar] [CrossRef]
- Villavedra, M.; Carol, H.; Hjulstrom, M.; Holmgren, J.; Czerkinsky, C. “PERFEXT”: A direct method for quantitative assessment of cytokine production in vivo at the local level. Res. Immunol. 1997, 148, 257–266. [Google Scholar] [CrossRef]
- Johansson, E.L.; Rask, C.; Fredriksson, M.; Eriksson, K.; Czerkinsky, C.; Holmgren, J. Antibodies and antibody-secreting cells in the female genital tract after vaginal or intranasal immunization with cholera toxin B subunit or conjugates. Infect. Immun. 1998, 66, 514–520. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.H.; Kim, H.; Rho, S.; Shin, Y.K.; Song, M.; Walker, R.; Czerkinsky, C.; Kim, D.W.; Kim, J.O. Cross-Protective Shigella Whole-Cell Vaccine With a Truncated O-Polysaccharide Chain. Front. Microbiol. 2018, 9, 2609. [Google Scholar] [CrossRef]
- Fleming, J.A.; Gurley, N.; Knudson, S.; Kabore, L.; Bawa, J.T.; Dapaah, P.; Kumar, S.; Uranw, S.; Tran, T.; Mai, L.T.P.; et al. Exploring Shigella vaccine priorities and preferences: Results from a mixed-methods study in low- and middle-income settings. Vaccine X 2023, 15, 100368. [Google Scholar] [CrossRef] [PubMed]
- Nothaft, H.; Bian, X.; Shajahan, A.; Miller, W.G.; Bolick, D.T.; Guerrant, R.L.; Azadi, P.; Ng, K.K.S.; Szymanski, C.M. Detecting Glucose Fluctuations in the Campylobacter jejuni N-Glycan Structure. ACS Chem. Biol. 2021, 16, 2690–2701. [Google Scholar] [CrossRef]
- Nothaft, H.; Perez-Munoz, M.E.; Yang, T.; Murugan, A.V.M.; Miller, M.; Kolarich, D.; Plastow, G.S.; Walter, J.; Szymanski, C.M. Improving Chicken Responses to Glycoconjugate Vaccination Against Campylobacter jejuni. Front. Microbiol. 2021, 12, 734526. [Google Scholar] [CrossRef] [PubMed]
- Qadri, F.; Wierzba, T.F.; Ali, M.; Chowdhury, F.; Khan, A.I.; Saha, A.; Khan, I.A.; Asaduzzaman, M.; Akter, A.; Khan, A.; et al. Efficacy of a Single-Dose, Inactivated Oral Cholera Vaccine in Bangladesh. N. Engl. J. Med. 2016, 374, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Joshi, N.; Kumar Mandyal, A.; Nordqvist, S.L.; Lebens, M.; Kanchan, V.; Löfstrand, M.; Jeverstam, F.; Mainul Ahasan, M.; Khan, I.; et al. Development of Hillchol®, a low-cost inactivated single strain Hikojima oral cholera vaccine. Vaccine 2020, 38, 7998–8009. [Google Scholar] [CrossRef]
- Tobias, J.; Svennerholm, A.M. Strategies to overexpress enterotoxigenic Escherichia coli (ETEC) colonization factors for the construction of oral whole-cell inactivated ETEC vaccine candidates. Appl. Microbiol. Biotechnol. 2012, 93, 2291–2300. [Google Scholar] [CrossRef]
- Tobias, J.; Svennerholm, A.M.; Holmgren, J.; Lebens, M. Construction and expression of immunogenic hybrid enterotoxigenic Escherichia coli CFA/I and CS2 colonization fimbriae for use in vaccines. Appl. Microbiol. Biotechnol. 2010, 87, 1355–1365. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrinoni, M.; Holmgren, J.; Turbyfill, K.R.; Van De Verg, L.; Maier, N.; Walker, R. Potential for a Combined Oral Inactivated Whole-Cell Vaccine Against ETEC and Shigella: Preclinical Studies Supporting Feasibility. Vaccines 2025, 13, 513. https://doi.org/10.3390/vaccines13050513
Terrinoni M, Holmgren J, Turbyfill KR, Van De Verg L, Maier N, Walker R. Potential for a Combined Oral Inactivated Whole-Cell Vaccine Against ETEC and Shigella: Preclinical Studies Supporting Feasibility. Vaccines. 2025; 13(5):513. https://doi.org/10.3390/vaccines13050513
Chicago/Turabian StyleTerrinoni, Manuela, Jan Holmgren, Kevin Ross Turbyfill, Lillian Van De Verg, Nicole Maier, and Richard Walker. 2025. "Potential for a Combined Oral Inactivated Whole-Cell Vaccine Against ETEC and Shigella: Preclinical Studies Supporting Feasibility" Vaccines 13, no. 5: 513. https://doi.org/10.3390/vaccines13050513
APA StyleTerrinoni, M., Holmgren, J., Turbyfill, K. R., Van De Verg, L., Maier, N., & Walker, R. (2025). Potential for a Combined Oral Inactivated Whole-Cell Vaccine Against ETEC and Shigella: Preclinical Studies Supporting Feasibility. Vaccines, 13(5), 513. https://doi.org/10.3390/vaccines13050513





