Specific Features of Immune Response in Patients with Different Asthma Endotypes Following Immunization with a Conjugate Pneumococcal Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Conditions
2.3. Medical Intervention
2.4. Key Study Outcomes and Outcome Documentation
2.5. Ethical Review
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GINA. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2024. Available online: https://ginasthma.org/2024-report/ (accessed on 13 February 2025).
- Murdoch, J.R.; Lloyd, C.M. Chronic inflammation and asthma. Mutat. Res. 2010, 690, 24–39. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. Asthma: The importance of dysregulated barrier immunity. Eur. J. Immunol. 2013, 43, 3125–3137. [Google Scholar] [CrossRef]
- Hammad, H.; Lambrecht, B.N. The basic immunology of asthma. Cell 2021, 184, 1469–1485. [Google Scholar] [CrossRef]
- Samitas, K.; Zervas, E.; Gaga, M. T2-low asthma: Current approach to diagnosis and therapy. Curr. Opin. Pulm. Med. 2017, 23, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bhakta, N.R.; Woodruff, P.G. Human asthma phenotypes: From the clinic, to cytokines, and back again. Immunol. Rev. 2011, 242, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Kuruvilla, M.E.; Lee, F.E.H.; Lee, G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clinic. Rev. Allerg. Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Kraposhina, A.Y.; Sobko, E.A.; Demko, I.V.; Katser, A.B.; Kazmerchuk, O.V.; Abramov, Y.I. Modern Understanding of Severe Bronchial Asthma. Russ. Arch. Intern. Med. 2022, 12, 113–122. [Google Scholar] [CrossRef]
- Fitzpatrick, A.M.; Chipps, B.E.; Holguin, F.; Woodruff, P.G. T2-“low” asthma: Overview and management strategies. J. Allergy Clin. Immunol. Pract. 2020, 8, 452–463. [Google Scholar] [CrossRef]
- Pesek, R.; Lockey, R. Vaccination of adults with asthma and COPD. Allergy 2011, 66, 25–31. [Google Scholar] [CrossRef]
- Lee, T.A.; Weaver, F.M.; Weiss, K.B. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. J. Gen. Intern. Med. 2007, 22, 62–67. [Google Scholar] [CrossRef]
- Andreeva, N.P.; Protasov, A.V.; Kostinova, T.A.; Lezhenina, S.V. Effect of Vaccination against Pneumococcal Infection and Influenza on the Clinical Course of Bronchial Asthma. Epidemiol. Vaccinal. Prev. 2019, 18, 93–100. [Google Scholar] [CrossRef]
- Markelova, E.V.; Gushchina, Y.S.; Kostinov, M.P.; Zhuravleva, N.V. Clinical and immunological effect produced by vaccination with «Pneumo 23» of children with atopic bronchial asthma. J. Microbiol. Epidemiol. Immunobiol. 2005, 2, 83–85. [Google Scholar]
- Kim, B.G.; Ghosh, P.; Ahn, S.; Rhee, D.K. Pneumococcal pep27 mutant immunization suppresses allergic asthma in mice. Biochem. Biophys. Res. Commun. 2019, 514, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.N.; O’Sullivan, B.J.; Thomas, R.; Kumar, R.K.; Foster, P.S.; Gibson, P.G.; Hansbro, P.M. Pneumococcal conjugate vaccine-induced regulatory T cells suppress the development of allergic airways disease. Thorax 2010, 65, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, H.; Yang, T.; Yang, B.; Jiang, X.; Wang, L.; Fu, Z. Infant 7-valent pneumococcal conjugate vaccine immunization alters young adulthood CD4+ T cell subsets in allergic airway disease mouse model. Vaccine 2014, 32, 2079–2085. [Google Scholar] [CrossRef]
- Protasov, A.D.; Zhestkov, A.V.; Kostinov, M.P.; Zolotarev, P.N.; Tezikov, Y.V. The Method of Forming Immunological Memory for Antigens Streptococcus pneumoniae in Patients with Chronic Obstructive Pulmonary Disease. Patent for invention No. RU2544168C1, 17 January 2014. [Google Scholar]
- Drapkina, O. Brief Algorithms of Management of Patients at the Stage of Providing Primary Healthcare. In Manual for Therapists; Vidox: Moscow, Russia, 2019; 20p. [Google Scholar]
- Chuchalin, A.; Alexandrovsky, Y.; Ametov, A.; Apolikhin, O.; Belousov, Y.; Bogatyrev, V. Federal Guidelines for the Use of Drugs (Formulary System); Echo: Moscow, Russia, 2015; 1016p, Issue XVIII. [Google Scholar]
- Avdeev, S.N.; Alyeva, M.H.; Baranov, A.A.; Bikmieva, A.V.; Briko, N.I.; Bulgakova, V.A.; Vishneva, E.A.; Gorelov, A.V.; Demko, I.V.; Dobrynina, E.A.; et al. Federal Clinical Guidelines on Vaccination of pneumococcal infection in children and adults. Russ. J. Prev. Med. 2023, 26, 3–23. [Google Scholar] [CrossRef]
- Maison, N.; Omony, J.; Illi, S.; Thiele, D.; Skevaki, C.; Dittrich, A.; Bahmer, T.; Rabe, K.F.; Weckmann, M.; Happle, C.; et al. T2-high asthma phenotypes across lifespan. Eur. Respir. J. 2022, 60, e2102288. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Sprio, A.E.; Baroso, A.; Gallo, F.; Riccardi, E.; Bertolini, F.; Carriero, V.; Arrigo, E.; Ciprandi, G. Characterization of T2-Low and T2-High Asthma Phenotypes in Real-Life. Biomedicines 2021, 9, 1684. [Google Scholar] [CrossRef]
- Akar-Ghibril, N.; Casale, T.; Custovic, A.; Phipatanakul, W. Allergic endotypes and phenotypes of asthma. J. Allergy Clin. Immunol. Pract. 2020, 8, 429–440. [Google Scholar] [CrossRef]
- Ansaldi, F.; Turello, V.; Lai, P.; Bastone, G.; De Luca, S.; Rosselli, R.; Durando, P.; Sticchi, L.; Gasparini, R.; Delfino, E.; et al. Effectiveness of a 23-valent polysaccharide vaccine in preventing pneumonia and non-invasive pneumococcal infection in elderly people: A large-scale retrospective cohort study. J. Int. Med. Res. 2005, 33, 490–500. [Google Scholar] [CrossRef]
- Karakaş, T.; Şahiner, Ü.M.; Soyer, Ö.U.; Civelek, E.; Cokugras, H.; Şekerel, B.E. Paediatricians’ perspectives on the use of pneumococcal vaccine in healthy and asthmatic children. Allergol. Immunopathol. 2010, 38, 241–245. [Google Scholar] [CrossRef]
- Fletcher, M.A.; Schmoele-Thoma, B.; Vojicic, J.; Daigle, D.; Paradiso, P.R.; del Carmen Morales, G. Adult indication 13-valent pneumococcal conjugate vaccine clinical development overview: Formulation, safety, immunogenicity (dosing and sequence), coadministration, and efficacy. Expert Rev. Vaccines 2024, 23, 944–957. [Google Scholar] [CrossRef]
- Marra, F.; Khatri Vadlamudi, N. Efficacy and Safety of the Pneumococcal Conjugate-13 Valent Vaccine in Adults. Aging Dis. 2019, 10, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Kostinov, M.P.; Protasov, A.D.; Zhestkov, A.V.; Polishchuk, V.B. Perspective data of application of pneumococcal 13-valent conjugated vaccine in adult patients with chronic bronchopulmonary pathology. Pul’monologiya 2014, 4, 57–63. [Google Scholar] [CrossRef]
- Zhao, H.; Jung, J.A.; Briles, D.E.; Kita, H.; Tsigrelis, C.; Juhn, Y.J. Asthma and antibodies to pneumococcal virulence proteins. Infection 2013, 41, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.A.; Hirohito, K.; Ravneet, D.; Robert, M.J.; Moon, H.N.; Miguel, P.; Constantine, T.; Young, J.J. Influence of asthma status on serotype-specific pneumococcal antibody levels. Postgrad. Med. 2010, 122, 116–124. [Google Scholar] [CrossRef]
- Khan, A.Q.; Shen, Y.; Wu, Z.Q.; Wynn, T.A.; Snapper, C.M. Endogenous pro- and anti-inflammatory cytokines differentially regulate an in vivo humoral response to Streptococcus pneumoniae. Infect. Immun. 2002, 70, 749–761. [Google Scholar] [CrossRef]
- Kyriakopoulos, C.; Gogali, A.; Bartziokas, K.; Kostikas, K. Identification and treatment of T2-low asthma in the era of biologics. ERJ Open Res. 2021, 7, 00309–2020. [Google Scholar] [CrossRef]
- Kostinov, M.P.; Protasov, A.D.; Zhetkov, A.V.; Shteyner, M.L.; Tezikov, Y.V.; Lipatov, I.S. The Effect of Different Regimens of Vaccination against Pneumococcal Infection on the Clinical Course of Chronic Obstructive Pulmonary Disease: Focus on Changes in Sputum Microorganism Population. Tuberc. Lung Dis. 2021, 99, 7–17. [Google Scholar] [CrossRef]
- Protasov, A.D.; Kostinov, M.P.; Zhestkov, A.V.; Gorbachev, D.O.; Kostinov, A.M.; Elner, M.E.; Kozina, T.A. Changes in Sputum Microbiocenosis and Clinical Pattern Under Different Vaccination Protocols for Pneumococcal Infection in Patients with Bronchial Asthma. Glob. J. Respir. Care 2022, 8, 18–27. [Google Scholar] [CrossRef]
- Preston, J.A.; Essilfie, A.T.; Horvat, J.C.; Wade, M.A.; Beagley, K.W.; Gibson, P.G.; Foster, P.S.; Hansbro, P.M. Inhibition of allergic airways disease by immunomodulatory therapy with whole killed Streptococcus pneumoniae. Vaccine 2007, 25, 8154–8162. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Foster, P.S.; Gibson, P.G.; Hansbro, P.M. Components of Streptococcus pneumoniae suppress allergic airways disease and NKT cells by inducing regulatory T cells. J. Immunol. 2012, 188, 4611–4620. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, F.D.; Hogan, S.P.; Hershey, G.K.K.; Rothenberg, M.E.; Wills-Karp, M. Importance of cytokines in murine allergic airway disease and human asthma. J. Immunol. 2010, 184, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Raundhal, M.; Morse, C.; Khare, A.; Oriss, T.B.; Milosevic, J.; Trudeau, J.; Huff, R.; Pilewski, J.; Holguin, F.; Kolls, J.; et al. High IFN-γ and low SLPI mark severe asthma in mice and humans. J. Clin. Investig. 2015, 125, 3037–3050. [Google Scholar] [CrossRef] [PubMed]
- Zuercher, A.W.; Imboden, M.A.; Jampen, S.; Bosse, D.; Ulrich, M.; Chtioui, H.; Lauter-burg, B.H.; Lang, A.B. Cellular immunity in healthy volunteers treated with an octavalent conjugate Pseudomonas aeruginosa vaccine. Clin. Exp. Immunol. 2006, 143, 132–138. [Google Scholar] [CrossRef]
- Pleguezuelos, O.; Robinson, S.; Fernández, A.; Stoloff, G.A.; Mann, A.; Gilbert, A.; Balaratnam, G.; Wilkinson, T.; Lambkin-Williams, R.; Oxford, J.; et al. A Synthetic Influenza Virus Vaccine Induces a Cellular Immune Response That Correlates with Reduction in Symptomatology and Virus Shedding in a Randomized Phase Ib Live-Virus Challenge in Humans. Clin. Vaccine. Immunol. 2015, 22, 828–835. [Google Scholar] [CrossRef]
- Ogawa, Y.; Duru, E.A.; Ameredes, B.T. Role of IL-10 in the resolution of airway inflammation. Curr. Mol. Med. 2010, 8, 437–445. [Google Scholar] [CrossRef]

| Parameters | Values |
|---|---|
| Number of patients, abs. | 31 |
| Asthma duration, years; median (Q1–Q3) | 7.0 (3–20) |
| Number of exacerbations per patient in the last 12 months; mean (standard deviation) | 2.3 (2.3) |
| Number of hospitalizations per patient in the last 12 months; mean (standard deviation) | 0.7 (1.1) |
| Asthma severity; abs. (%): | |
| 12 (38.7) |
| 13 (41.9) |
| 6 (19.4) |
| Level of asthma control; abs. (%): | |
| 10 (32.2) |
| 2 (6.5) |
| 19 (61.3) |
| Personal history of atopic diseases; abs. (%) | 10 (32.3) |
| Asthma endotype; abs (%): | |
| 14 (45.2) |
| 17 (54.8) |
| Daily dose of ICS; abs (%): | |
| 5 (16.1) |
| 9 (29.0) |
| 17 (54.8) |
| 0 (0.0) |
| Parameters | Time Periods | Changes | ||
|---|---|---|---|---|
| 12 Months Before Vaccination | 12 Months After Vaccination | |||
| Number of patients with exacerbations; abs. (%) | T2-high asthma | 12 (85.7) | 3 (21.4) | ↓ (75.0) p < 0.001 |
| T2-low asthma | 15 (88.2) | 2 (11.8) | ↓ (86.7) p < 0.001 | |
| Total | 27 (87.1) | 5 (16.1) | ↓ 22 ↓ (81.5) p < 0.001 | |
| Number of patients free from hospitalization; abs. (%) | T2-high asthma | 9 (64.3) | 14 (100.0) | ↑ (55.6) p < 0.001 |
| T2-low asthma | 8 (47.1) | 16 (94.1) | ↑ (100.0) p < 0.001 | |
| Total | 17 (54.8) | 30 (96.8) | ↑ 13 ↑ (76.5) p < 0.001 | |
| Number of patients with controlled asthma; abs. (%) | T2-high asthma | 8 (57.1) | 7 (50.0) | ↓ (12.5) |
| T2-low asthma | 2 (11.8) | 8 (47.1) | ↑ (300.0) p < 0.05 | |
| Total | 10 (32.3) | 15 (48.4) | ↑ 5 ↑ (50.0) | |
| Number of patients on low daily doses of ICS (or free of ICS) at the end of the follow-up period; abs. (%) | T2-high asthma | 5 (35.7) | 12 (85.7) | ↑ 7 ↑ (140.0) p < 0.05 |
| T2-low asthma | 9 (52.9) | 11 (64.7) | ↑ 2 ↑ (22.2) | |
| Total | 14 (45.2) | 23 (74.2) | ↑ 9 ↑ (64.3) p < 0.05 | |
| Mean FEV1 at the end of the follow-up period; (SD) [min; median; max] | T2-high asthma | 73.41 (15.38) [38.10; 79.50; 88.90] | 77.64 (14.10) [57.00; 79.55; 96.20] | ↑ (5.8) |
| T2-low asthma | 70.51 (17.17) [31.60; 74.00; 92.80] | 77.12 (15.87) [40.90; 83.90; 95.60] | ↑ (9.4) p < 0.01 | |
| Total | 71.82 (16.19) [31.60; 74.90; 92.80] | 77.35 (14.85) [40.90; 82.70; 96.20] | ↑ (7.7) p < 0.01 | |
| Mean ACQ-5 score at the end of the follow-up period; (SD) [min; median; max] | T2-high asthma | 1.10 (1.06) [0.00; 0.60; 3.20] | 0.73 (0.52) [0.00; 0.70; 1.80] | ↓ (33.6) |
| T2-low asthma | 2.18 (1.12) [0.00; 2.00; 4.60] | 1.12 (1.20) [0.00; 0.80; 4.20] | ↓ (48.6) p < 0.01 | |
| Total | 1.69 (1.21) [0.00; 1.80; 4.60] | 0.94 (0.96) [0.00; 0.80; 4.20] | ↓ (44.4) p < 0.01 | |
| Cytokines | Time Points | |||
|---|---|---|---|---|
| Baseline | 6 Weeks | 6 Months | 12 Months | |
| IL-4 | 1.33 [0.00; 2.87] | 1.18 [0.50; 2.26] | 1.56 [0.09; 2.53] | 0.78 [0.00; 1.87] |
| IL-6 | 1.44 [0.82; 2.62] | 2.05 [0.88; 3.52] | 1.52 [0.80; 2.82] | 1.33 [0.47; 2.25] |
| IL-8 | 5.93 [4.84; 8.67] | 6.63 [5.19; 9.20] | 6.87 [4.90; 8.37] | 6.01 [2.69; 7.94] |
| IL-10 | 1.46 [0.51; 2.68] | 1.08 [0.16; 3.38] | 1.70 [0.09; 3.54] | 0.57 [0.00; 2.57] |
| IL-18 | 155.50 [99.30; 248.00] | 210.72 (124.35) [0.00; 178.50; 525.00] | 182.06 (108.63) [4.63; 167.00; 461.00] | 135.00 [87.40; 216.00] |
| IFN-γ | 0.00 [0.00; 1.24] | 0.15 * [0.00; 2.62] | 0.00 [0.00; 1.15] | 0.00 [0.00; 0.97] |
| TNF-α | 5.23 [3.61; 6.94] | 5.50 [3.09; 7.11] | 5.48 [5.04; 6.91] | 6.02 [5.04; 7.48] |
| MCP-1 | 144.00 [84.10; 185.00] | 167.39 (89.52) [4.40; 180.00; 361.00] | 161.50 [71.40; 193.00] | 162.62 (132.87) [0.00; 160.00; 519.00] |
| Parameters | Time Points | ||||
|---|---|---|---|---|---|
| Baseline | 6 Weeks | 6 Months | 12 Months | p Value | |
| Asthma endotype | |||||
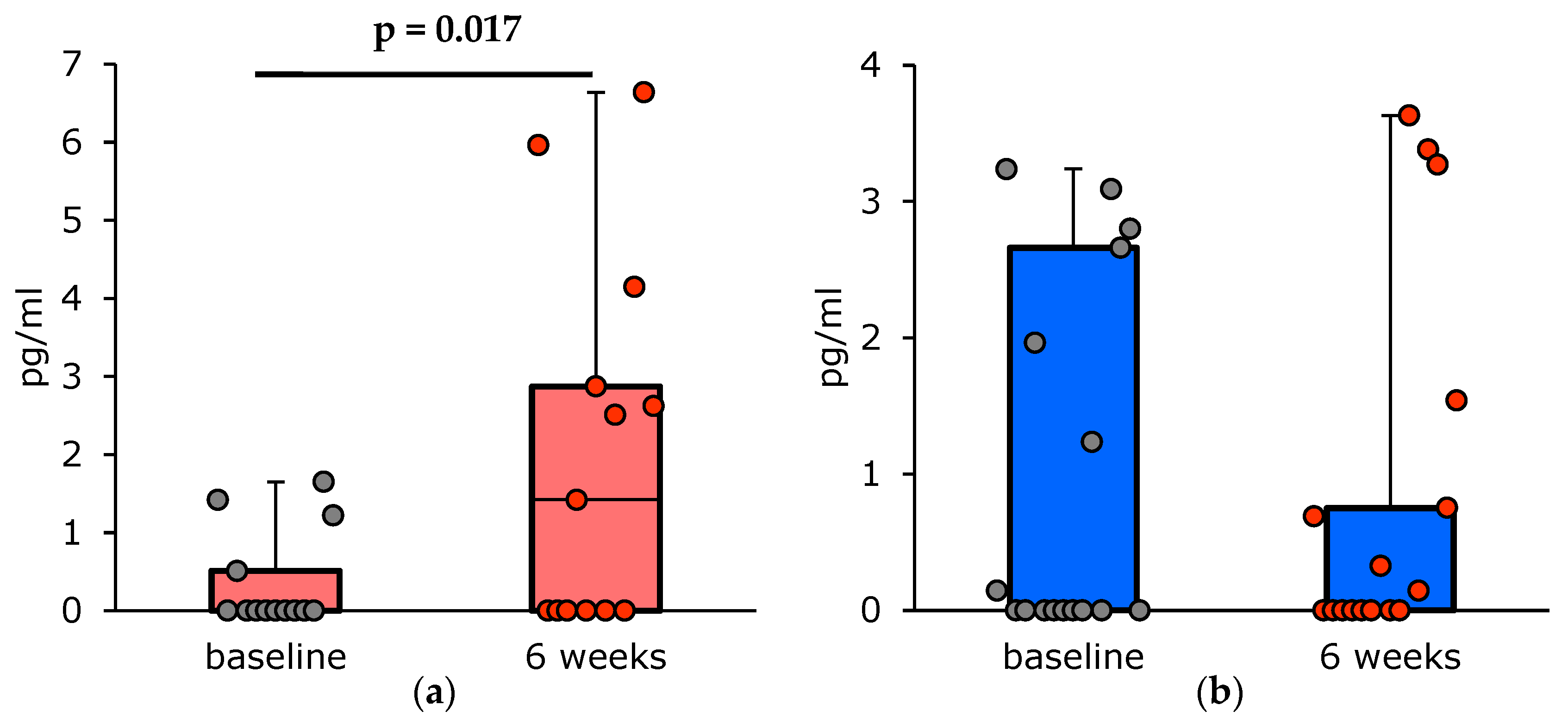
| T2-high asthma | 0.00 [0.00; 0.51] | 1.42 [0.00; 2.87] | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.51] | 0.003 |
| T2-low asthma | 0.00 [0.00; 2.66] | 0.00 [0.00; 0.75] | 0.00 [0.00; 2.41] | 0.00 [0.00; 2.33] | 0.480 |
| History of atopic disorders | |||||
| No | 0.00 [0.00; 1.60] | 0.00 [0.00; 1.15] | 0.00 [0.00; 2.41] | 0.00 [0.00; 1.20] | 0.589 |
| Yes | 0.00 [0.00; 1.22] | 2.52 (2.40) [0.00; 2.51; 6.64] | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.69] | 0.002 |
| Level of total IgE at visit 1 (baseline) | |||||
| Normal | 0.00 [0.00; 1.24] | 0.24 [0.00; 2.51] | 0.00 [0.00; 2.30] | 0.00 [0.00; 1.26] | 0.154 |
| Increased | 0.00 [0.00; 1.42] | 0.00 [0.00; 4.15] | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.51] | 0.025 |
| Parameters | Time Points | ||||
|---|---|---|---|---|---|
| Baseline | 6 Weeks | 6 Months | 12 Months | p Value | |
| Asthma severity | |||||
| mild | 0.00 [0.00; 1.23] | 0.78 [0.00; 3.51] | 0.00 [0.00; 2.30] | 0.00 [0.00; 1.79] | 0.011 |
| moderate | 0.00 [0.00; 1.96] | 0.00 [0.00; 0.75] | 0.00 [0.00; 2.69] | 0.00 [0.00; 1.14] | 0.963 |
| severe | 0.00 [0.00; 0.15] | 1.56 (1.49) [0.00; 1.60; 3.27] | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.69] | 0.036 |
| Changes in the number of exacerbations (compared to the 12 months before the vaccination) | |||||
| decreased | 0.00 [0.00; 1.42] | 0.51 [0.00; 2.87] | 0.00 [0.00; 2.30] | 0.00 [0.00; 1.26] | 0.006 |
| unchanged | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.00] | – |
| increased | – | – | – | – | – |
| Changes in the number of hospitalizations (compared to the 12 months before the vaccination) | |||||
| decreased | 0.00 [0.00; 0.00] | 0.15 [0.00; 1.54] | 0.00 [0.00; 0.00] | 0.00 [0.00; 0.97] | 0.432 |
| unchanged | 0.07 [0.00; 1.13] | 0.16 [0.00; 3.51] | 0.00 [0.00; 2.30] | 0.00 [0.00; 0.88] | 0.007 |
| increased | – | – | – | – | – |
| Parameters | Time Points | |
|---|---|---|
| 6 Weeks | 6 Months | |
| Asthma endotype | ||
| T2-high asthma | 2.12 (2.00) [0.00; 1.61; 5.49] | 2.86 (2.61) [0.00; 2.55; 7.60] |
| T2-low asthma | 0.61 [0.00; 2.88] | 1.05 [0.03; 3.54] |
| p value | 0.430 | 0.196 |
| History of atopic disorders | ||
| No | 1.08 [0.20; 4.05] | 1.42 (1.75) [0.00; 0.53; 5.05] |
| Yes | 0.71 [0.00; 1.67] | 0.53 [0.09; 2.87] |
| p value | 0.391 | 0.302 |
| Level of total IgE at visit 1 (baseline) | ||
| Normal | 0.88 [0.00; 2.88] | 0.56 [0.03; 3.32] |
| Increased | 2.27 (2.02) [0.00; 1.67; 5.49] | 3.58 (2.35) [0.16; 3.49; 7.50] |
| p value | 0.282 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostinov, A.M.; Konishcheva, A.Y.; Protasov, A.D.; Kostinov, M.P.; Polishchuk, V.B.; Zhestkov, A.V.; Yastrebova, N.E.; Kostinova, A.M.; Musagulova, Z.S.; Prutskova, E.V. Specific Features of Immune Response in Patients with Different Asthma Endotypes Following Immunization with a Conjugate Pneumococcal Vaccine. Vaccines 2025, 13, 459. https://doi.org/10.3390/vaccines13050459
Kostinov AM, Konishcheva AY, Protasov AD, Kostinov MP, Polishchuk VB, Zhestkov AV, Yastrebova NE, Kostinova AM, Musagulova ZS, Prutskova EV. Specific Features of Immune Response in Patients with Different Asthma Endotypes Following Immunization with a Conjugate Pneumococcal Vaccine. Vaccines. 2025; 13(5):459. https://doi.org/10.3390/vaccines13050459
Chicago/Turabian StyleKostinov, Anton M., Anna Yu. Konishcheva, Andrey D. Protasov, Mikhail P. Kostinov, Valentina B. Polishchuk, Alexander V. Zhestkov, Natalia E. Yastrebova, Aristitsa M. Kostinova, Zhanar Sh. Musagulova, and Ekaterina V. Prutskova. 2025. "Specific Features of Immune Response in Patients with Different Asthma Endotypes Following Immunization with a Conjugate Pneumococcal Vaccine" Vaccines 13, no. 5: 459. https://doi.org/10.3390/vaccines13050459
APA StyleKostinov, A. M., Konishcheva, A. Y., Protasov, A. D., Kostinov, M. P., Polishchuk, V. B., Zhestkov, A. V., Yastrebova, N. E., Kostinova, A. M., Musagulova, Z. S., & Prutskova, E. V. (2025). Specific Features of Immune Response in Patients with Different Asthma Endotypes Following Immunization with a Conjugate Pneumococcal Vaccine. Vaccines, 13(5), 459. https://doi.org/10.3390/vaccines13050459






