Abstract
Monkeypox (mpox) is a zoonotic disease (zoonose) caused by the monkeypox virus (MPXV). MPXV, a member of the Orthopoxviridae family, is categorized into two clades, Central Africa (I) and West Africa (II), each of which is further subdivided into subclades a and b. Clade I generally causes more serious illness and higher mortality rates, while Clade II results in milder illness. Historically, mpox epidemics were localized to specific regions and countries in Africa. Since 2022, the mpox epidemic, fueled by MPXV Clade IIb, has swiftly spread across various nations and regions, jeopardizing public health and safety. However, starting in 2024, Clade Ib gradually replaced Clade IIb. The notable genetic variation in Clade Ib may provide MPXV with new opportunities to evade the immune system and adapt to hosts. According to the World Health Organization (WHO), from 1 January 2022, to 24 November 2024, there were 117,663 confirmed cases and 2 probable cases, resulting in 263 deaths across 127 Member States in all six WHO regions. As of 9 January 2025, 12 countries outside Africa have reported imported MPXV Clade Ib cases, with secondary cases emerging in the United Kingdom, Germany, and China. Due to the incomplete development of a vaccine specifically for MPXV, the smallpox vaccine remains in use for preventing mpox or for emergency vaccination post-exposure. Therefore, the persistent spread of mpox is still a major concern, requiring greater awareness and vaccination efforts in populations at high risk. This paper aims to summarize the etiological characteristics, epidemic situation, and vaccine prevention efforts for mpox, offering a reference for managing this serious epidemic and ensuring effective scientific prevention and control.
1. Introduction
As a new and re-emerging infectious disease, mpox has broken out and spread worldwide, posing a threat to public safety and health. A global outbreak and epidemic of MPXV Clade II occurred in 2022–2023, the first sustained transmission outside Africa since the first human cases of mpox were identified in 1970 [1]. In 2023, Clade Ib cases were diagnosed in the Democratic Republic of the Congo (DRC) and subsequently spread to neighboring countries, leading to an increasingly serious epidemic [2]. On 14 August 2024, the WHO declared mpox a Public Health Emergency of International Concern (PHEIC) for the second time in two years [3]. Under the emergency prevention and control measures of various countries and regions around the world, along with the importation of relevant vaccines, the number of mpox cases worldwide is generally declining. However, some countries and regions are still experiencing outbreaks or epidemics [4] (Figure 1). Because MPXV-specific vaccines are still not fully developed, smallpox vaccines can be used to prevent mpox or for emergency use. These mainly include first-generation vaccines (Dryvax®), second-generation vaccines (ACAM1000®, ACAM2000®), third-generation vaccines (LC16m8®), and fourth-generation vaccines (OrthopoxVac®) [5]. Notable approved vaccines include JYNNEOS® (MVA-BN), LC16m8®, ACAM2000®, and OrthopoxVac® [6,7]. OrthopoxVac®, a vaccine of the fourth generation, was primarily created and authorized in Russia, utilizing a weakened version of Orthopoxvirus to prevent smallpox. However, its use has not been widely discussed due to the lack of clinical research data on mpox [7]. Widely used during the global mpox outbreak, the first three vaccines provide protection by stimulating a cross-immune response that results in the creation of neutralizing antibodies [8]. Additional mRNA and recombinant protein vaccines are under development. It can be observed from the two epidemics that some countries and regions in Africa have not established a public health surveillance and information reporting system, resulting in a lack of case detection, diagnosis, surveillance, and reporting [9]. Moreover, due to objective factors, such as a lack of equipment, weak technology, insufficient medical staff, and a shortage of vaccines, misdiagnoses, missed diagnoses, inaccurate epidemic assessments, and the expansion of the epidemic are common [10,11]. Therefore, the long-term transmission of mpox cannot be ignored. Studies on the etiology, epidemic status, and vaccine-based prevention of mpox are needed to provide references for severe epidemic forms and effective scientific prevention and control.
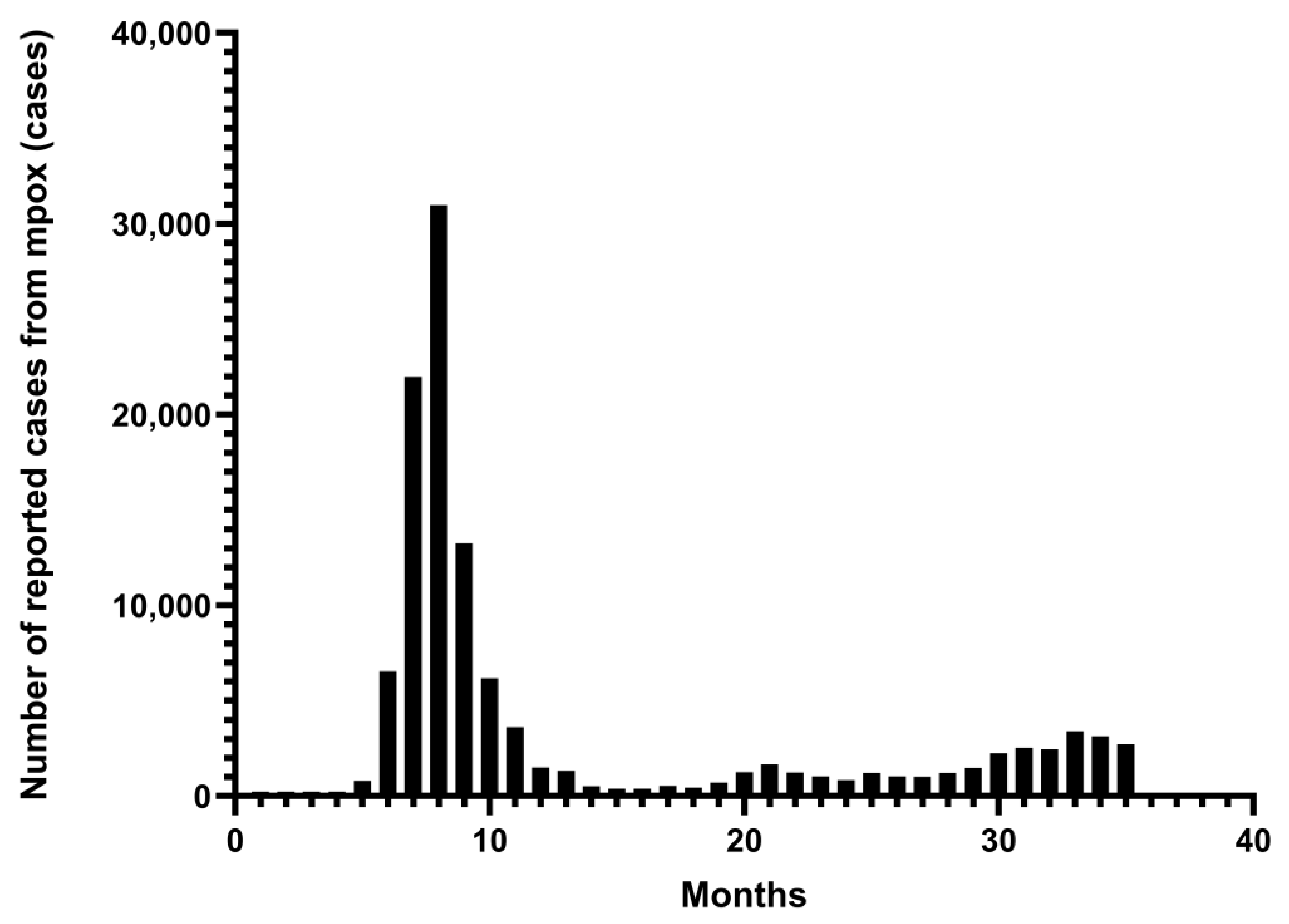
Figure 1.
From January 2022 to November 2024. Monthly number of mpox cases reported in 128 Member States across all six WHO regions. The data are from the WHO Global Aggregate of Mpox Cases. This figure was drawn by Graphpad Pism 9.5.
2. The Etiological Characteristics of Mpox
MPXV is an enveloped, double-stranded DNA virus of the genus Orthopoxvirus in the family Poxviridae [12]. Subtype MPXV is divided into two clades: Central Africa (I) and West Africa (II). Each of these clades can be divided into subclades a and b [9,13] (Table 1). Clade I, found in Central African countries, has higher mortality (up to 11%) [14], while Clade II, prevalent in West African countries, is less severe, with a 6% mortality rate [15]. The global mpox epidemic in 2022 was primarily caused by clade IIb [8], transmitted predominantly through sexual contact, and was more common among men who have sex with men [16,17]. Human-to-human transmission can also occur through close contact with lesions, respiratory droplets, body fluids, and contaminated materials [18]. Human infection with Clade IIb has an incubation period of 7 to 14 days and symptom duration is 14 to 21 days [19]. In 2024, Clade Ib was the reason for the global mpox epidemic [3], which mainly occurred through close family contact, animal contact, and unprotected medical care contact [20,21]. Family or community were the main transmission locations. Human infection with Clade Ib has an incubation period of 5 to 13 days, and the symptoms may last from 4 to 21 days [22]. The main hosts of MPXV in Central, East, and West Africa are rodents (squirrels) and primates (monkeys) [14]. Current exploration of the mode of transmission is mostly limited to close animal-to-human or human-to-human contact [23], and human-to-animal transmission may also occur [24]. At present, polymerase chain reaction (PCR) is the primary method for laboratory diagnosis. MPXV can be detected by PCR in saliva, oropharynx, upper respiratory tract swabs, blood, semen, vaginal fluid, urine, and anal swabs from infected individuals [25,26]. The stability of MPXV enables it to survive for a certain amount of time in vitro, using contaminated surfaces or environments as a medium. It has a half-life of up to 38.75 days in dried blood, 4.57 days in dried semen, and 5.74 days in wastewater [27]. By comparing the different genomes, significant differences between clades can be observed. Some genes may be completely absent or truncated in a given clade, resulting in changes in gene content. The VACV-Cop E5R ortholog was completely absent from Clade I, along with three other genes, including the VACV-Cop A47L and B11R orthologs, as well as K1R, which were truncated. Unlike in Clade II, all four genes (D14L, D15L, D16L, and D17L) were completely deleted, and three genes (D4L, B14L, and B15L) were truncated [28]. This may be associated with the biological properties of different clades through the deletion or truncation of the genes. However, the genetic diversity of Clade Ib is 54% higher than that of Clade I, with the number of nucleotide changes increasing from approximately 96 to around 149. Among these, many mutations are related to genes involved in host immune regulation and viral replication. For example, the B21R (OPG210) gene exhibited three consensus amino acid substitutions (D209N, P722S, and M1741I), which might exist to impair the immune escape ability and host fitness of MPXV. This remarkable genetic variation is driven mostly by APOBEC3-mediated cytosine deamination [29], and it results in a dramatic increase in CT mutations within the MPXV genome [30]. This mutation pattern not only reflects the critical role of APOBEC3 in MPXV evolution but also the rapid and sustained spread of Clade Ib in the population [31]. In addition, the genomes of the MPXV Clade Ib differ significantly from those of other Clade I virus strains that were previously sequenced in the DRC. There was a deletion of approximately 1 kbp in the genome of Clade Ib (relative to the Clade I reference genome NC_003310) that may have interfered with the detection power of Clade I-specific diagnostic PCR-based assays [32]. Therefore, continuous monitoring and in-depth research on the etiology, transmission, pathogenesis, and evolution of, as well as variations in, mpox are urgently needed for the better prevention and diagnosis of MPXV.

Table 1.
Comparison of similarities and differences between four mpox clades.
3. The Prevalence and Characteristics of Mpox
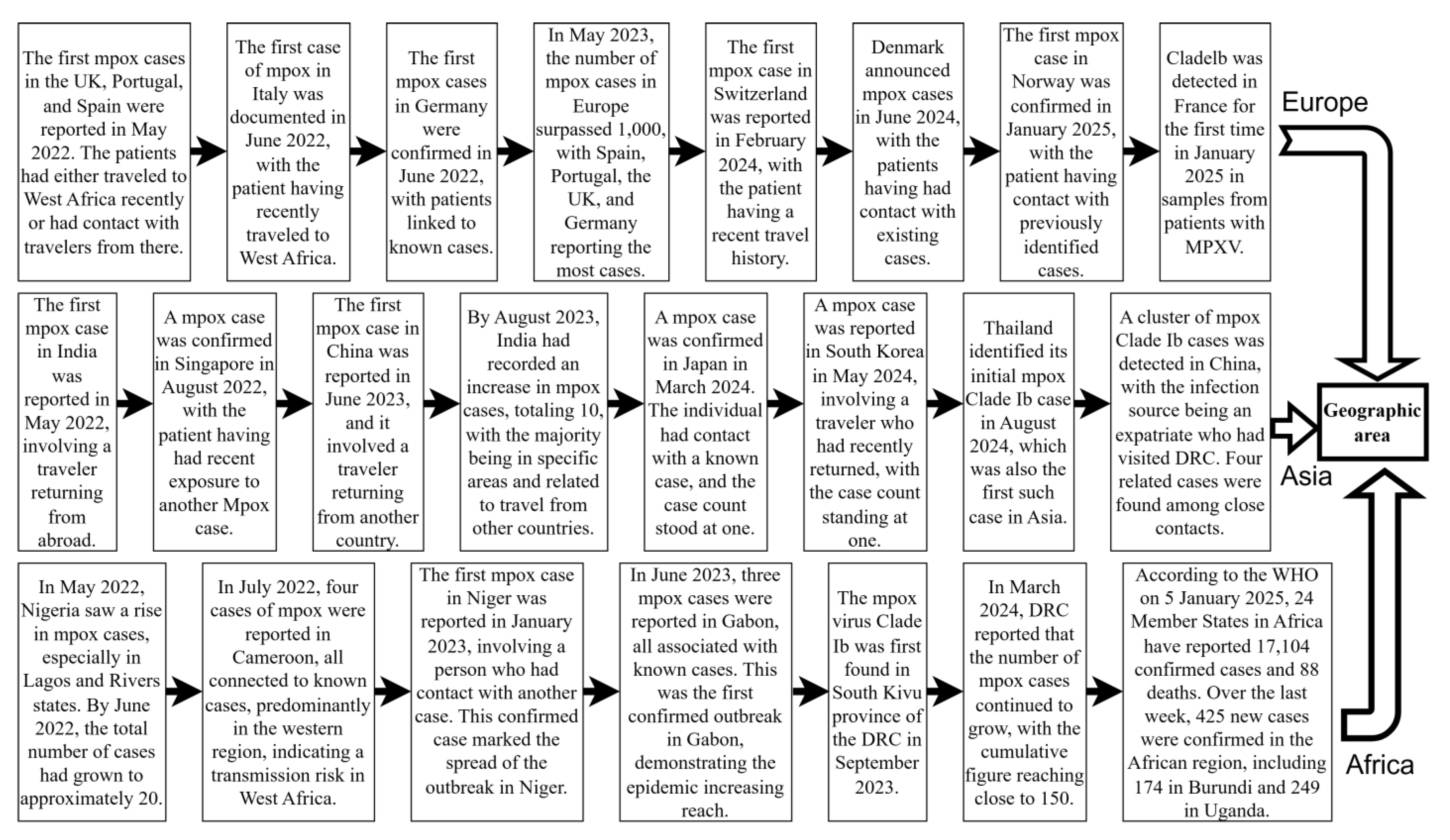
Monkeypox is a zoonose that was previously restricted to outbreaks or epidemics in selected countries in Central and West Africa [9]. In May 2022, MPXV Clade IIb outbreaks emerged in non-endemic regions such as Europe, America, and Asia. By 23 July, the WHO designated the mpox outbreak as a PHEIC. The outbreak spread across six WHO regions, resulting in 92,783 confirmed cases and 171 deaths [33]. Sexual activity played a key role in transmission during this outbreak, particularly among men who have sex with men [34,35,36]. Vertical transmission from mother to child can occur, which can also easily lead to fetal death and abortion [37]. Indirect transmission occurs through contaminated medical devices in hospitals [38]. With the gradual and effective control of the Clade IIb outbreak, despite the WHO’s declaration on 11 May 2023 that mpox outbreaks no longer constituted a PHEIC [39,40], Clade Ib began to gradually replace the effects of Clade IIb. Since the 1970s, the DRC has reported only cases of Clade I mpox, transmitted through human-to-human contact, mostly in small households or via community outbreaks [21]. In April 2023, the DRC reported the first Clade I mpox cases, transmitted through contact in Kango Province, with multiple cases among sex workers. Subsequently, the Kinshasa and South Kivu provinces reported their first Clade I outbreaks due to sexual contact in August and September 2023 [4]. In July 2024, MPXV Clade Ib began spreading from the DRC to neighboring countries, causing sustained community transmission. This led to the WHO once again declaring the mpox outbreak a PHEIC on 14 August 2024 [3]. As of 9 January 2025, 12 non-African countries had reported imported cases of MPXV Clade Ib infection, with Germany and China experiencing multiple secondary cases [41] (Figure 2). Unlike Clade IIb, which is mainly transmitted through sexual contact, the newly emerging Clade Ib is primarily transmitted through close household contact [42]. As a result, among all age groups, infants and children are more likely to be affected by Clade Ib, influenced by factors such as vaccine inequity, low immunity, and malnutrition [13]. At least 500 related deaths have been reported so far [43]. Among the total cases in all 26 provinces of the DRC, children under 15 years of age accounted for 66% of the reported cases and over 82% of deaths [44]. Studies in the DRC have shown that MPXV Clade Ib has a higher transmission probability through female sex workers, higher incidence in females, and a greater risk of mother-to-child vertical transmission. This indicates that Clade Ib has higher transmissibility and may be more virulent [32] (Figure 3).
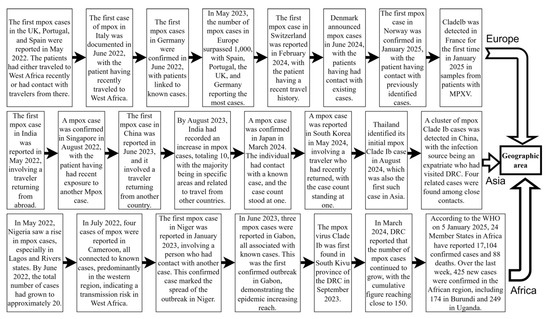
Figure 2.
Global mpox outbreaks and related important events since 2022. This figure was drawn using Figdraw2.0.
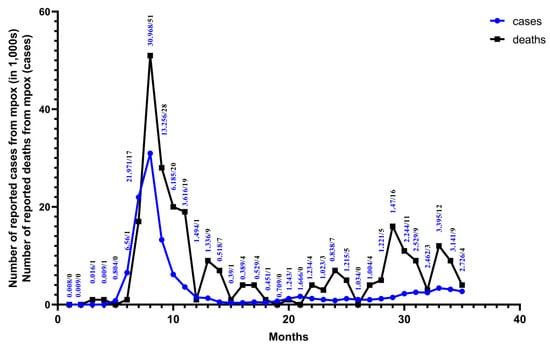
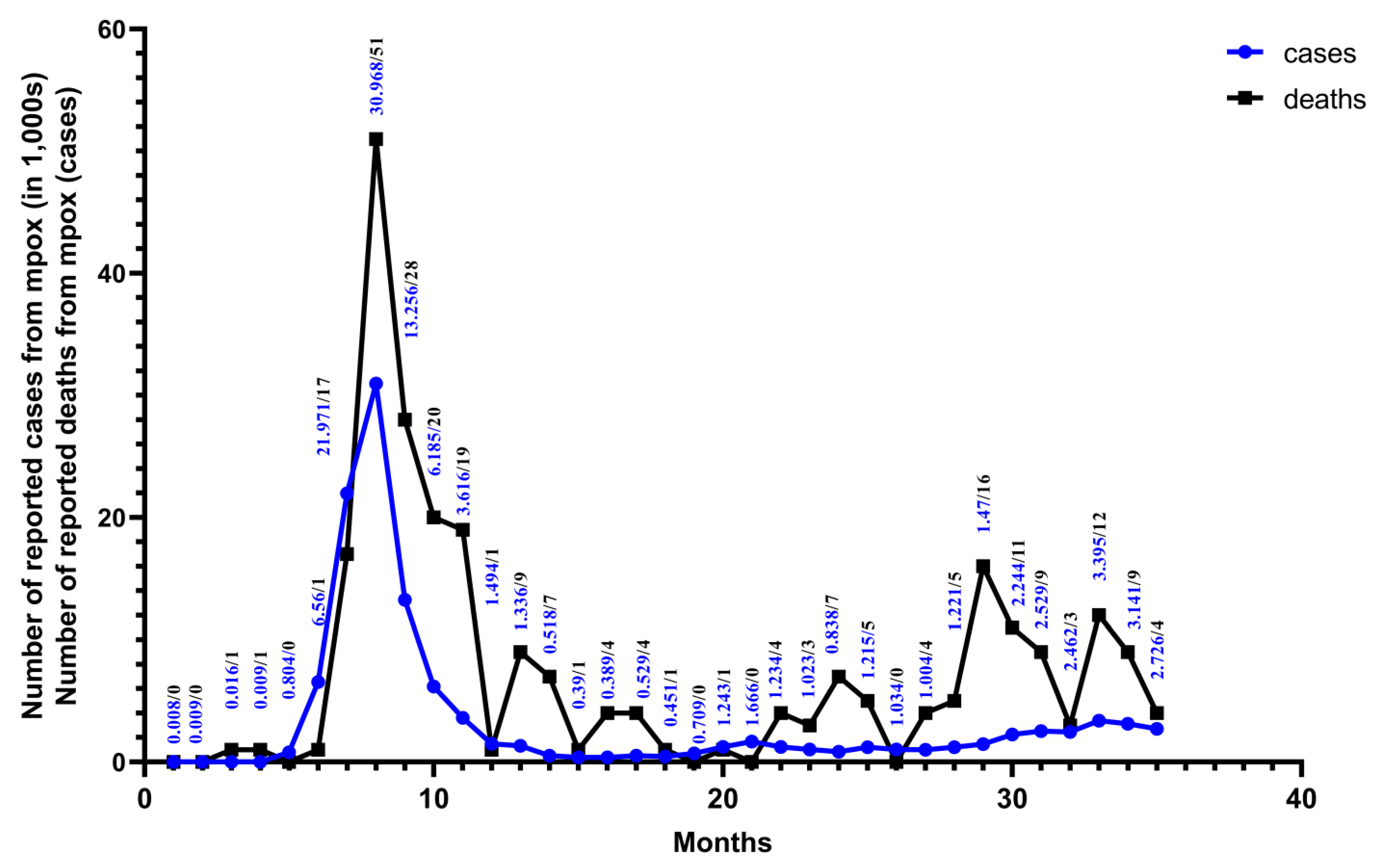
Figure 3.
From January 2022 to November 2024. Monthly numbers of cases and deaths from mpox are reported for 128 Member States across all six WHO regions. The data are from the WHO Global Aggregate of Mpox Cases. This figure was drawn using Graphpad Pism.
4. Advances in Mpox Vaccines
4.1. Second-Generation Smallpox Vaccine-ACAM2000
ACAM2000, a live attenuated vaccine capable of replication, is derived from a monoclonal virus isolate of the first-generation Dryvax vaccine. Typically, one dose is administered percutaneously to actively immunize groups identified as being at high risk of smallpox infection [45]. ACAM2000 has been shown to be non-inferior to the first-generation vaccine (Dryvax) in immunogenicity tests, with a better safety profile and a lower incidence of side effects [46]. Common side effects include localized reactions such as pain, itching, and swelling at the injection site, as well as systemic symptoms like muscle pain, headache, rash, fever, fatigue, tearing, blurred vision, eye pain, and flu-like symptoms [47]. However, serious side effects include severe allergic reactions, encephalitis, encephalomyelitis, encephalopathy, pericarditis, and even death. A study by Decker et al. involving 897,227 individuals who received the ACAM2000 vaccine over an 18-year period reported an incidence of pericarditis at approximately 20 cases per 100,000 recipients [48]. However, according to data from the United States (US) Food and Drug Administration (FDA), the incidence is higher, with approximately 1 case per 175 vaccinated individuals [47]. In addition, the use of ACAM2000 is strictly contraindicated in individuals with impaired cardiac function, pregnant women, newborns, and immunocompromised individuals (those with leukemia, human immunodeficiency virus, etc.) [8,49]. Therefore, it is only suitable for individuals who are not pregnant and have a functioning immune system [50]. The risk of infection is greatly minimized by vaccination, but since no vaccine is 100% effective, contracting mpox is still possible, particularly during outbreaks.
4.2. Third-Generation Smallpox Vaccine
4.2.1. JYNNEOS (MVA-BN)
JYNNEOS is a non-replicating live vaccine derived from the Modified Vaccinia Ankara (MVA) virus. In 2019, JYNNEOS was officially licensed in the US [51]. JYNNEOS has been effective in the emergency prevention of mpox in a number of animal models. In August 2022, the FDA granted emergency authorization of the JYNNEOS vaccine for the prevention of mpox, which has proven effective in multiple animal models [52]. The clinical trial evidence suggests that JYNNEOS provides 66% to 85.9% effectiveness when it is administered as two subcutaneous injections (SC) spaced 4 weeks apart [53,54]. Individuals need a booster shot every 2–10 years if they remain exposed to MPXV [55]. In addition, WHO reported that, in eight separate studies that assessed 1222 participants, the seroconversion rates were consistently higher than 98 percent, proving the efficacy of the vaccine [56]. Typically, the JYNNEOS vaccine causes mild side effects, including injection site reactions and transient systemic symptoms (pain, fatigue, itching, chills, and nausea) [57,58]. No adverse effects of myocarditis or pericarditis were observed among 9713 vaccinated individuals enrolled in 19 randomized controlled trials [47]. Based on their studies, Rao et al. concluded that JYNNEOS is safe for immunocompromised people, but with reduced immunogenicity compared to healthy people, after conducting three randomized controlled trials and 15 observational studies of 5775 vaccinated individuals [59]. According to Fontán-Vela et al., the MVA-BN vaccine proved effective in minimizing the risk of MPXV infection in high-risk HIV pre-exposure prophylaxis cases, with reductions of 79% at 75 days and 14% at 79 days after vaccination [60]. Consequently, JYNNEOS offers a safer alternative to ACAM2000. The MVA-BN vaccine is the preferred choice for individuals with weakened immune systems and can be used for initial and post-exposure prophylaxis in pregnant and breastfeeding women [50]. The risk of infection is greatly minimized by vaccination, but since no vaccine is 100% effective, contracting mpox is still possible, particularly during outbreaks.
4.2.2. LC16m8
The LC16m8 vaccine is a live, minimally replicating, attenuated vaccine derived from the Lister (Elstree) strain. It is administered as a single-dose scratch vaccination using a bifurcated needle. LC16m8 was developed in 1975 by the Kaketsuken Research Institute in Japan and was approved for mpox prevention in Japan in August 2022 [61]. Clinical studies on the immunogenicity of LC16m8 have demonstrated the seroconversion of neutralizing antibodies against MPXV by day 28 post-vaccination [62,63]. The vaccine has a favorable safety profile, with side effects being mild and transient. These include injection site reactions and mild systemic symptoms such as swollen lymph nodes, fatigue, fever, rash, redness, and swelling at the injection site [8,58]. According to a WHO report dated 23 August 2024, research on the efficacy of LC16m8 is ongoing. The inferred clinical efficacy of LC16m8 for smallpox and mpox derives from indirect experiments, such as studies on its protective effects in various animal models. LC16m8 vaccination has provided protection to mice, rabbits, and monkeys against deadly MPXV assaults [56]. Although MVA-BN and LC16m8 have been authorized for post-exposure vaccination in children in the US and Japan, vaccination should only be administered when the benefits clearly outweigh the potential risks, particularly in vulnerable populations [50]. The risk of infection is greatly minimized by vaccination, but since no vaccine is 100% effective, contracting mpox is still possible, particularly during outbreaks.
4.3. Recent Advances in Vaccine Development
Currently, the global approaches to developing mpox vaccines include live attenuated vaccines, mRNA vaccines, recombinant protein vaccines, and fourth-generation vaccines. Significant progress has been made in mRNA and recombinant protein research. mRNA vaccines can be quickly developed and designed using viral gene sequences. When the virus mutates, companies can swiftly modify the vaccine formula to avert immune escape. Upon successful development, the production process is easy to manage and can be scaled up to satisfy the demand for mass vaccination [64]. The study by Mucker et al. was the first to evaluate the effectiveness of an mRNA vaccine (mRNA-1769) and MVA in preventing mpox in a non-human primate model [65]. Overall, the results showed that the mRNA-1769 vaccine induced fewer lesions and lower viral replication, in addition to stronger, more active viral neutralizing and functional antibodies that improved the ability to control viral replication and alleviate symptoms of disease. Just like BNT166, an mRNA vaccine from BioNTech, showed high immunogenicity, good protection in preclinical models, and 100% protection in mouse and rhesus monkeys against MPXV and related orthopox viruses [66].
Sometime soon, Chinese homegrown mRNA vaccines will enter clinical trials. The Chinese biological vaccine research and development team is leading in this field. By encoding the A35R and M1R proteins of MPXV, they have developed three mRNA vaccines (VGPox 1, VGPox 2, and VGPox 3) targeting MPXV [67]. All three vaccines rapidly elicited antibodies against A35R in mouse studies, and two mRNA vaccine (VGPox 1, VGPox 2) generated anti-A35R and anti-M1R IgG antibodies very rapidly and well, with strong virus neutralization [67]. Additionally, researchers at the Seventh Affiliated Hospital of Sun Yat-sen University developed a multivalent mpox mRNA vaccine containing four immunogenic targets from mpox Clade II isolates, such as A27, A33, B5, and L1, in 2003. The team encapsulated the mRNAs for these antigens in lipid nanoparticles (LNPs). The mRNA-LNP vaccine with four antigens can effectively trigger a humoral immune response at a high dose (5 μg). Even at a low dose of 0.5 μg, it can ensure 100% survival of mice [64]. In addition, researchers at the University of Science and Technology of China created the MPXV-1103 tetravalent vaccine, targeting the B6, A35, A29, and M1 MPXV proteins at an mRNA immunogenicity equivalent to that of conventional smallpox vaccines ACAM2000 and MVA and inducing strong humoral immunity and T cell responses against MPXV [68,69]. Notably, MPXV-1103 generated high levels of neutralizing antibodies even at a low dose of 1 μg, making it a highly efficient and safe vaccine candidate. It is expected to become a critical tool in preventing mpox infections [69]. In another innovative approach using antigenic structure-guided multi-epitope chimerism, the Institute of Microbiology of the Chinese Academy of Sciences designed a ‘two-in-one’ MPXV recombinant protein vaccine called DAM. DAM provides comprehensive protection against MPXV infectious virus particles with a single immunogen, and its neutralizing power is 28 times higher than that of standard live attenuated vaccines [70]. Overall, global research on mpox vaccines and treatments is advancing rapidly. The ongoing mutations of MPXV complicate vaccine development, underscoring the need for a deeper understanding of its pathogenicity and mutation potential.
5. Discussion
The usability of MPXV vaccines is determined using the balance between their immunogenicity and safety, with effectiveness and side effects serving as key evaluation criteria. ACAM2000, derived from a live attenuated cowpox virus, provides significant cross-protection against both smallpox and mpox, demonstrating high immunogenicity [71]. However, it carries a higher risk of side effects. Uncontrolled viral replication may lead to accidental or self-inoculation, resulting in conditions such as cowpox eczema and progressive cowpox [47,72]. In contrast, JYNNEOS does not pose this risk. It utilizes a non-replicating Modified Vaccinia Ankara (MVA) virus, making it a safer option, particularly for immunocompromised individuals. Although it may induce a weaker immune response compared to ACAM2000, JYNNEOS avoids the skin reactions at the vaccination site commonly associated with ACAM2000 [16]. Nevertheless, this weaker immune response means that JYNNEOS offers less protection than the more potent but potentially riskier ACAM2000 [65]. On the other hand, LC16m8, derived from a highly attenuated cowpox virus strain, strikes a balance between safety and immunogenicity. It reduces side effects while inducing a robust immune response. However, further clinical evidence is needed to fully validate its efficacy [73] (Table 2). The aforementioned three smallpox vaccines are crucial in fighting the ongoing mpox outbreak. Their effectiveness in MPXV control and prevention is not entirely adequate, and their side effects hinder their protective function in specific demographics [8]. Therefore, in response to the current mpox outbreak, there is an urgent need for a safer, more effective vaccine specifically designed to target MPXV.

Table 2.
Similarities and differences between ACAM2000, JYNNEOS, and LC16m8 vaccines.
This research goal was successfully achieved through mRNA and recombinant protein vaccines. mRNA vaccines have attracted attention in research due to their fast development, robust response to viral mutations, and simple mass production. The research has indicated that mRNA vaccines and recombinant protein vaccines possess excellent immunogenicity and provide protection in animal models [75]. For instance, the mRNA-1769 vaccine can significantly decrease lesions and viral replication while eliciting a robust immune response. The DAM recombinant protein vaccine, developed by the Institute of Microbiology at the Chinese Academy of Sciences, has also demonstrated notable protective effects and robust neutralization capabilities. Compared with existing vaccines, the new vaccines show significant advantages in multiple aspects. In terms of immunogenicity, mRNA vaccines elicit a stronger immune response than MVA-BN and match or even surpass ACAM2000 in effectiveness [65]. The recombinant protein vaccine DAM offers a protective efficacy comparable to traditional live-attenuated vaccines but with enhanced safety [70]. In contrast, JYNNEOS has relatively weaker immunogenicity. Regarding safety, the new vaccines represent a marked improvement. While ACAM2000 carries a high risk of side effects, mRNA and recombinant protein vaccines are associated with milder adverse reactions and are safer for immunocompromised individuals. In terms of their adaptability, the new vaccines have a broader application scope, which better satisfies the demand for diverse vaccination needs and provides more robust protection across different populations [76]. The ongoing mutations of the MPXV present a challenge for vaccine development, necessitating an exploration of its pathogenic mechanisms and mutation traits to advance more effective, long-lasting, and safe vaccines. Moving forward, large-scale randomized clinical trials will be necessary to assess the safety and effectiveness of the new mpox vaccines.
Current treatment strategies for mpox encompass antiviral drugs, supportive care, and immunomodulatory therapy. Tecovirimat, an FDA-approved antiviral drug for the treatment of mpox virus infection, is effective in alleviating patients’ symptoms and shortening the disease course [77]. It is particularly suitable for severely ill patients and those who are immunocompromised [12]. Cidofovir, which interferes with viral DNA replication by inhibiting the viral DNA polymerase, has a more restricted application due to its nephrotoxicity [78,79]. Brincidofovir, a prodrug of Cidofovir, boasts better oral bioavailability and lower nephrotoxicity. It demonstrated effectiveness against MPXV in animal models [80]. Supportive therapy centers on providing symptomatic treatment and wound care. For instance, non-steroidal anti-inflammatory drugs can be employed to relieve symptoms such as pain and fever. It is also crucial to keep the wound clean and dry to prevent secondary infection [81]. Among the available immunomodulatory therapies, immunoglobulin therapy can be administered to severely immunocompromised patients. However, anti-inflammatory treatments should be used cautiously to avoid undermining the immune system’s ability to combat the virus. To explore combination therapy options, researchers are investigating the simultaneous use of multiple antiviral drugs with different mechanisms of action, such as combining Tecovirimat with PA104, with the aim of enhancing treatment efficacy and reducing the likelihood of viral resistance [19]. Cutting-edge therapeutic approaches, including gene therapy and immunomodulatory therapies, also create new hope for mpox treatment [82]. Drug repurposing studies have identified several drugs with potential antiviral activity by screening existing FDA-approved drugs, like doxycycline, niclosamide, sunitinib, oestradiol, and medroxyprogesterone acetate [83]. Monoclonal antibody studies are focused on developing monoclonal antibodies against specific antigens of the MPXV to specifically neutralize it [19]. Additionally, research into traditional and alternative therapies has identified certain plant extracts and natural compounds with antiviral and anti-inflammatory properties, which may provide new ideas for future treatments [82].
An effective response to the global mpox outbreak should involve more than just vaccination or treatment strategies, and consider socioeconomic factors such as poverty, healthcare access, and urbanization [84]. Poverty drives people deeper into forests in search of food and resources, increasing exposure to wildlife—the natural host of the MPXV. Hunting, handling, and consuming wildlife further heighten infection risks. Moreover, poverty restricts access to healthcare, delaying effective treatment and accelerating viral spread [85]. Rapid urbanization and population growth have created crowded living conditions, facilitating human-to-human transmission. In urban areas with poor sanitation and limited medical resources, the virus spreads more rapidly [86]. Furthermore, urbanization drives large-scale population movements, accelerating regional virus spread and triggering transnational public health crises. The interplay of poverty and urbanization intensifies the severity of the mpox epidemic [84,87]. To effectively address mpox, these factors must be considered in the development of targeted public health strategies and interventions. These include improving the economic conditions of poor areas, increasing employment opportunities, and reducing reliance on wildlife; enhancing urban public health infrastructure to improve healthcare access and quality; and strengthening mpox surveillance and prevention, particularly in high-risk, impoverished, and rapidly urbanizing areas [88]. In addition to other problems, the fair distribution of vaccines globally is a pressing issue that needs resolution.
6. Conclusions
Given the current progress of vaccine research, both domestically and internationally, the immunity now provided via the smallpox vaccine is the only protection against MPXV. Second- and third-generation vaccines, which are safer and have better immune efficacy, have replaced first-generation smallpox vaccines [47]. Additionally, clinical trials provided evidence that smallpox vaccines, such as MVA-BN, caused no serious adverse reactions in immunocompromised subjects. This finding offers a key foundation for expanding the target population for vaccination and eliminating mortality due to mpox infection [89]. MPXV Clade Ib remains widespread and continues to spread globally due to mutations that increase the pathogenicity and transmissibility of MPXV. To address this challenge, an MPXV-specific vaccine should be developed with the ability to rapidly adapt to emerging strains, ensuring effective targeting of evolving MPXV variants. Key objectives include improving vaccine safety and efficacy while reducing viral pathogenicity and transmissibility. The developed vaccines are more suitable for those with immune system disorders, skin problems, or cardiovascular problems [50]. The mRNA-1769 vaccine is currently being evaluated in an ongoing Phase I/II clinical trial (NCT05995275) to assess the safety and immunogenicity of an mRNA-based Orthopoxvirus vaccine [65]. BNT166 is also in a phase 1/2 clinical trial to determine its safety and immunogenicity [66]. At the pilot stage, DAM was developed by Wang et al. in collaboration with Shanghai Junshi Biological Products, which developed a one-step purification of industrially produced cell lines and DAM with a yield of 1.18 g/L. This innovation offers a safer and easier-to-scale vaccine option for the prevention and control of MPXV [70]. Overall, new advances in vaccines are boosting confidence in the next round of vaccines to prevent and treat mpox. Despite this progress, there are still some gaps in vaccine distribution, the accessibility of therapeutic drugs, and regional equity. In addition, the surveillance, diagnosis, treatment, and management of mpox presents challenges in some or varied countries or regions. Although PCR is the most commonly used MPXV diagnostic because of its high sensitivity and specificity, it cannot be used in point-of-care testing (POCT) due to the equipment and environmental demands [90]. Within this context, CRISPR/Cas technology is emerging as a new means of nucleic acid detection with no requirement for dedicated laboratory equipment and a controlled environment; this is expected to serve as a POCT candidate. This technology can quickly and precisely test mpox nucleic acids in various countries and regions [91]. Therefore, a better understanding of mpox and an improvement in the entire prevention system could provide valuable insights for dealing with severe cases and implementing effective scientific prevention and control.
Author Contributions
X.Z. and D.-A.L.: framework construction, literature collection, collation of data, draft—writing. Y.Q., R.H., S.C., Y.X., K.C. and J.Y.: review and editing. X.L.: resources, writing—review and editing, supervision, project administration, and funding. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by Zhejiang Shuren University Basic Scientific Research Special Funds (2024XZ011). The funder did not participate in the designing, performing or reporting in the current study.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- World Health Organization. Monkeypox-United Kingdom of Great Britain and Northern Ireland. 2022. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON383 (accessed on 18 May 2022).
- Nachega, J.B.; Sam-Agudu, N.A.; Ogoina, D.; Mbala-Kingebeni, P.; Ntoumi, F.; Nakouné, E.; Njouom, R.; Lewis, R.F.; Gandhi, M.; Rosenthal, P.J.; et al. The surge of mpox in Africa: A call for action. Lancet Glob. Health 2024, 12, e1086–e1088. [Google Scholar] [CrossRef]
- World Health Organization. WHO Director-General Declares Mpox Outbreak a Public Health Emergency of International Concern. 2024. Available online: https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern#:~:text=In%20July%202022%2C%20the%20multi-country%20outbreak%20of%20mpox,where%20the%20virus%20had%20not%20been%20seen%20before (accessed on 14 August 2024).
- World Health Organization. 2022–2024 Mpox (Monkeypox) Outbreak: Global Trends. 2025. Available online: https://reliefweb.int/report/world/2022-24-mpox-monkeypox-outbreak-global-trends (accessed on 27 November 2024).
- Danladi, N.P.; Agboola, P.; Olaniyi, P.; Eze, S.; Oladapo, O.; Obiwulu, D.; Akano, O.S.; Adeola, O.A.; Olawale, K.; Adiatu, A.I.; et al. Challenges in Global Distribution and Equitable Access to Monkeypox Vaccines. Viruses 2024, 16, 1815. [Google Scholar] [CrossRef]
- Karagoz, A.; Tombuloglu, H.; Alsaeed, M.; Tombuloglu, G.; AlRubaish, A.A.; Mahmoud, A.; Smajlović, S.; Ćordić, S.; Rabaan, A.A.; Alsuhaimi, E. Monkeypox (mpox) virus: Classification, origin, transmission, genome organization, antiviral drugs, and molecular diagnosis. J. Infect. Public Health 2023, 16, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Pischel, L.; Martini, B.A.; Yu, N.; Cacesse, D.; Tracy, M.; Kharbanda, K.; Ahmed, N.; Patel, K.M.; Grimshaw, A.A.; Malik, A.A.; et al. Vaccine effectiveness of 3rd generation mpox vaccines against mpox and disease severity: A systematic review and meta-analysis. Vaccine 2024, 42, 126053. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, Z.; Sheng, S.; Song, R.; Jin, R. The Current State and Progress of Mpox Vaccine Research. China CDC Wkly. 2024, 6, 118–125. [Google Scholar] [CrossRef]
- Adetifa, I.; Muyembe, J.-J.; Bausch, D.G.; Heymann, D.L. Mpox neglect and the smallpox niche: A problem for Africa, a problem for the world. Lancet 2023, 401, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.M.; Adesola, R.O.; Priyadarsini, S.; Singh, R.; Shaheen, M.N.F.; Ogundijo, O.A.; Gulumbe, B.H.; Lounis, M.; Samir, M.; Govindan, K.; et al. Unveiling the Global Surge of Mpox (Monkeypox): A comprehensive review of current evidence. Microbe 2024, 4, 100141. [Google Scholar] [CrossRef]
- Mercy, K.; Tibebu, B.; Fallah, M.; Faria, N.R.; Ndembi, N.; Tebeje, Y.K. Mpox continues to spread in Africa and threatens global health security. Nat. Med. 2024, 30, 1225–1226. [Google Scholar] [CrossRef]
- Kmiec, D.; Kirchhoff, F. Monkeypox: A New Threat? Int. J. Mol. Sci. 2022, 23, 7866. [Google Scholar] [CrossRef]
- Beeson, A.M.; Haston, J.; McCormick, D.W.; Reynolds, M.; Chatham-Stephens, K.; McCollum, A.M.; Godfred-Cato, S. Mpox in Children and Adolescents: Epidemiology, Clinical Features, Diagnosis, and Management. Pediatrics 2023, 151, e2022060179. [Google Scholar] [CrossRef]
- Gromowski, G.; Bunge, E.M.; Hoet, B.; Chen, L.; Lienert, F.; Weidenthaler, H.; Baer, L.R.; Steffen, R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Neglected Trop. Dis. 2022, 16, e0010141. [Google Scholar] [CrossRef]
- Shafaati, M.; Zandi, M. State-of-the-art on monkeypox virus: An emerging zoonotic disease. Infection 2022, 50, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J.G.; Lippi, G.; Henry, B.M.; Forthal, D.N.; Rizk, Y. Prevention and Treatment of Monkeypox. Drugs 2022, 82, 957–963. [Google Scholar] [CrossRef]
- Aden, D.; Zaheer, S.; Kumar, R.; Ranga, S. Monkeypox (Mpox) outbreak during COVID-19 pandemic—Past and the future. J. Med. Virol. 2023, 95, e28701. [Google Scholar] [CrossRef]
- Kaler, J.; Hussain, A.; Flores, G.; Kheiri, S.; Desrosiers, D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus 2022, 14, e26531. [Google Scholar] [CrossRef]
- Lu, J.; Xing, H.; Wang, C.; Tang, M.; Wu, C.; Ye, F.; Yin, L.; Yang, Y.; Tan, W.; Shen, L. Mpox (formerly monkeypox): Pathogenesis, prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 458. [Google Scholar] [CrossRef] [PubMed]
- Beeson, A.; Styczynski, A.; Hutson, C.L.; Whitehill, F.; Angelo, K.M.; Minhaj, F.S.; Morgan, C.; Ciampaglio, K.; Reynolds, M.G.; McCollum, A.M.; et al. Mpox respiratory transmission: The state of the evidence. Lancet Microbe 2023, 4, e277–e283. [Google Scholar] [CrossRef]
- World Health Organization. Mpox (monkeypox)-Democratic Republic of the Congo. 2023. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON522 (accessed on 14 June 2024).
- Beiras, C.G.; Malembi, E.; Escrig-Sarreta, R.; Ahuka, S.; Mbala, P.; Mavoko, H.M.; Subissi, L.; Abecasis, A.B.; Marks, M.; Mitjà, O. Concurrent outbreaks of mpox in Africa—An update. Lancet 2025, 405, 86–96. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, S.; Jain, S.; Mohanty, A.; Thapa, N.; Poudel, P.; Bhusal, K.; Al-Qaim, Z.H.; Barboza, J.J.; Padhi, B.K.; et al. The Global Monkeypox (Mpox) Outbreak: A Comprehensive Review. Vaccines 2023, 11, 1093. [Google Scholar] [CrossRef]
- Seang, S.; Burrel, S.; Todesco, E.; Leducq, V.; Monsel, G.; Le Pluart, D.; Cordevant, C.; Pourcher, V.; Palich, R. Evidence of human-to-dog transmission of monkeypox virus. Lancet 2022, 400, 658–659. [Google Scholar] [CrossRef]
- Coppens, J.; Vanroye, F.; Brosius, I.; Liesenborghs, L.; van Henten, S.; Vanbaelen, T.; Bracke, S.; Berens-Riha, N.; De Baetselier, I.; Kenyon, C.; et al. Alternative sampling specimens for the molecular detection of mpox (formerly monkeypox) virus. J. Clin. Virol. 2023, 159, 105372. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Junior, M.A.; Andrade, B.S.; Guevara-Vega, M.; de Melo, I.S.; Cunha, T.M.; Jardim, A.C.G.; Sabino-Silva, R. Oral Infection, Oral Pathology and Salivary Diagnostics of Mpox Disease: Relevance in Dentistry and OMICs Perspectives. Int. J. Mol. Sci. 2023, 24, 14362. [Google Scholar] [CrossRef] [PubMed]
- Yinda, C.K.; Morris, D.H.; Fischer, R.J.; Gallogly, S.; Weishampel, Z.A.; Port, J.R.; Bushmaker, T.; Schulz, J.E.; Bibby, K.; van Doremalen, N.; et al. Stability of Monkeypox Virus in Body Fluids and Wastewater. Emerg. Infect. Dis. 2023, 29, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Alakunle, E.; Kolawole, D.; Diaz-Cánova, D.; Alele, F.; Adegboye, O.; Moens, U.; Okeke, M.I. A comprehensive review of monkeypox virus and mpox characteristics. Front. Cell. Infect. Microbiol. 2024, 6, 1360586. [Google Scholar] [CrossRef]
- Pecori, R.; Di Giorgio, S.; Paulo Lorenzo, J.; Nina Papavasiliou, F. Functions and consequences of AID/APOBEC-mediated DNA and RNA deamination. Nat. Rev. Genet. 2022, 23, 505–518. [Google Scholar] [CrossRef]
- Delamonica, B.; Davalos, L.; Larijani, M.; Anthony, S.J.; Liu, J.; MacCarthy, T. Evolutionary potential of the monkeypox genome arising from interactions with human APOBEC3 enzymes. Virus Evol. 2023, 9, vead047. [Google Scholar] [CrossRef]
- Martinez, T.; Shapiro, M.; Bhaduri-McIntosh, S.; MacCarthy, T. Evolutionary effects of the AID/APOBEC family of mutagenic enzymes on human gamma-herpesviruses. Virus Evol. 2019, 5, vey040. [Google Scholar] [CrossRef]
- Vakaniaki, E.H.; Kacita, C.; Kinganda-Lusamaki, E.; O’Toole, Á.; Wawina-Bokalanga, T.; Mukadi-Bamuleka, D.; Amuri-Aziza, A.; Malyamungu-Bubala, N.; Mweshi-Kumbana, F.; Mutimbwa-Mambo, L.; et al. Sustained Human Outbreak of a New MPXV Clade I Lineage in Eastern Democratic Republic of the Congo. Nat. Med. 2024, 30, 2791–2795. [Google Scholar] [CrossRef]
- World Health Organization. Multi-Country Outbreak of Mpox. 2023. Available online: https://www.who.int/publications/m/item/multi-country-outbreak-of-mpox--external-situation-report-31---22-december-2023 (accessed on 22 December 2023).
- Iñigo Martínez, J.; Gil Montalbán, E.; Jiménez Bueno, S.; Martín Martínez, F.; Nieto Juliá, A.; Sánchez Díaz, J.; Marín, N.G.; Deorador, E.C.; Forte, A.N.; García, M.A.; et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance 2022, 27, 2200471. [Google Scholar] [CrossRef]
- Mailhe, M.; Beaumont, A.-L.; Thy, M.; Le Pluart, D.; Perrineau, S.; Houhou-Fidouh, N.; Deconinck, L.; Bertin, C.; Ferré, V.M.; Cortier, M.; et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: An observational cohort study. Clin. Microbiol. Infect. 2023, 29, 233–239. [Google Scholar] [CrossRef]
- Palich, R.; Burrel, S.; Monsel, G.; Nouchi, A.; Bleibtreu, A.; Seang, S.; Bérot, V.; Brin, C.; Gavaud, A.; Wakim, Y.; et al. Viral loads in clinical samples of men with monkeypox virus infection: A French case series. Lancet Infect. Dis. 2023, 23, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Ramnarayan, P.; Mitting, R.; Whittaker, E.; Marcolin, M.; O’Regan, C.; Sinha, R.; Bennett, A.; Moustafa, M.; Tickner, N.; Gilchrist, M.; et al. Neonatal Monkeypox Virus Infection. N. Engl. J. Med. 2022, 387, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.B.; Casadio, L.V.B.; Polly, M.; Nastri, A.C.; Turdo, A.C.; de Araujo Eliodoro, R.H.; Sabino, E.C.; Levin, A.S.; de Proença, A.C.T.; Higashino, H.R. Monkeypox Virus Transmission to Healthcare Worker through Needlestick Injury, Brazil. Emerg. Infect. Dis. 2022, 28, 2334–2336. [Google Scholar] [CrossRef]
- Lee, S.-S.; Bockarie, M.J.; Al-Tawfiq, J.A. Was the public health emergency status of mpox ended too soon? Int. J. Infect. Dis. 2023, 134, 301–302. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing–11 May 2023. 2023. Available online: https://reliefweb.int/report/world/who-director-generals-opening-remarks-media-briefing-11-may-2023 (accessed on 11 May 2023).
- Branda, F.; Ceccarelli, G.; Ciccozzi, M.; Scarpa, F. First cases of mpox Clade I outside of Africa: Genetic insights on its evolution. Infect. Dis. 2024, 56, 1003–1005. [Google Scholar] [CrossRef]
- Del Giudice, P.; Fribourg, A.; Roudiere, L.; Gillon, J.; Decoppet, A.; Reverte, M. Familial Monkeypox Virus Infection Involving 2 Young Children. Emerg. Infect. Dis. 2023, 29, 437–439. [Google Scholar] [CrossRef]
- Ishoso, D.K.; Danovaro-Holliday, M.C.; Cikomola, A.M.-W.; Lungayo, C.L.; Mukendi, J.-C.; Mwamba, D.; Ngandu, C.; Mafuta, E.; Dikassa, P.S.L.; Lulebo, A.; et al. “Zero Dose” Children in the Democratic Republic of the Congo: How Many and Who Are They? Vaccines 2023, 11, 900. [Google Scholar] [CrossRef] [PubMed]
- Adepoju, P. Mpox declared a public health emergency. Lancet 2024, 404, e1–e2. [Google Scholar] [CrossRef]
- Petersen, B.W.; Harms, T.J.; Reynolds, M.G.; Harrison, L.H. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses-Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 257–262. [Google Scholar] [CrossRef]
- Frey, S.E.; Newman, F.K.; Kennedy, J.S.; Ennis, F.; Abate, G.; Hoft, D.F.; Monath, T.P. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000 and Dryvax® in healthy vaccinia-naive adults. Vaccine 2009, 27, 1637–1644. [Google Scholar] [CrossRef]
- Sah, R.; Paul, D.; Mohanty, A.; Shah, A.; Mohanasundaram, A.S.; Padhi, B.K. Monkeypox (Mpox) vaccines and their side effects: The other side of the coin. Int. J. Surg. 2023, 109, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Decker, M.D.; Garman, P.M.; Hughes, H.; Yacovone, M.A.; Collins, L.C.; Fegley, C.D.; Lin, G.; DiPietro, G.; Gordon, D.M. Enhanced safety surveillance study of ACAM2000 smallpox vaccine among US military service members. Vaccine 2021, 39, 5541–5547. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Tang, L.-K.; Xiao, F.-F.; Zhang, P.; Lu, C.-M.; Hu, L.-Y.; Wang, L.-S.; Cheng, G.-Q.; Zhou, W.-H. Monkeypox and the perinatal period: What does maternal–fetal medicine need to know? World J. Pediatr. 2022, 19, 213–223. [Google Scholar] [CrossRef]
- Rana, J.; Patel, S.K.; Agrawal, A.; Channabasappa, N.K.; Niranjan, A.K.; Chandra Das, B.; Pandey, M.K.; Tiwari, S.P.; Gaihre, M.M. Mpox vaccination in global perspective: Priorities and challenges. Ann. Med. Surg. 2023, 85, 2243–2246. [Google Scholar] [CrossRef] [PubMed]
- Cohn, H.; Bloom, N.; Cai, G.Y.; Clark, J.J.; Tarke, A.; Bermúdez-González, M.C.; Altman, D.R.; Lugo, L.A.; Lobo, F.P.; Marquez, S.; et al. Mpox vaccine and infection-driven human immune signatures: An immunological analysis of an observational study. Lancet Infect. Dis. 2023, 23, 1302–1312. [Google Scholar] [CrossRef]
- Sah, R.; Humayun, M.; Baig, E.; Farooq, M.; Hussain, H.G.; Shahid, M.U.; Cheema, H.A.; Chandran, D.; Yatoo, M.I.; Sharma, A.K.; et al. FDA’s authorized “Jynneos” vaccine for counteracting monkeypox global public health emergency; an update–Correspondence. Int. J. Surg. 2022, 107, 106971. [Google Scholar] [CrossRef]
- Dalton, A.F.; Diallo, A.O.; Chard, A.N.; Moulia, D.L.; Deputy, N.P.; Fothergill, A.; Kracalik, I.; Wegner, C.W.; Markus, T.M.; Pathela, P.; et al. Estimated Effectiveness of Jynneos Vaccine in Preventing MpoxA Multijurisdictional Case-Control Study—United States, 19 August 2022–31 March 2023. Morb. Mortal. Wkly. Rep. 2023, 72, 553–558. [Google Scholar] [CrossRef]
- Deputy, N.P.; Deckert, J.; Chard, A.N.; Sandberg, N.; Moulia, D.L.; Barkley, E.; Dalton, A.F.; Sweet, C.; Cohn, A.C.; Little, D.R.; et al. Vaccine Effectiveness of Jynneos against Mpox Disease in the United States. N. Engl. J. Med. 2023, 388, 2434–2443. [Google Scholar] [CrossRef]
- Kumar, D.; Malviya, R.; Srivastava, S.; Sridhar, S.B.; Talath, S.; Shareef, J.; Prajapati, B.G. Personalized immunization against Mpox Clades I and Ib: Strategies to combat the emerging epidemic. Infect. Med. 2025, 4, 100166. [Google Scholar] [CrossRef]
- World Health Organization. Smallpox and Mpox (Orthopoxviruses): WHO Position Paper, August 2024. 2024. Available online: https://www.who.int/publications/i/item/who-wer-9934-429-456 (accessed on 23 August 2024).
- Petersen, B.W.; Kabamba, J.; McCollum, A.M.; Lushima, R.S.; Wemakoy, E.O.; Muyembe Tamfum, J.-J.; Nguete, B.; Hughes, C.M.; Monroe, B.P.; Reynolds, M.G. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antivir. Res. 2019, 162, 171–177. [Google Scholar] [CrossRef]
- Garcia-Atutxa, I.; Mondragon-Teran, P.; Huerta-Saquero, A.; Villanueva-Flores, F. Advancements in monkeypox vaccines development: A critical review of emerging technologies. Front. Immunol. 2024, 15, 1456060. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of Jynneos (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices-United States, 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 734–742. [Google Scholar] [CrossRef]
- Fontán-Vela, M.; Hernando, V.; Olmedo, C.; Coma, E.; Martínez, M.; Moreno-Perez, D.; Lorusso, N.; Torres, M.V.; del Buey, J.F.B.; Roig-Sena, J.; et al. Effectiveness of Modified Vaccinia Ankara-Bavaria Nordic Vaccination in a Population at High Risk of Mpox: A Spanish Cohort Study. Clin. Infect. Dis. 2024, 78, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Ghazy, R.M.; Elrewany, E.; Gebreal, A.; ElMakhzangy, R.; Fadl, N.; Elbanna, E.H.; Tolba, M.M.; Hammad, E.M.; Youssef, N.; Abosheaishaa, H.; et al. Systematic Review on the Efficacy, Effectiveness, Safety, and Immunogenicity of Monkeypox Vaccine. Vaccines 2023, 11, 1708. [Google Scholar] [CrossRef]
- Tomita, N.; Morino, E.; Terada-Hirashima, J.; Uemura, Y.; Shimizu, Y.; Saito, S.; Suzuki, T.; Okumura, N.; Iwasaki, H.; Ebihara, H.; et al. Evaluating the Immunogenicity and Safety of a Smallpox Vaccine to Monkeypox in Healthy Japanese Adults: A Single-Arm Study. Life 2023, 13, 787. [Google Scholar] [CrossRef] [PubMed]
- Yano, R.; Terada-Hirashima, J.; Uemura, Y.; Tomita, N.; Shimizu, Y.; Iwasaki, H.; Okumura, N.; Suzuki, T.; Saito, S.; Ujiie, M.; et al. Efficacy and Safety of the Smallpox Vaccine for Postexposure Prophylaxis in Monkeypox: Protocol for an Open-Labeled, Single-Armed Study. JMIR Res. Protoc. 2023, 12, e46955. [Google Scholar] [CrossRef]
- Hou, F.; Zhang, Y.; Liu, X.; Murad, Y.M.; Xu, J.; Yu, Z.; Hua, X.; Song, Y.; Ding, J.; Huang, H.; et al. mRNA vaccines encoding fusion proteins of monkeypox virus antigens protect mice from vaccinia virus challenge. Nat. Commun. 2023, 14, 5925. [Google Scholar] [CrossRef]
- Mucker, E.M.; Freyn, A.W.; Bixler, S.L.; Cizmeci, D.; Atyeo, C.; Earl, P.L.; Natarajan, H.; Santos, G.; Frey, T.R.; Levin, R.H.; et al. Comparison of protection against mpox following mRNA or modified vaccinia Ankara vaccination in nonhuman primates. Cell 2024, 187, 5540–5553.e5510. [Google Scholar] [CrossRef]
- Zuiani, A.; Dulberger, C.L.; De Silva, N.S.; Marquette, M.; Lu, Y.-J.; Palowitch, G.M.; Dokic, A.; Sanchez-Velazquez, R.; Schlatterer, K.; Sarkar, S.; et al. A multivalent mRNA monkeypox virus vaccine (BNT166) protects mice and macaques from orthopoxvirus disease. Cell 2024, 187, 1363–1373.e1312. [Google Scholar] [CrossRef]
- Fang, Z.; Monteiro, V.S.; Renauer, P.A.; Shang, X.; Suzuki, K.; Ling, X.; Bai, M.; Xiang, Y.; Levchenko, A.; Booth, C.J.; et al. Polyvalent mRNA vaccination elicited potent immune response to monkeypox virus surface antigens. Cell Res. 2023, 33, 407–410. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, X.; Zhu, Y.; Mo, O.; Yu, H.; Zhu, L.; Zhang, J.; Kuang, L.; Gao, Y.; Cao, R.; et al. Multi-valent mRNA vaccines against monkeypox enveloped or mature viron surface antigens demonstrate robust immune response and neutralizing activity. Sci. China Life Sci. 2023, 66, 2329–2341. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Gong, Q.; Zhang, J.; Guo, X.; Xie, W.; Chen, D.; Shen, Y.; Hong, D.; Li, Z.; Wang, Q.; et al. An mpox quadrivalent mRNA vaccine protects mice from lethal vaccinia virus challenge. Antivir. Res. 2024, 230, 105974. [Google Scholar] [CrossRef]
- Wang, H.; Yin, P.; Zheng, T.; Qin, L.; Li, S.; Han, P.; Qu, X.; Wen, J.; Ding, H.; Wu, J.; et al. Rational design of a ‘two-in-one’ immunogen DAM drives potent immune response against mpox virus. Nat. Immunol. 2024, 25, 307–315. [Google Scholar] [CrossRef]
- Tack, D.M.; Karem, K.L.; Montgomery, J.R.; Collins, L.; Bryant-Genevier, M.G.; Tiernan, R.; Cano, M.; Lewis, P.; Engler, R.J.; Damon, I.K.; et al. Unintentional transfer of vaccinia virus associated with smallpox vaccines. Hum. Vaccines Immunother. 2013, 9, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S. Pathological skin manifestations following smallpox vaccination (ACAM2000) in US military personnel, 2009–2023: A systematic review. Bayl. Univ. Med. Cent. Proc. 2024, 37, 851–857. [Google Scholar] [CrossRef]
- Saito, T.; Fujii, T.; Kanatani, Y.; Saijo, M.; Morikawa, S.; Yokote, H.; Takeuchi, T.; Kuwabara, N. Clinical and immunological response to attenuated tissue-cultured smallpox vaccine LC16m8. J. Am. Med. Assoc. 2009, 301, 1025–1033. [Google Scholar] [CrossRef]
- Hajjo, R.; Abusara, O.H.; Sabbah, D.A.; Bardaweel, S.K. Advancing the understanding and management of Mpox: Insights into epidemiology, disease pathways, prevention, and therapeutic strategies. BMC Infect. Dis. 2025, 25, 529. [Google Scholar] [CrossRef] [PubMed]
- Chuai, X.; Ye, T.; Zhao, B.; Wu, Y.; Guo, C.; Li, F.; Zhou, J.; Zhang, K.; Wang, Y.; Liu, Y.; et al. Long-Lasting Protection and Dose Optimization of Mpxv Polyvalent Mpox mRNA Vaccines Against Lethal Vaccinia Virus Challenge in Mice. J. Med. Virol. 2024, 97, e70309. [Google Scholar] [CrossRef]
- Tai, W.; Tian, C.; Shi, H.; Chai, B.; Yu, X.; Zhuang, X.; Dong, P.; Li, M.; Yin, Q.; Feng, S.; et al. An mRNA vaccine against monkeypox virus inhibits infection by co-activation of humoral and cellular immune responses. Nat. Commun. 2025, 16, 2971. [Google Scholar] [CrossRef]
- Tripathi, P.; Pandey, S.; Yadav, D.; Joshi, S. Emergence and evolution of monkeypox virus: Epidemiology, pathology, clinical symptoms, preventative and treatment measures. Int. Immunopharmacol. 2025, 152, 114448. [Google Scholar] [CrossRef]
- Andrei, G.; Snoeck, R. Cidofovir Activity against Poxvirus Infections. Viruses 2010, 2, 2803–2830. [Google Scholar] [CrossRef] [PubMed]
- Shamim, M.A.; Padhi, B.K.; Satapathy, P.; Veeramachaneni, S.D.; Chatterjee, C.; Tripathy, S.; Akhtar, N.; Pradhan, A.; Dwivedi, P.; Mohanty, A.; et al. The use of antivirals in the treatment of human monkeypox outbreaks: A systematic review. Int. J. Infect. Dis. 2023, 127, 150–161. [Google Scholar] [CrossRef]
- Siegrist, E.A.; Sassine, J. Antivirals With Activity Against Mpox: A Clinically Oriented Review. Clin. Infect. Dis. 2023, 76, 155–164. [Google Scholar] [CrossRef]
- Patel, A.; Bilinska, J.; Tam, J.C.H.; Da Silva Fontoura, D.; Mason, C.Y.; Daunt, A.; Snell, L.B.; Murphy, J.; Potter, J.; Tuudah, C.; et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: Descriptive case series. Bmj 2022, 378, e072410. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Chandran, D.; Mohapatra, R.K.; Alagawany, M.; El-Shall, N.A.; Sharma, A.K.; Chakraborty, C.; Dhama, K. Clinical management, antiviral drugs and immunotherapeutics for treating monkeypox. An update on current knowledge and futuristic prospects. Int. J. Surg. 2022, 105, 106847. [Google Scholar] [CrossRef]
- Yousaf, M.A.; Michel, M.; Khan, A.T.-A.; Noreen, M.; Bano, S. Repurposing doxycycline for the inhibition of monkeypox virus DNA polymerase: A comprehensive computational study. Silico Pharmacol. 2025, 13, 27. [Google Scholar] [CrossRef]
- Akingbola, A.; Adegbesan, C.A.; Adewole, O.; Idahor, C.; Odukoya, T.; Nwaeze, E.; Mayowa, S.; Abdullahi, O.; Mariaria, P.K.; Akingbola, A.; et al. Understanding the resurgence of mpox: Key drivers and lessons from recent outbreaks in Africa. Trop. Med. Health 2025, 53, 47. [Google Scholar] [CrossRef]
- Ogunleye, S.C.; Akinsulie, O.C.; Aborode, A.T.; Olorunshola, M.M.; Gbore, D.; Oladoye, M.; Adesola, R.O.; Gbadegoye, J.O.; Olatoye, B.J.; Lawal, M.A.; et al. The re-emergence and transmission of Monkeypox virus in Nigeria: The role of one health. Front. Public Health 2024, 11, 1334238. [Google Scholar] [CrossRef] [PubMed]
- Moyo, E.; Musuka, G.; Murewanhema, G.; Moyo, P.; Dzinamarira, T. Monkeypox outbreak: A perspective on Africa’s diagnostic and containment capacity. Int. J. Infect. Dis. 2022, 123, 127–130. [Google Scholar] [CrossRef]
- Gao, S.; Zeng, Z.; Xin, Q.; Yang, M.; Feng, X.; Liu, X.; Kan, W.; Chen, F.; Chen, Y.; Chen, Z. Global transboundary transmission path and risk of Mpox revealed with Least Cost Path model. Int. J. Infect. Dis. 2024, 146, 107101. [Google Scholar] [CrossRef]
- Olawade, D.B.; Wada, O.Z.; Fidelis, S.C.; Oluwole, O.S.; Alisi, C.S.; Orimabuyaku, N.F.; David-Olawade, A.C. Strengthening Africa’s response to Mpox (monkeypox): Insights from historical outbreaks and the present global spread. Sci. One Health 2024, 3, 100085. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mohapatra, R.K.; Chandran, D.; Alagawany, M.; Sv, P.; Islam, M.A.; Chakraborty, C.; Dhama, K. Monkeypox vaccines and vaccination strategies: Current knowledge and advances. An update–Correspondence. Int. J. Surg. 2022, 105, 106869. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Heymann, D.L.; Teo, Y.-Y.; Garcia, P.J. Diagnostics for COVID-19: Moving from pandemic response to control. Lancet 2022, 399, 757–768. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, R.; Hu, X.; Wang, X. The current status and future prospects of CRISPR-based detection of monkeypox virus: A review. Anal. Chim. Acta 2025, 1336, 343295. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).