Shigella Mutant with Truncated O-Antigen as an Enteric Multi-Pathogen Vaccine Platform
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Preparation of Bacterial Strains for Challenge Studies
2.3. Preparation of the STM in Flask and Fermentor Culture
2.3.1. Flask Growth
2.3.2. Fermentor Growth
2.4. Expression of the C. jejuni N-Glycan on the Surface of the STM
2.5. Characterization of STM and STM-Cj by Western Blot
2.6. Flow Cytometry Analysis of Surface Exposure of PSSP-1 on Whole Cells
2.7. Intranasal Immunizations and Challenges with Shigella
2.8. Enzyme-Linked Immunosorbent Assay
2.9. Statistical Analysis
3. Results
3.1. Growth of STM in Fermentor Compared to Flask
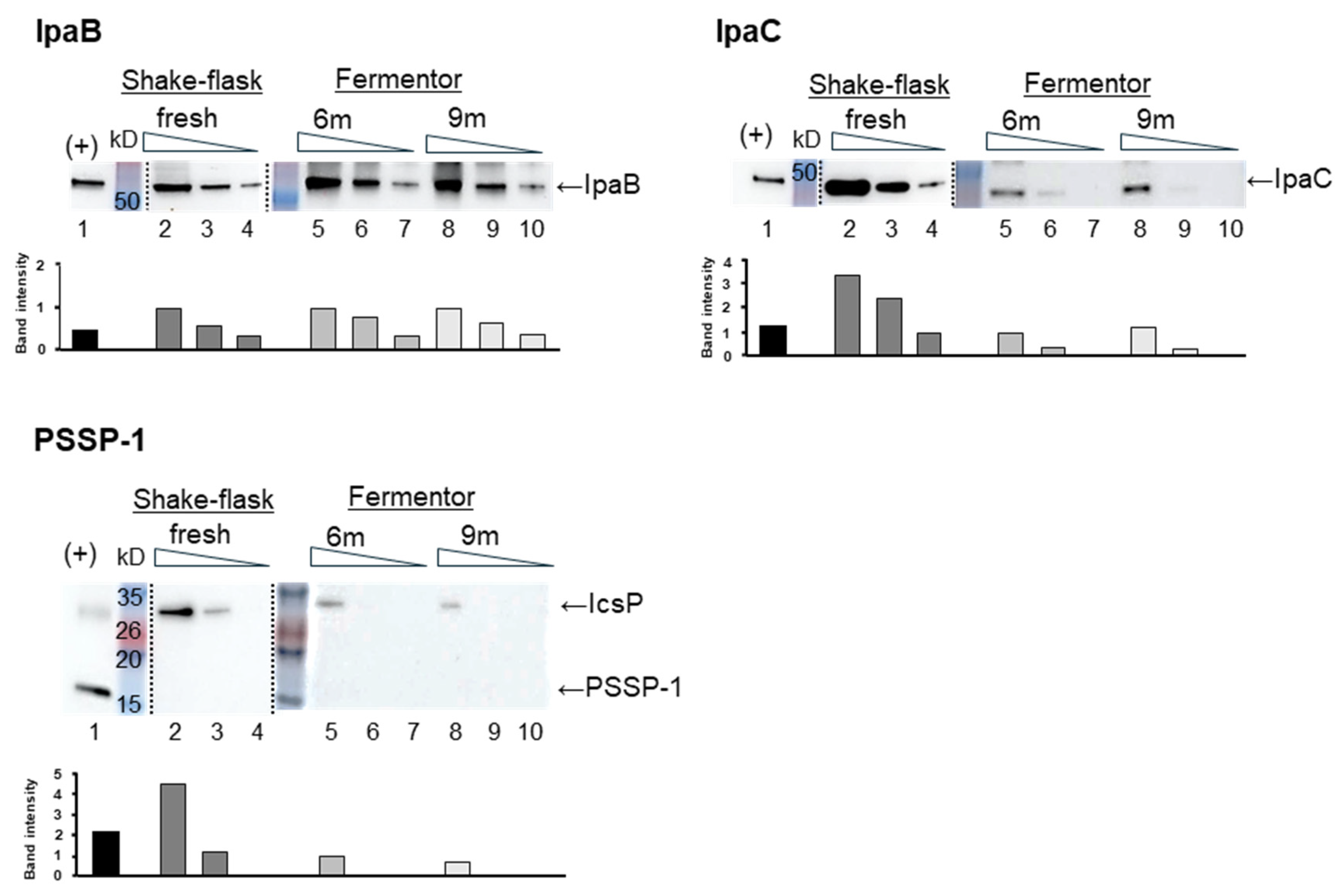
3.2. Expression of Key Shigella Antigens in STM Grown by Fermentation with Animal-Free Medium
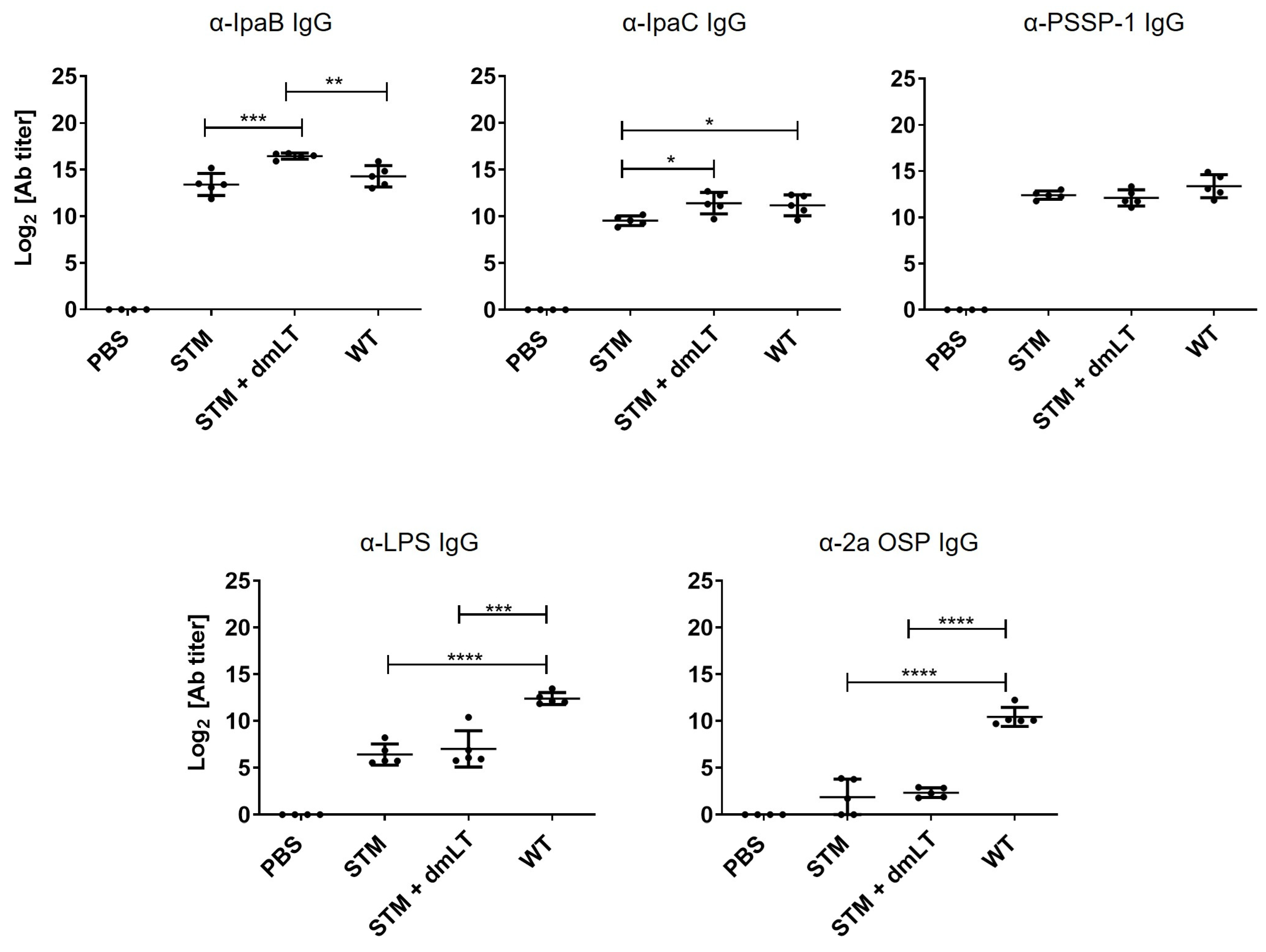
3.3. Immune Responses Induced by Fermentor-Cultured STM
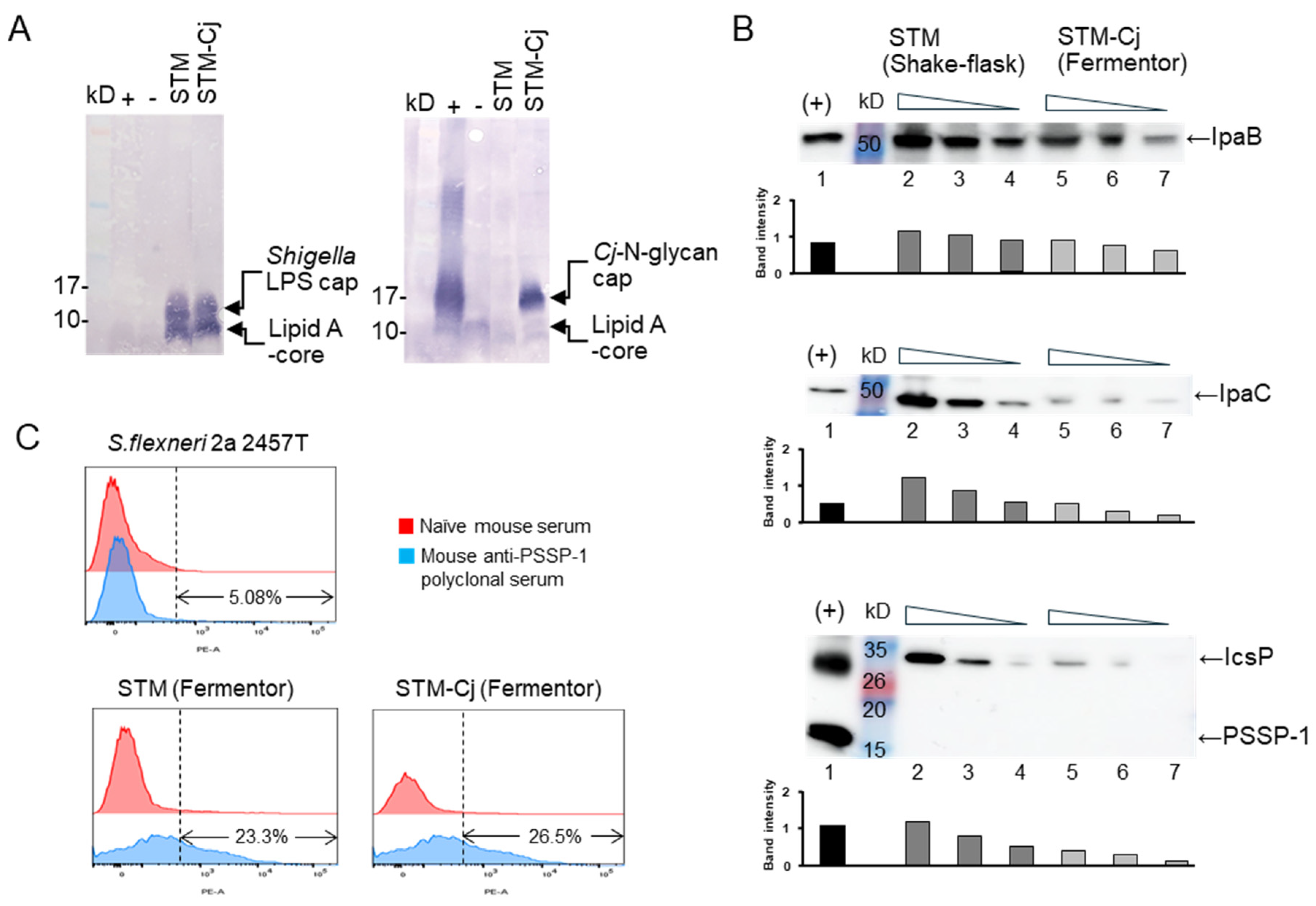
3.4. Characterization of STM-Cj
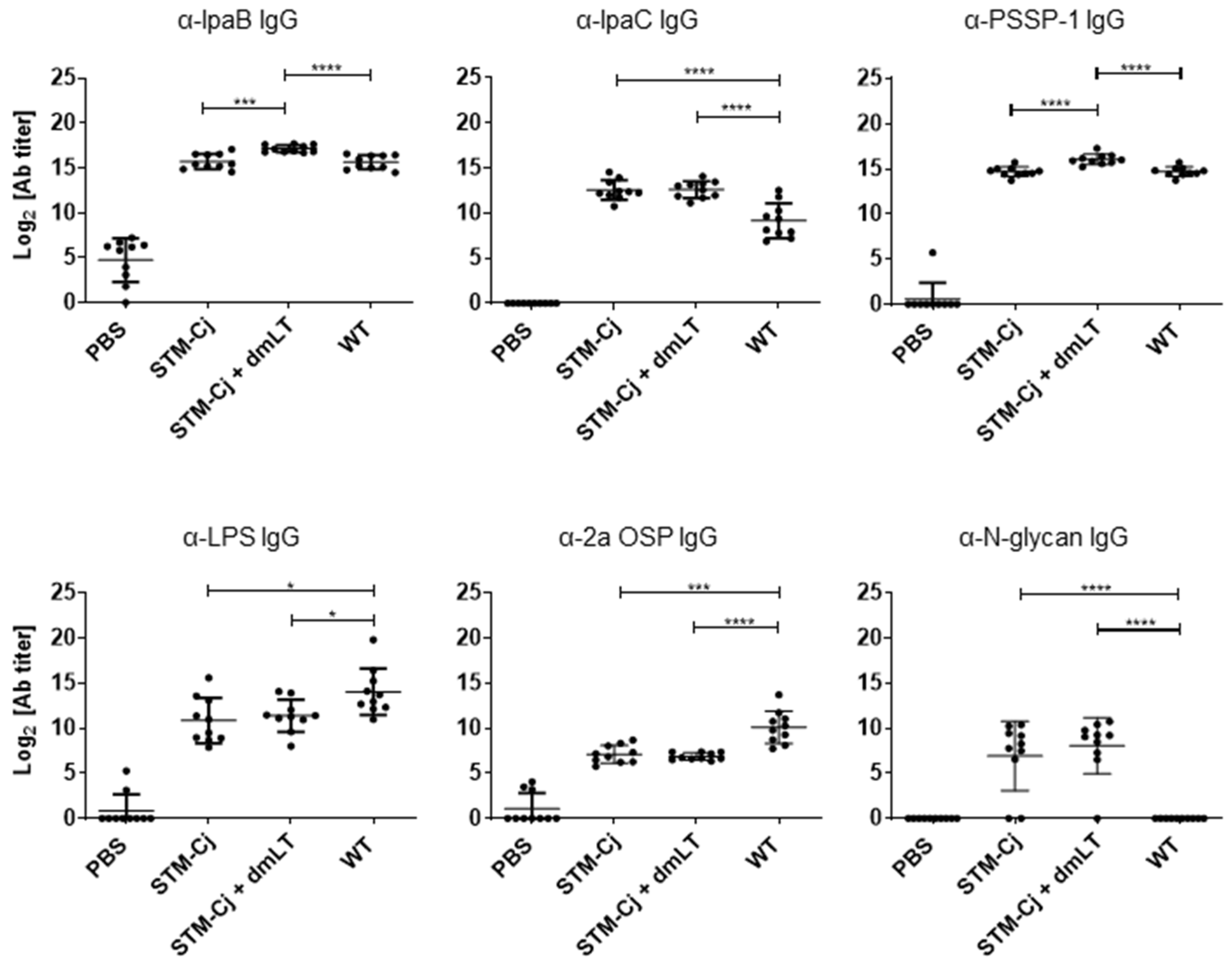
3.5. Evaluation of STM-Cj Immunogenicity in Mice Against Shigella and Campylobacter Antigens
3.6. Protection Against Shigella Following STM-Cj Immunization in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacLennan, C.A.; Steele, A.D. Frontiers in Shigella Vaccine Development. Vaccines 2022, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef]
- Fleming, J.A.; Gurley, N.; Knudson, S.; Kabore, L.; Bawa, J.T.; Dapaah, P.; Kumar, S.; Uranw, S.; Tran, T.; Mai, L.T.P.; et al. Exploring Shigella vaccine priorities and preferences: Results from a mixed-methods study in low- and middle-income settings. Vaccine X 2023, 15, 100368. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, W.P.; Anderson, J.D.t.; Bagamian, K.H.; Bourgeois, A.L.; Mills, M.; Sawe, F.; Scheele, S.; Talaat, K.; Giersing, B.K. Vaccine value profile for Shigella. Vaccine 2023, 41 (Suppl. 2), S76–S94. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Preferred Product Characteristics for Vaccines Against Shigella; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Levine, M.M.; Kotloff, K.L.; Barry, E.M.; Pasetti, M.F.; Sztein, M.B. Clinical trials of Shigella vaccines: Two steps forward and one step back on a long, hard road. Nat. Rev. Microbiol. 2007, 5, 540–553. [Google Scholar] [CrossRef]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef]
- Barry, E.; Cassels, F.; Riddle, M.; Walker, R.; Wierzba, T. Vaccines Against Shigella and Enterotoxigenic Escherichia coli: A summary of the 2018 VASE Conference. Vaccine 2019, 37, 4768–4774. [Google Scholar] [CrossRef]
- McKenzie, R.; Walker, R.I.; Nabors, G.S.; Van De Verg, L.L.; Carpenter, C.; Gomes, G.; Forbes, E.; Tian, J.H.; Yang, H.H.; Pace, J.L.; et al. Safety and immunogenicity of an oral, inactivated, whole-cell vaccine for Shigella sonnei: Preclinical studies and a Phase I trial. Vaccine 2006, 24, 3735–3745. [Google Scholar] [CrossRef]
- Chakraborty, S.; Harro, C.; DeNearing, B.; Bream, J.; Bauers, N.; Dally, L.; Flores, J.; Van de Verg, L.; Sack, D.A.; Walker, R. Evaluation of the Safety, Tolerability, and Immunogenicity of an Oral, Inactivated Whole-Cell Shigella flexneri 2a Vaccine in Healthy Adult Subjects. Clin. Vaccine Immunol. 2016, 23, 315–325. [Google Scholar] [CrossRef]
- Kaminski, R.W.; Wu, M.; Turbyfill, K.R.; Clarkson, K.; Tai, B.; Bourgeois, A.L.; Van De Verg, L.L.; Walker, R.I.; Oaks, E.V. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin. Vaccine Immunol. 2014, 21, 366–382. [Google Scholar] [CrossRef]
- Osorio, M.; Bray, M.D.; Walker, R.I. Vaccine potential for inactivated shigellae. Vaccine 2007, 25, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Kantele, A.; Riekkinen, M.; Jokiranta, T.S.; Pakkanen, S.H.; Pietilä, J.P.; Patjas, A.; Eriksson, M.; Khawaja, T.; Klemets, P.; Marttinen, K.; et al. Safety and immunogenicity of ETVAX®, an oral inactivated vaccine against enterotoxigenic Escherichia coli diarrhoea: A double-blinded, randomized, placebo-controlled trial amongst Finnish travellers to Benin, West Africa. J. Travel. Med. 2023, 30. [Google Scholar] [CrossRef] [PubMed]
- Malembaka, E.B.; Bugeme, P.M.; Hutchins, C.; Xu, H.; Hulse, J.D.; Demby, M.N.; Gallandat, K.; Saidi, J.M.; Rumedeka, B.B.; Itongwa, M.; et al. Effectiveness of one dose of killed oral cholera vaccine in an endemic community in the Democratic Republic of the Congo: A matched case-control study. Lancet Infect. Dis. 2024, 24, 514–522. [Google Scholar] [CrossRef]
- Norton, E.B.; Lawson, L.B.; Freytag, L.C.; Clements, J.D. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 2011, 18, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Svennerholm, A.M.; Lundgren, A.; Leach, S.; Akhtar, M.; Qadri, F. Mucosal Immune Responses Against an Oral Enterotoxigenic Escherichia coli Vaccine Evaluated in Clinical Trials. J. Infect. Dis. 2021, 224, S821–S828. [Google Scholar] [CrossRef]
- Sukwa, N.; Mubanga, C.; Hatyoka, L.M.; Chilyabanyama, O.N.; Chibuye, M.; Mundia, S.; Munyinda, M.; Kamuti, E.; Siyambango, M.; Badiozzaman, S.; et al. Safety, tolerability, and immunogenicity of an oral inactivated ETEC vaccine (ETVAX(R)) with dmLT adjuvant in healthy adults and children in Zambia: An age descending randomised, placebo-controlled trial. Vaccine 2023, 41, 6884–6894. [Google Scholar] [CrossRef]
- Muthuramalingam, M.; Whittier, S.K.; Picking, W.L.; Picking, W.D. The Shigella Type III Secretion System: An Overview from Top to Bottom. Microorganisms 2021, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Becerra, F.J.; Kissmann, J.M.; Diaz-McNair, J.; Choudhari, S.P.; Quick, A.M.; Mellado-Sanchez, G.; Clements, J.D.; Pasetti, M.F.; Picking, W.L. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect. Immun. 2012, 80, 1222–1231. [Google Scholar] [CrossRef]
- Turbyfill, K.R.; Clarkson, K.A.; Oaks, E.V.; Kaminski, R.W. From Concept to Clinical Product: A Brief History of the Novel Shigella Invaplex Vaccine’s Refinement and Evolution. Vaccines 2022, 10, 548. [Google Scholar] [CrossRef]
- Martinez-Becerra, F.J.; Chen, X.; Dickenson, N.E.; Choudhari, S.P.; Harrison, K.; Clements, J.D.; Picking, W.D.; Van De Verg, L.L.; Walker, R.I.; Picking, W.L. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect. Immun. 2013, 81, 4470–4477. [Google Scholar] [CrossRef]
- Kim, J.O.; Rho, S.; Kim, S.H.; Kim, H.; Song, H.J.; Kim, E.J.; Kim, R.Y.; Kim, E.H.; Sinha, A.; Dey, A.; et al. Shigella outer membrane protein PSSP-1 is broadly protective against Shigella infection. Clin. Vaccine Immunol. 2015, 22, 381–388. [Google Scholar] [CrossRef]
- Ndungo, E.; Randall, A.; Hazen, T.H.; Kania, D.A.; Trappl-Kimmons, K.; Liang, X.; Barry, E.M.; Kotloff, K.L.; Chakraborty, S.; Mani, S.; et al. A Novel Shigella Proteome Microarray Discriminates Targets of Human Antibody Reactivity following Oral Vaccination and Experimental Challenge. mSphere 2018, 3, e00260-18. [Google Scholar] [CrossRef]
- Heine, S.J.; Franco-Mahecha, O.L.; Chen, X.; Choudhari, S.; Blackwelder, W.C.; van Roosmalen, M.L.; Leenhouts, K.; Picking, W.L.; Pasetti, M.F. Shigella IpaB and IpaD displayed on L. lactis bacterium-like particles induce protective immunity in adult and infant mice. Immunol. Cell Biol. 2015, 93, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Moon, Y.H.; Kim, H.; Rho, S.; Shin, Y.K.; Song, M.; Walker, R.; Czerkinsky, C.; Kim, D.W.; Kim, J.O. Cross-Protective Shigella Whole-Cell Vaccine with a Truncated O-Polysaccharide Chain. Front. Microbiol. 2018, 9, 2609. [Google Scholar] [CrossRef] [PubMed]
- Ascari, A.; Morona, R. Recent insights into Wzy polymerases and lipopolysaccharide O-antigen biosynthesis. J. Bacteriol. 2025, 207, e0041724. [Google Scholar] [CrossRef]
- Nothaft, H.; Perez-Muñoz, M.E.; Yang, T.; Murugan, A.V.M.; Miller, M.; Kolarich, D.; Plastow, G.S.; Walter, J.; Szymanski, C.M. Improving Chicken Responses to Glycoconjugate Vaccination Against Campylobacter jejuni. Front. Microbiol. 2021, 12, 734526. [Google Scholar] [CrossRef]
- Cain, J.A.; Dale, A.L.; Sumer-Bayraktar, Z.; Solis, N.; Cordwell, S.J. Identifying the targets and functions of N-linked protein glycosylation in Campylobacter jejuni. Mol. Omics 2020, 16, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Badjo, A.O.R.; Kabore, N.F.; Zongo, A.; Gnada, K.; Ouattara, A.; Muhigwa, M.; Ouangraoua, S.; Poda, A.; Some, S.A.; Schubert, G.; et al. Burden and epidemiology of Campylobacter species in acute enteritis cases in Burkina Faso. BMC Infect. Dis. 2024, 24, 808. [Google Scholar] [CrossRef]
- Zong Minko, O.; Mabika Mabika, R.; Moyen, R.; Mounioko, F.; Ondjiangui, L.F.; Yala, J.F. The Impact of Campylobacter, Salmonella, and Shigella in Diarrheal Infections in Central Africa (1998–2022): A Systematic Review. Int. J. Environ. Res. Public Health 2024, 21, 1635. [Google Scholar] [CrossRef]
- Paintsil, E.K.; Masanta, W.O.; Dreyer, A.; Ushanov, L.; Smith, S.I.; Frickmann, H.; Zautner, A.E. Campylobacter in Africa—A specific viewpoint. Eur. J. Microbiol. Immunol. 2023, 13, 107–124. [Google Scholar] [CrossRef]
- Amour, C.; Gratz, J.; Mduma, E.; Svensen, E.; Rogawski, E.T.; McGrath, M.; Seidman, J.C.; McCormick, B.J.; Shrestha, S.; Samie, A.; et al. Epidemiology and Impact of Campylobacter Infection in Children in 8 Low-Resource Settings: Results from the MAL-ED Study. Clin. Infect. Dis. 2016, 63, 1171–1179. [Google Scholar] [CrossRef]
- Wei, J.; Goldberg, M.B.; Burland, V.; Venkatesan, M.M.; Deng, W.; Fournier, G.; Mayhew, G.F.; Plunkett, G., 3rd; Rose, D.J.; Darling, A.; et al. Complete genome sequence and comparative genomics of Shigella flexneri serotype 2a strain 2457T. Infect. Immun. 2003, 71, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Romanowska, E.; Katzenellenbogen, E.; Lugowski, C.; Gamian, A.; Bogulska, M. Immunochemical characteristics of Shigella sonnei and serotype 6 Shigella flexneri lipopolysaccharides and enterobacterial common antigen. Arch. Immunol. Et Ther. Exp. 1978, 26, 249–254. [Google Scholar]
- Holt, K.E.; Baker, S.; Weill, F.-X.; Holmes, E.C.; Kitchen, A.; Yu, J.; Sangal, V.; Brown, D.J.; Coia, J.E.; Kim, D.W. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat. Genet. 2012, 44, 1056. [Google Scholar] [CrossRef] [PubMed]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef]
- Ranallo, R.T.; Barnoy, S.; Thakkar, S.; Urick, T.; Venkatesan, M.M. Developing live Shigella vaccines using λ Red recombineering. FEMS Immunol. Med. Microbiol. 2006, 47, 462–469. [Google Scholar] [CrossRef]
- Nothaft, H.; Bian, X.; Shajahan, A.; Miller, W.G.; Bolick, D.T.; Guerrant, R.L.; Azadi, P.; Ng, K.K.S.; Szymanski, C.M. Detecting Glucose Fluctuations in the Campylobacter jejuni N-Glycan Structure. ACS Chem. Biol. 2021, 16, 2690–2701. [Google Scholar] [CrossRef]
- Venkatesan, M.M.; Buysse, J.M.; Kopecko, D.J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc. Natl. Acad. Sci. USA 1988, 85, 9317–9321. [Google Scholar] [CrossRef]
- Fukuda, I.; Suzuki, T.; Munakata, H.; Hayashi, N.; Katayama, E.; Yoshikawa, M.; Sasakawa, C. Cleavage of Shigella surface protein VirG occurs at a specific site, but the secretion is not essential for intracellular spreading. J. Bacteriol. 1995, 177, 1719–1726. [Google Scholar] [CrossRef][Green Version]
- Nothaft, H.; Scott, N.E.; Vinogradov, E.; Liu, X.; Hu, R.; Beadle, B.; Fodor, C.; Miller, W.G.; Li, J.; Cordwell, S.J.; et al. Diversity in the protein N-glycosylation pathways within the Campylobacter genus. Mol. Cell Proteom. 2012, 11, 1203–1219. [Google Scholar] [CrossRef]
- Tran, E.N.; Doyle, M.T.; Morona, R. LPS unmasking of Shigella flexneri reveals preferential localisation of tagged outer membrane protease IcsP to septa and new poles. PLoS ONE 2013, 8, e70508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, X.K.; Zhao, G.; Zhi, Y.D.; Bu, X.; Ying, T.Y.; Feng, E.L.; Wang, J.; Zhang, X.M.; Huang, P.T.; et al. Dynamic proteome changes of Shigella flexneri 2a during transition from exponential growth to stationary phase. Genom. Proteom. Bioinform. 2007, 5, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Caboni, M.; Pédron, T.; Rossi, O.; Goulding, D.; Pickard, D.; Citiulo, F.; MacLennan, C.A.; Dougan, G.; Thomson, N.R.; Saul, A.; et al. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog. 2015, 11, e1004749. [Google Scholar] [CrossRef]
- Zamora, C.Y.; Ward, E.M.; Kester, J.C.; Chen, W.L.K.; Velazquez, J.G.; Griffith, L.G.; Imperiali, B. Application of a gut-immune co-culture system for the study of N-glycan-dependent host-pathogen interactions of Campylobacter jejuni. Glycobiology 2020, 30, 374–381. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-O.; Nothaft, H.; Moon, Y.; Jeong, S.; Vortherms, A.R.; Song, M.; Szymanski, C.M.; White, J.; Walker, R. Shigella Mutant with Truncated O-Antigen as an Enteric Multi-Pathogen Vaccine Platform. Vaccines 2025, 13, 506. https://doi.org/10.3390/vaccines13050506
Kim J-O, Nothaft H, Moon Y, Jeong S, Vortherms AR, Song M, Szymanski CM, White J, Walker R. Shigella Mutant with Truncated O-Antigen as an Enteric Multi-Pathogen Vaccine Platform. Vaccines. 2025; 13(5):506. https://doi.org/10.3390/vaccines13050506
Chicago/Turabian StyleKim, Jae-Ouk, Harald Nothaft, Younghye Moon, Seonghun Jeong, Anthony R. Vortherms, Manki Song, Christine M. Szymanski, Jessica White, and Richard Walker. 2025. "Shigella Mutant with Truncated O-Antigen as an Enteric Multi-Pathogen Vaccine Platform" Vaccines 13, no. 5: 506. https://doi.org/10.3390/vaccines13050506
APA StyleKim, J.-O., Nothaft, H., Moon, Y., Jeong, S., Vortherms, A. R., Song, M., Szymanski, C. M., White, J., & Walker, R. (2025). Shigella Mutant with Truncated O-Antigen as an Enteric Multi-Pathogen Vaccine Platform. Vaccines, 13(5), 506. https://doi.org/10.3390/vaccines13050506





