Strategies, Barriers, and Facilitators for Healthcare Professionals to Recommend HPV Vaccination: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Extraction
2.4. Quality and Risk of Bias Assessment
2.5. Synthesis of Results
3. Results
3.1. Identification of Studies
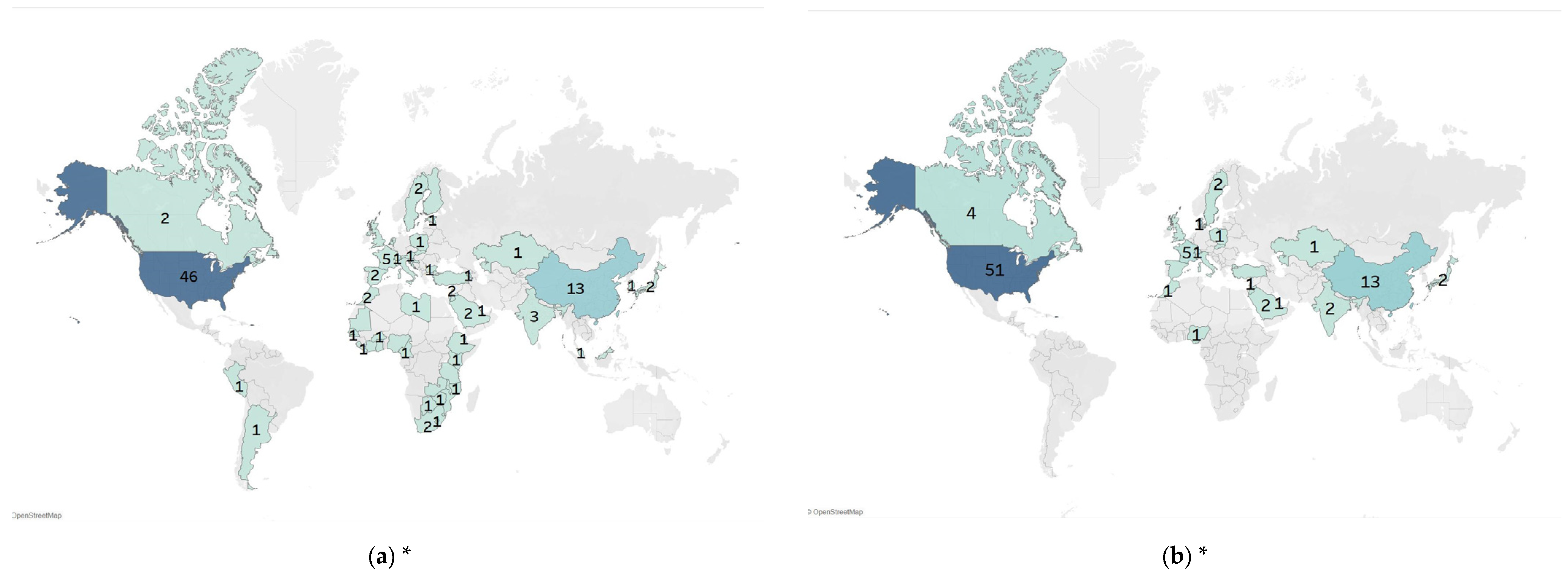
3.2. Characteristics of Included Studies
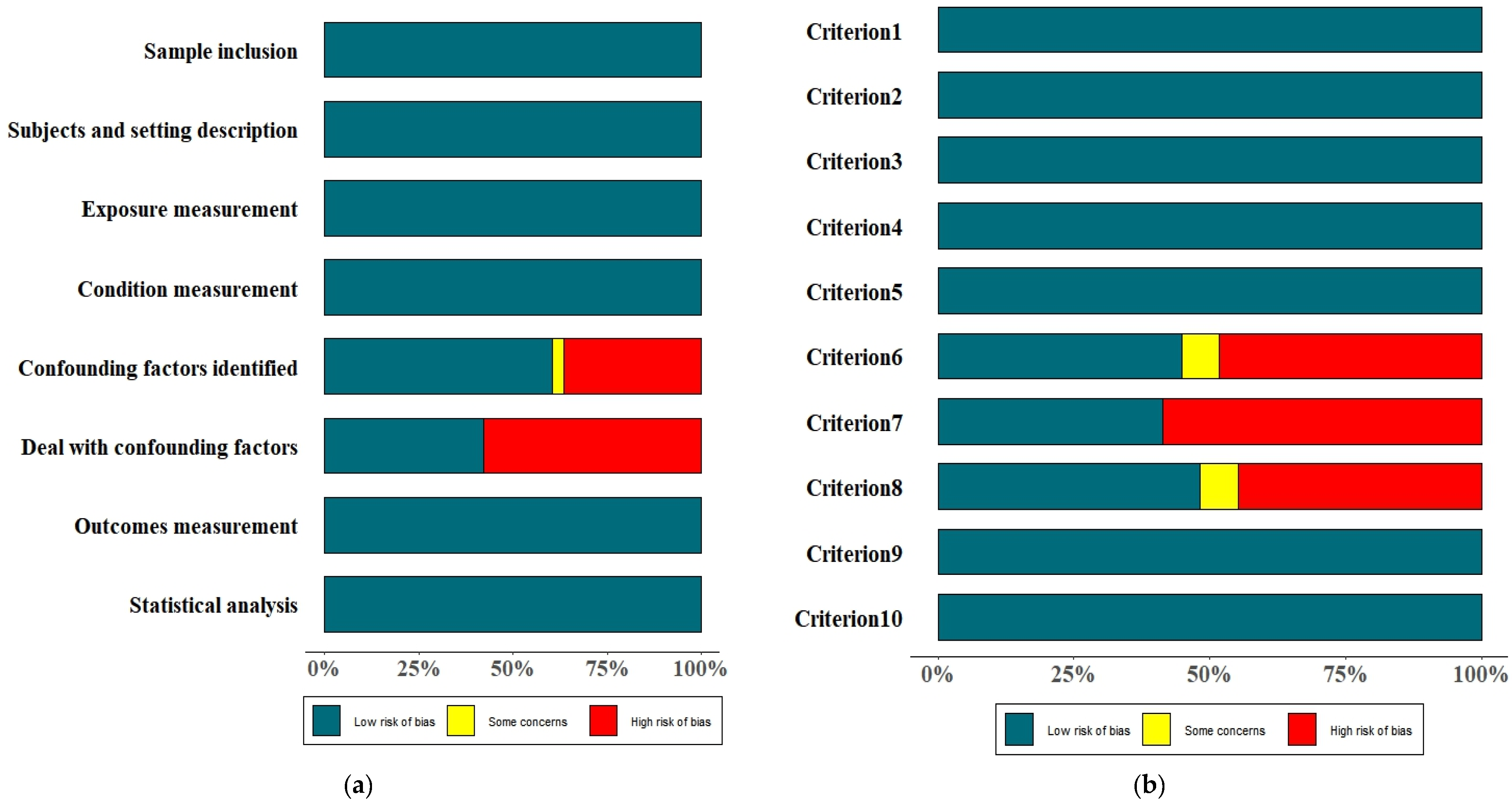
3.3. Quality Assessment
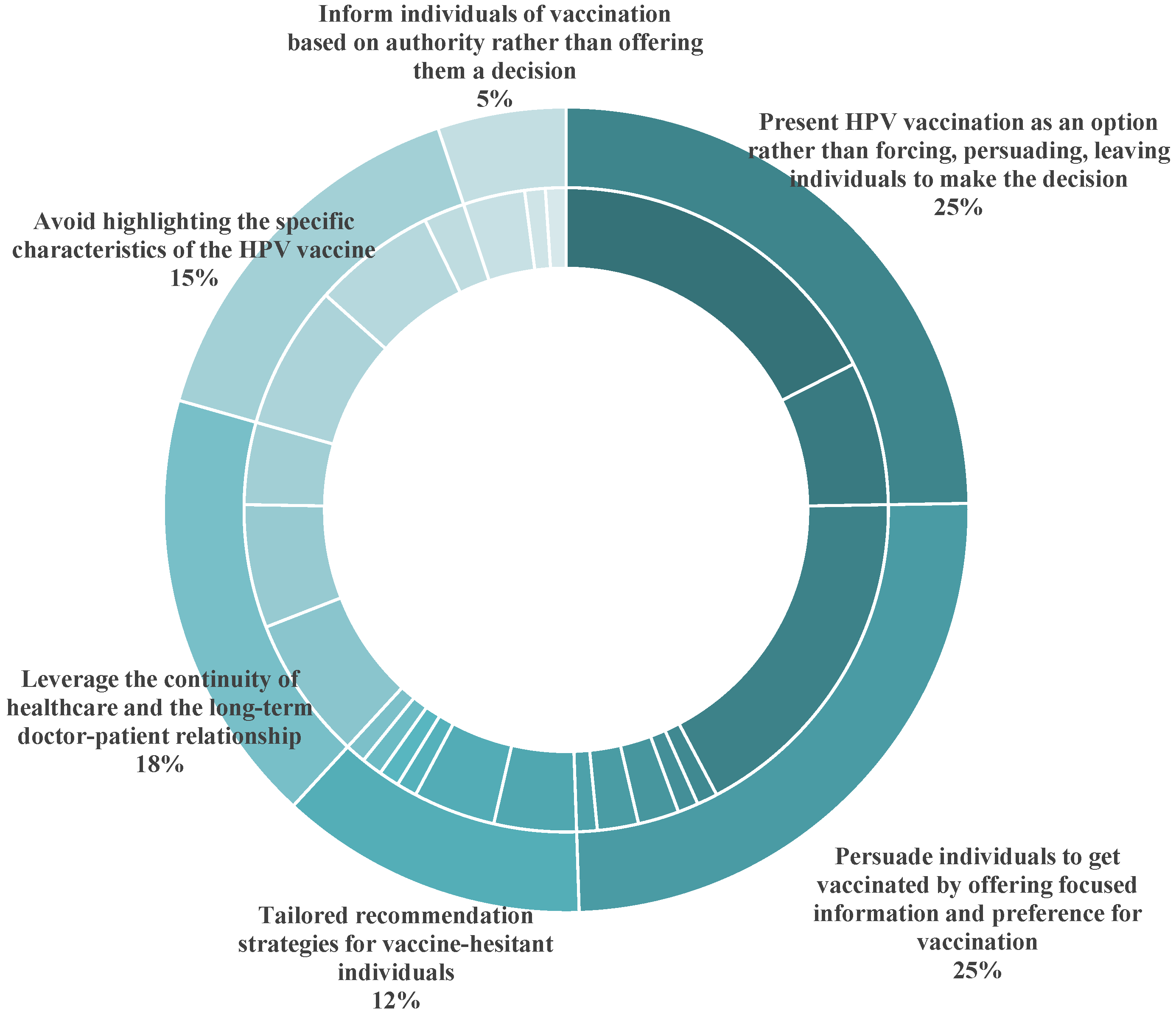
3.4. Strategies Used by Healthcare Professionals to Recommend HPV Vaccination
3.5. Willingness of Healthcare Professionals to Recommend HPV Vaccination
3.5.1. Practices of International Healthcare Professionals
3.5.2. Practices of Chinese Healthcare Professionals
3.5.3. Differences of Willingness to Recommend HPV Vaccination
4. Discussion
4.1. Strategies of Healthcare Professionals for Recommending HPV Vaccination and Associated Factors Were Different in HICs and LMICs
4.2. Willingness of Healthcare Professionals to Recommend HPV Vaccination and Associated Factors Were Different in HICs and LMICs
4.3. Policy Suggestions
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPV | Human papilloma virus |
| WHO | World Health Organization |
| HICs | High-income countries |
| LMICs | Low- and middle-income countries |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-Analyses |
| NIP | National Immunization Program |
| JBI | Joanna Briggs Institute |
| ACIP | Advisory Committee on Immunization Practices |
| CHIP | Children’s Health Insurance Program |
| ACA | The 2010 Patient Protection and Affordable Care Act |
| GPs | General practitioners |
| Gavi | Gavi, the Vaccine Alliance |
| MICs | Middle-income countries |
| LICs | Low-income countries |
| UNICEF | The United Nations Children’s Fund |
| PAHO | Pan American Health Organization |
References
- Bansal, A.; Singh, M.P.; Rai, B. Human papillomavirus-associated cancers: A growing global problem. Int. J. Appl. Basic. Med. Res. 2016, 6, 84–89. [Google Scholar] [PubMed]
- World Health Organization. Human Papillomavirus and Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/human-papilloma-virus-and-cancer (accessed on 9 April 2025).
- Näsman, A.; Du, J.; Dalianis, T. A global epidemic increase of an HPV-induced tonsil and tongue base cancer—Potential benefit from a pan-gender use of HPV vaccine. J. Intern. Med. 2020, 287, 134–152. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018: A worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- World Health Organization. A Cervical Cancer-Free Future: First-Ever Global Commitment to Eliminate a Cancer. Available online: https://www.who.int/news/item/17-11-2020-a-cervical-cancer-free-future-first-ever-global-commitment-to-eliminate-a-cancer (accessed on 9 April 2025).
- World Health Organization. Introduction of HPV (Human Papilloma Virus) Vaccine. Available online: https://immunizationdata.who.int/global/wiise-detail-page/introduction-of-hpv-(human-papilloma-virus)-vaccine?ISO_3_CODE=&YEAR= (accessed on 9 April 2025).
- Wang, H.; Wang, Y.; Wang, Z.; Wang, Y.; Sun, X.; Xu, D.; Li, M.; Li, Y.; Li, Z.; Yu, W.; et al. Expert consensus on immunoprophylaxis of human papillomavirus-related diseases (abridged). Chin. J. Vaccines Immun. 2019, 25, 718–735. [Google Scholar]
- World Health Organization. Immunization Coverage. Available online: https://www.who.int/zh/news-room/fact-sheets/detail/immunization-coverage (accessed on 9 April 2025).
- MacDonald, N.E. Vaccine hesitancy: Definition, scope and determinants. Vaccine 2015, 33, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ten Threats to Global Health in 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 9 April 2025).
- World Health Organization. Global Vaccine Market Report 2024. Available online: https://www.who.int/publications/i/item/B09198 (accessed on 9 April 2025).
- Bakare, D.; Gobbo, E.; Akinsola, K.O.; Bakare, A.A.; Salako, J.; Hanson, C.; Herzig van Wees, S.; Falade, A.; King, C. Healthcare worker practices for HPV vaccine recommendation: A systematic review and meta-analysis. Hum. Vaccines Immunother. 2024, 20, 2402122. [Google Scholar] [CrossRef]
- Thanasa, E.; Thanasa, A.; Kamaretsos, E.; Paraoulakis, I.; Balafa, K.; Gerokostas, E.E.; Kontogeorgis, G.; Koutalia, N.; Stamouli, D.; Grapsidi, V.; et al. Awareness Regarding Human Papilloma Virus Among Health Professionals and Will to Accept Vaccination: A Systematic Review. Cureus 2022, 14, e30855. [Google Scholar] [CrossRef]
- Yan, H.; Su, Z.; Liu, S.; Fan, J.; Qiao, Y. Knowledge and attitude toward HPV vaccination promotion after COVID-19 epidemic in various populations in China. Chin. J. Public. Health 2021, 37, 1731–1736. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. Chapter 7: Systematic reviews of etiology and risk. JBI Man. Evid. Synthesis. 2020, 57, 444–465. [Google Scholar]
- Lockwood, C.; Munn, Z.; Porritt, K. Qualitative research synthesis: Methodological guidance for systematic reviewers utilizing meta-aggregation. Int. J. Evid. Based Healthc. 2015, 13, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wang, S.; Zou, X.; Jia, X.; Tong, C.; Yin, J.; Lian, X.; Qiao, Y. Parental willingness of HPV vaccination in Mainland China: A meta-analysis. Hum. Vaccin. Immunother. 2024, 20, 2314381. [Google Scholar] [CrossRef]
- Kong, W.Y.; Bustamante, G.; Pallotto, I.K.; Margolis, M.A.; Carlson, R.; McRee, A.-L.; Gilkey, M.B. Disparities in Healthcare Providers’ Recommendation of HPV Vaccination for US Adolescents: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1981–1992. [Google Scholar] [CrossRef]
- Rosen, B.L.; Shepard, A.; Kahn, J.A. US Health Care Clinicians’ Knowledge, Attitudes, and Practices Regarding Human Papillomavirus Vaccination: A Qualitative Systematic Review. Acad. Pediatr. 2018, 18, S53–S65. [Google Scholar] [CrossRef]
- Beavis, A.L.; Krishnamoorthi, M.S.; Adler, S.; Fleszar, L.G.; Moran, M.B.; Rositch, A.F. Contemporary provider perspectives on how to address HPV vaccine hesitancy in the US: A qualitative study. Vaccine X 2024, 20, 100533. [Google Scholar] [CrossRef]
- Apaydin, K.Z.; Fontenot, H.B.; Shtasel, D.L.; Mayer, K.H.; Keuroghlian, A.S. Primary Care Provider Practices and Perceptions Regarding HPV Vaccination and Anal Cancer Screening at a Boston Community Health Center. J. Community Health 2018, 43, 792–801. [Google Scholar] [CrossRef]
- Dilley, S.E.; Peral, S.; Straughn, J.M.; Scarinci, I.C. The challenge of HPV vaccination uptake and opportunities for solutions: Lessons learned from Alabama. Prev. Med. 2018, 113, 124–131. [Google Scholar] [CrossRef]
- Enskar, I.; Enskar, K.; Neveus, T.; Engstrom, A.H.; Grandahl, M. Barriers in the School-Based Pan-Gender HPV Vaccination Program in Sweden: Healthcare Providers’ Perspective. Vaccines 2023, 11, 310. [Google Scholar] [CrossRef]
- Fenton, A.T. Abandoning Medical Authority: When Medical Professionals Confront Stigmatized Adolescent Sex and the Human Papillomavirus (HPV) Vaccine. J. Health Soc. Behav. 2019, 60, 240–256. [Google Scholar] [CrossRef]
- Garcia, M.A.; Schlecht, N.F.; Rokitka, D.A.; Attwood, K.M.; Rodriguez, E.M. Examining the Barriers and Opportunities for Human Papillomavirus Vaccine Delivery in Cancer Care Settings: A Mixed-Methods Study. Cancer Prev. Res. 2023, 16, 581–590. [Google Scholar] [CrossRef]
- Grace, D.; Gaspar, M.; Rosenes, R.; Grewal, R.; Burchell, A.N.; Grennan, T.; Salit, I.E. Economic barriers, evidentiary gaps, and ethical conundrums: A qualitative study of physicians’ challenges recommending HPV vaccination to older gay, bisexual, and other men who have sex with men. Int. J. Equity Health 2019, 18, 159. [Google Scholar] [CrossRef]
- Jackson, C.; Nielsen, S.M.; Simonyan, B.; Kirakosyan, M.; Hovhannisyan, M.; Sahakyan, G.; Habersaat, K.B. Medical specialists’ attitudes and practices towards childhood vaccination: A qualitative study in Armenia. BMC Pediatr. 2022, 22, 620. [Google Scholar] [CrossRef] [PubMed]
- Kataria, I.; Siddiqui, M.; Treiman, K.; Foley, S.; Anand, M.; Biswas, S.; Shastri, D.; Bhatla, N.; Radhakrishnan, D.; Mamidi, P.; et al. Awareness, perceptions, and choices of physicians pertaining to human papillomavirus (HPV) vaccination in India: A formative research study. Vaccine X 2022, 12, 100228. [Google Scholar] [CrossRef] [PubMed]
- Lake, P.; Kasting, M.L.; Malo, T.; Giuliano, A.R.; Vadaparampil, S.T. An environmental scan to examine stakeholder perspectives on human papillomavirus vaccination: A mixed methods study. Vaccine 2019, 37, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Moya, E.M.; Garcia, A.; Joyce Ponder, A.; Frietze, G. Addressing knowledge gaps: The key role of community health workers and healthcare providers in human papillomavirus prevention and vaccine uptake in a border community. Front. Public. Health 2023, 11, 1243539. [Google Scholar] [CrossRef]
- Murciano-Gamborino, C.; Diez-Domingo, J.; Fons-Martinez, J.; Consortium, P.E. Healthcare Professionals’ Perspectives on HPV Recommendations: Themes of Interest to Different Population Groups and Strategies for Approaching Them. Vaccines 2024, 12, 748. [Google Scholar] [CrossRef]
- Richman, A.R.; Torres, E.; Wu, Q.; Eldridge, D.; Lawson, L. HPV vaccine recommendation practices of current and future physicians in North Carolina: An exploratory study. Health Educ. Res. 2022, 37, 213–226. [Google Scholar] [CrossRef]
- Runngren, E.; Eriksson, M.; Blomberg, K. Balancing Between Being Proactive and Neutral: School Nurses’ Experiences of Offering Human Papilloma Virus Vaccination to Girls. J. Sch. Nurs. Off. Publ. Natl. Assoc. Sch. Nurses 2022, 38, 270–278. [Google Scholar] [CrossRef]
- Ganeshkumar, P.; Tank, J.; Choudhury, S.S.; Acharya, V.; Gaur, Y.; Srivastava, R.; Janakiraman, R.; Ganeshkumar, A. Roadmap to Success: Illustrating Insights from a KAP Study on Cervical Cancer Prevention and HPV Vaccination. South Asian J. Cancer 2024. [Google Scholar] [CrossRef]
- Dionne, M.; Sauvageau, C.; Kiely, M.; Rathwell, M.; Bandara, T.; Neudorf, C.; Dubé, È. “The problem is not lack of information”: A qualitative study of parents and school nurses’ perceptions of barriers and potential solutions for HPV vaccination in schools. Vaccine 2023, 41, 6654–6660. [Google Scholar] [CrossRef] [PubMed]
- Garbutt, J.M.; Dodd, S.; Walling, E.; Lee, A.A.; Kulka, K.; Lobb, R. Barriers and facilitators to HPV vaccination in primary care practices: A mixed methods study using the Consolidated Framework for Implementation Research. BMC Fam. Pract. 2018, 19, 53. [Google Scholar] [CrossRef]
- Gilkey, M.B.; Grabert, B.K.; Malo, T.L.; Hall, M.E.; Brewer, N.T. Physicians’ rhetorical strategies for motivating HPV vaccination. Soc. Sci. Med. 2020, 266, 113441. [Google Scholar] [CrossRef]
- Shuto, M.; Kim, Y.; Okuyama, K.; Ouchi, K.; Ueichi, H.; Nnadi, C.; Larson, H.J.; Perez, G.; Sasaki, S. Understanding confidence in the human papillomavirus vaccine in Japan: A web-based survey of mothers, female adolescents, and healthcare professionals. Hum. Vaccines Immunother. 2021, 17, 3102–3112. [Google Scholar] [CrossRef]
- Tron, A.; Schlegel, V.; Pinot, J.; Bruel, S.; Ecollan, M.; Bel, J.L.; Rossignol, L.; Gauchet, A.; Gagneux-Brunon, A.; Mueller, J.; et al. Barriers and facilitators to the HPV vaccine: A multicenter qualitative study of French general practitioners. Arch. Public. Health 2024, 82, 2. [Google Scholar] [CrossRef]
- Biancarelli, D.L.; Drainoni, M.L.; Perkins, R.B. Provider Experience Recommending HPV Vaccination Before Age 11 Years. J. Pediatr. 2020, 217, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Bouchez, M.; Ward, J.K.; Bocquier, A.; Benamouzig, D.; Peretti-Watel, P.; Seror, V.; Verger, P. Physicians’ decision processes about the HPV vaccine: A qualitative study. Vaccine 2021, 39, 521–528. [Google Scholar] [CrossRef]
- Brennan, L.P.; Rodriguez, N.M.; Head, K.J.; Zimet, G.D.; Kasting, M.L. Obstetrician/gynecologists’ HPV vaccination recommendations among women and girls 26 and younger. Prev. Med. Rep. 2022, 27, 101772. [Google Scholar] [CrossRef]
- Hopfer, S.; Wright, M.E.; Pellman, H.; Wasserman, R.; Fiks, A.G. HPV vaccine recommendation profiles among a national network of pediatric practitioners: Understanding contributors to parental vaccine hesitancy and acceptance. Hum. Vaccines Immunother. 2019, 15, 1776–1783. [Google Scholar] [CrossRef]
- Odebunmi, O.O.; Spees, L.P.; Biddell, C.B.; Yemeke, T.; Yanguela, J.; Higgins, C.; Gilkey, M.; Ozawa, S.; Wheeler, S.B. Benefits, challenges, and strategies related to using presumptive recommendations for HPV vaccination: A qualitative study with rural and non-rural-serving primary care professionals. Hum. Vaccines Immunother. 2024, 20, 2347018. [Google Scholar] [CrossRef] [PubMed]
- Habermacher, C.; Lalloué, B.; Lamouille, V.; Thilly, N.; Agrinier, N. Family physicians’ practices and attitudes towards HPV vaccination since extension of HPV vaccination to males. Infect. Dis. Now. 2023, 53, 104669. [Google Scholar] [CrossRef]
- Hurtaud, A.; Tara, A.A.; Bouazzi, L.; Pacquelet, Y.; Boiteux-Chabrier, M.; Pham, B.N.; Pierre Cavard, H.; Barbe, C. Practices of French General Practitioners Regarding Vaccination of Boys Against Human Papillomavirus (HPV), One Year After the Application of Its Official Recommendation. J. Cancer Educ. 2024, 39, 271–278. [Google Scholar] [CrossRef]
- Darville-Sanders, G.; Reinoso, H.; MacInnes, J.; Corluyan, E.; Munroe, D.; Mathis, M.W.; Madden, S.L.; Hamrick, J.; Dickerson, L.; Gaddis, C. HPV Vaccine Communication Competency Scale for Medical Trainees: Interdisciplinary Development Study. JMIR Form. Res. 2022, 6, e38164. [Google Scholar] [CrossRef]
- Kong, W.Y.; Queen, T.L.; O’Shea, N.G.; Heisler-MacKinnon, J.; Liu, A.; Ozawa, S.; Brewer, N.T.; Gilkey, M.B. Impact of visit characteristics on intention to recommend HPV vaccine: An experiment with US health care professionals. Prev. Med. 2024, 179, 107841. [Google Scholar] [CrossRef]
- Tsui, J.; Vincent, A.; Anuforo, B.; Btoush, R.; Crabtree, B.F. Understanding primary care physician perspectives on recommending HPV vaccination and addressing vaccine hesitancy. Hum. Vaccines Immunother. 2021, 17, 1961–1967. [Google Scholar] [CrossRef]
- Grabert, B.K.; Heisler-MacKinnon, J.; Liu, A.; Margolis, M.A.; Cox, E.D.; Gilkey, M.B. Prioritizing and implementing HPV vaccination quality improvement programs in healthcare systems: The perspective of quality improvement leaders. Hum. Vaccines Immunother. 2021, 17, 3577–3586. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.W.M.; Avashia, S.; Duc, J.; Spielberg, F. What Impacts HPV Vaccination Recommendations? An Exploration of Medical Residents’ Knowledge, Training, Barriers, and Practices. Fam. Med. 2020, 52, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Kacew, A.J.; Jacobson, S.; Sheade, J.; Patel, A.A.; Hlubocky, F.J.; Lee, N.K.; Henderson, T.O.; Schneider, J.A.; Strohbehn, G.W. Provider-Level Barriers to Human Papillomavirus Vaccination in Survivors of Childhood and Young Adult Cancers. J. Adolesc. Young Adult Oncol. 2022, 11, 284–289. [Google Scholar] [CrossRef]
- Janio, E.A.; Walker, C.; Steere, E.; Seaman, A.T.; Askelson, N.; Pagedar, N.A. A Qualitative Study of Attitudes Toward HPV Vaccine Recommendation in Otolaryngology Clinics. Laryngoscope Investig. Otolaryngol. 2025, 10, e70085. [Google Scholar] [CrossRef]
- Fernandes, A.; Wang, D.; Domachowske, J.B.B.; Suryadevara, M. HPV vaccine knowledge, attitudes, and practices among New York State medical providers, dentists, and pharmacists. Hum. Vaccines Immunother. 2023, 19, 2219185. [Google Scholar] [CrossRef] [PubMed]
- Chido-Amajuoyi, O.G.; Osaghae, I.; Onyeaka, H.K.; Shete, S. Barriers to the assessment and recommendation of HPV vaccination among healthcare providers in Texas. Vaccine X 2024, 18, 100471. [Google Scholar] [CrossRef] [PubMed]
- Bozigar, M.; Faith, T.D.; White, A.A.; Drayton, K.l.D.; Fabick, A.; Cartmell, K.B. A Cross-Sectional Survey to Evaluate Potential for Partnering With School Nurses to Promote Human Papillomavirus Vaccination. Prev. Chronic Dis. 2020, 17, E111. [Google Scholar] [CrossRef]
- Alcalá, H.E.; Maxwell, G.L.; Keim-Malpass, J.; Mitchell, E.; Balkrishnan, R. Examining HPV Vaccination Practices and Differences Among Providers in Virginia. Value Health 2018, 21, S69. [Google Scholar] [CrossRef]
- Topazian, H.M.; Kundu, D.; Peebles, K.; Ramos, S.; Morgan, K.; Kim, C.J.; Richter, K.L.; Brewer, N.T.; Peris, M.; Smith, J.S. HPV Vaccination Recommendation Practices among Adolescent Health Care Providers in 5 Countries. J. Pediatr. Adolesc. Gynecol. 2018, 31, 575–582.e572. [Google Scholar] [CrossRef]
- Agyei-Baffour, P.; Asare, M.; Lanning, B.; Koranteng, A.; Millan, C.; Commeh, M.E.; Montealegre, J.R.; Mamudu, H.M. Human papillomavirus vaccination practices and perceptions among Ghanaian Healthcare Providers: A qualitative study based on multi-theory model. PLoS ONE 2020, 15, e0240657. [Google Scholar] [CrossRef]
- Waters, A.R.; Weir, C.; Kramer, H.S.; van Thiel Berghuijs, K.M.; Wu, Y.; Kepka, D.; Kirchhoff, A.C. Implementation barriers and considerations for recommending and administering the human papillomavirus (HPV) vaccination in oncology settings. J. Cancer Surviv. Res. Pract. 2024, 18, 1481–1491. [Google Scholar] [CrossRef]
- Llavall, A.C.; de Wildt, G.; Meza, G.; Tattsbridge, J.; Jones, L. Nurses’ and teachers’ perceived barriers and facilitators to the uptake of the Human Papilloma Virus (HPV) vaccination program in Iquitos, Peru: A qualitative study. PLoS ONE 2021, 16, e0255218. [Google Scholar] [CrossRef]
- Vu, M.; King, A.R.; Jang, H.M.; Bednarczyk, R.A. Practice-, provider- and patient-level facilitators of and barriers to HPV vaccine promotion and uptake in Georgia: A qualitative study of healthcare providers’ perspectives. Health Educ. Res. 2020, 35, 512–523. [Google Scholar] [CrossRef]
- Halista, C.E.; Kline, R.J.; Bepko, J. Understanding Barriers to HPV Vaccination: Perspectives From Air Force Family Medicine Physicians and Active Duty Air Force Males. Mil. Med. 2020, 185, e878–e886. [Google Scholar] [CrossRef]
- Miller, M.E.; Rahim, M.Q.; Coven, S.L.; Jacob, S.A.; Zimet, G.D.; Meagher, C.G.; Ott, M.A. Pediatric hematology and oncology physician and nurse practitioner views of the HPV vaccine and barriers to administration. Hum. Vaccines Immunother. 2023, 19, 2224089. [Google Scholar] [CrossRef] [PubMed]
- Balogun, F.M.; Omotade, O.O. Facilitators and barriers of healthcare workers’ recommendation of HPV vaccine for adolescents in Nigeria: Views through the lens of theoretical domains framework. BMC Health Serv. Res. 2022, 22, 824. [Google Scholar] [CrossRef]
- Francis, J.K.R.; Rodriguez, S.A.; Dorsey, O.; Blackwell, J.-M.; Balasubramanian, B.A.; Kale, N.; Day, P.; Preston, S.M.; Thompson, E.L.; Pruitt, S.L.; et al. Provider perspectives on communication and dismissal policies with HPV vaccine hesitant parents. Prev. Med. Rep. 2021, 24, 101562. [Google Scholar] [CrossRef] [PubMed]
- Rosen, B.L.; Rhodes, D.; Visker, J.; Cox, C.; Banez, J.C.; Lasser, B. Factors Associated with School Nurses’ and Personnel’s Professional Practice to Encourage Parents to Vaccinate Against Human Papillomavirus. J. Sch. Health 2019, 89, 569–577. [Google Scholar] [CrossRef]
- Yacouti, A.; Baddou, R.; Bourissi, H.; Ez-Zaouy, S.; Amayou, H.; Elmalki, K.; Got, A.E.; Benider, A.; Assoumou, S.Z.; Mouallif, M. Human PapillomaVirus Vaccine Uptake: Attitudes and Practices Among Moroccan Physicians. J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ. 2024, 39, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Dickson, T.; Hirko, K.A.; Ford, S. Provider Confidence and Perceived Barriers when Recommending the Human Papillomavirus Vaccine to Parents. J. Cancer Educ. Off. J. Am. Assoc. Cancer Educ. 2023, 38, 1193–1199. [Google Scholar] [CrossRef]
- Filakovska Bobakova, D.; Plavnicka, J.; Urbancikova, I.; Edelstein, M.; Jansen, D.; Dankulincova Veselska, Z. Barriers to HPV vaccination in marginalized Roma communities in Slovakia. Front. Public Health 2023, 11, 1239963. [Google Scholar] [CrossRef]
- Qaqish, A.; Abdo, N.; Abbas, M.M.; Saadeh, N.; Alkhateeb, M.; Msameh, R.; Tarawneh, S.; Al-Masri, M. Awareness and knowledge of physicians and residents on the non-sexual routes of human papilloma virus (HPV) infection and their perspectives on anti-HPV vaccination in Jordan. PLoS ONE 2023, 18, e0291643. [Google Scholar] [CrossRef]
- Della Polla, G.; Napolitano, F.; Pelullo, C.P.; De Simone, C.; Lambiase, C.; Angelillo, I.F. Investigating knowledge, attitudes, and practices regarding vaccinations of community pharmacists in Italy. Hum. Vaccin. Immunother. 2020, 16, 2422–2428. [Google Scholar] [CrossRef]
- Brewington, M.K.; Queen, T.L.; Heisler-MacKinnon, J.; Calo, W.A.; Weaver, S.; Barry, C.; Kong, W.Y.; Kennedy, K.L.; Shea, C.M.; Gilkey, M.B. Who are vaccine champions and what implementation strategies do they use to improve adolescent HPV vaccination? Findings from a national survey of primary care professionals. Implement. Sci. Commun. 2024, 5, 28. [Google Scholar] [CrossRef]
- Çataklı, T.; Duyan-çamurdan, A.; Aksakal-Baran, F.N.; Güven, A.E.; Beyazova, U. Attitudes of physicians concerning vaccines not included in the national immunization schedule. Turk. J. Pediatr. 2018, 60, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhao, Y.; Zhang, L.; Li, J.; Abdullah, A.S.; Zheng, P.; Wang, F. Frequency of health care provider recommendations for HPV vaccination: A survey in three large cities in China. Front. Public Health 2023, 11, 1203610. [Google Scholar] [CrossRef]
- Abi Jaoude, J.; Saad, H.; Farha, L.; Dagher, H.; Khair, D.; Kaafarani, M.A.; Jamaluddine, Z.; Cherfan, P. Barriers, Attitudes and Clinical Approach of Lebanese Physicians Towards HPV Vaccination; A Cross- Sectional Study. Asian Pac. J. Cancer Prev. 2019, 20, 3181–3187. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Hanley, S.; Sitnik, E.; Berry, W.; Blatt, S.; Seserman, M.; Formica, M.K. Attitudes towards HPV Vaccination Policy Strategies to Improve Adolescent Vaccination Coverage among Pediatric Providers in New York State. Vaccines 2023, 11, 1359. [Google Scholar] [CrossRef]
- Sypień, P.; Marek, W.; Zielonka, T.M. Awareness and Attitude of Polish Gynecologists and General Practitioners towards Human Papillomavirus Vaccinations. Healthcare 2023, 11, 1076. [Google Scholar] [CrossRef]
- Thaker, J.; Albers, A.N.; Newcomer, S.R. Nurses’ perceptions, experiences, and practices regarding human papillomavirus vaccination: Results from a cross-sectional survey in Montana. BMC Nurs. 2023, 22, 211. [Google Scholar] [CrossRef] [PubMed]
- Domgue, J.F.; Dille, I.; Kapambwe, S.; Yu, R.; Gnangnon, F.; Chinula, L.; Murenzi, G.; Mbatani, N.; Pande, M.; Sidibe, F.; et al. HPV vaccination in Africa in the COVID-19 era: A cross-sectional survey of healthcare providers’ knowledge, training, and recommendation practices. Front. Public Health 2024, 12, 1343064. [Google Scholar]
- Jaeger, L.; Senn, O.; Rosemann, T.; Plate, A. Awareness, Attitudes and Clinical Practices Regarding Human Papillomavirus Vaccination among General Practitioners and Pediatricians in Switzerland. Vaccines 2021, 9, 332. [Google Scholar] [CrossRef]
- Napolitano, F.; Pelullo, C.P.; Polla, G.D.; Angelillo, I.F. HPV vaccination attitudes and behaviors among general practitioners in italy. Vaccines 2021, 9, 63. [Google Scholar] [CrossRef]
- Abi Jaoude, J.; Khair, D.; Dagher, H.; Saad, H.; Cherfan, P.; Kaafarani, M.A.; Jamaluddine, Z.; Ghattas, H. Factors associated with Human Papilloma Virus (HPV) vaccine recommendation by physicians in Lebanon, a cross-sectional study. Vaccine 2018, 36, 7562–7567. [Google Scholar] [CrossRef]
- Btoush, R.; Kohler, R.K.; Carmody, D.P.; Hudson, S.V.; Tsui, J. Factors that Influence Healthcare Provider Recommendation of HPV Vaccination. Am. J. Health Promot. 2022, 36, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Schneiter, M.K.; Levinson, K.; Rositch, A.F.; Stone, R.L.; Nickles Fader, A.; Stuart Ferriss, J.; Wethington, S.L.; Beavis, A.L. Gynecologic oncology HPV vaccination practice patterns: Investigating practice barriers, knowledge gaps and opportunities for maximizing cervical cancer prevention. Gynecol. Oncol. Rep. 2022, 40, 100952. [Google Scholar] [CrossRef] [PubMed]
- Kasting, M.L.; Head, K.J.; DeMaria, A.L.; Neuman, M.K.; Russell, A.L.; Robertson, S.E.; Rouse, C.E.; Zimet, G.D. A National Survey of Obstetrician/Gynecologists’ Knowledge, Attitudes, and Beliefs Regarding Adult Human Papillomavirus Vaccination. J. Womens Health 2021, 30, 1476–1484. [Google Scholar] [CrossRef]
- Narayana, G.; Suchitra, J.; Kavya Suma, G.; Deepthi, G.N.; Divya Jyothi, C.; Pradeep Kumar, B. Physician’s Knowledge, Attitude, and Practice towards Human Papilloma Virus (HPV) Vaccine Recommendation in Anantapur District, Andhra Pradesh, India. Arch. Pharm. Pract. 2020, 11, 137–144. [Google Scholar]
- Steben, M.; Durand, N.; Guichon, J.R.; Greenwald, Z.R.; McFaul, S.; Blake, J. A National Survey of Canadian Physicians on HPV: Knowledge, Barriers, and Preventive Practices. J. Obs. Gynaecol. Can. 2019, 41, 599–607.e593. [Google Scholar] [CrossRef]
- Kara Elitok, G.; Bulbul, L.; Altuntas, S.B.; Altuntas, B.; Günindi, G.; Haltaş, M.; Yuvarlan, A.; Toprak, D.; Bulbul, A. Recommending immunizations to adolescents in Turkey: A study of the knowledge, attitude, and practices of physicians. Hum. Vaccines Immunother. 2020, 16, 1132–1138. [Google Scholar] [CrossRef]
- Sherman, S.M.; Cohen, C.R.; Denison, H.J.; Bromhead, C.; Patel, H. A survey of knowledge, attitudes and awareness of the human papillomavirus among healthcare professionals across the UK. Eur. J. Public Health 2020, 30, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Almughais, E.S.; Alfarhan, A.; Salam, M. Awareness of primary health care physicians about human papilloma virus infection and its vaccination: A cross-sectional survey from multiple clinics in Saudi Arabia. Infect. Drug Resist. 2018, 11, 2257–2267. [Google Scholar] [CrossRef]
- Alosaimi, B.; Fallatah, D.I.; Abd ElHafeez, S.; Saleeb, M.; Alshanbari, H.M.; Awadalla, M.; Ahram, M.; Khalil, M.A. Predictors of Human Papillomavirus (HPV) Vaccine Acceptability Among Physicians, Their Knowledge on Cervical Cancer, and Factors Influencing Their Decision to Recommend It. J. Multidiscip. Healthc. 2024, 17, 5177–5188. [Google Scholar] [CrossRef]
- Albayat, S.S.; Mundodan, J.M.; Elmardi, K.; Hasnain, S.; Khogali, H.; Baaboura, R.; Al-Romaihi, H.E.; AlKubaisi, N.J.; Bougmiza, M.I. Knowledge, attitude, and practices regarding human papilloma virus vaccination among physicians in Qatar. Women’s Health 2024, 20, 17455057241227360. [Google Scholar] [CrossRef]
- Dufour, L.; Carrouel, F.; Dussart, C. Human Papillomaviruses in Adolescents: Knowledge, Attitudes, and Practices of Pharmacists Regarding Virus and Vaccination in France. Viruses 2023, 15, 778. [Google Scholar] [CrossRef]
- Hurley, L.P.; O’Leary, S.T.; Markowitz, L.E.; Crane, L.A.; Cataldi, J.R.; Brtnikova, M.; Beaty, B.L.; Gorman, C.; Meites, E.; Lindley, M.C.; et al. US primary care physicians’ viewpoints on HPV vaccination for adults 27 to 45 years. J. Am. Board. Fam. Med. 2021, 34, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Kasting, M.L.; Christy, S.M.; Sutton, S.K.; Lake, P.; Malo, T.L.; Roetzheim, R.G.; Schechtman, T.; Zimet, G.D.; Walkosz, B.J.; Salmon, D.; et al. Florida physicians’ reported use of AFIX-based strategies for human papillomavirus vaccination. Prev. Med. 2018, 116, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Khamisy-Farah, R.; Adawi, M.; Jeries-Ghantous, H.; Bornstein, J.; Farah, R.; Bragazzi, N.L.; Odeh, M. Knowledge of human papillomavirus (HPV), attitudes and practices towards anti-HPV vaccination among Israeli pediatricians, gynecologists, and internal medicine doctors: Development and validation of an ad hoc questionnaire. Vaccines 2019, 7, 157. [Google Scholar] [CrossRef] [PubMed]
- Sakanishi, Y.; Takeuchi, J.; Suganaga, R.; Nakayama, K.; Nishioka, Y.; Chiba, H.; Kishi, T.; MacHino, A.; Mastumura, M.; Okada, T.; et al. Association between administration or recommendation of the human papillomavirus vaccine and primary care physicians’ knowledge about vaccination during proactive recommendation suspension: A nationwide cross-sectional study in Japan. BMJ Open 2023, 13, e074305. [Google Scholar] [CrossRef]
- Schneiter, M.; Rositch, A.; Levinson, K.; Stone, R.; Fader, A.; Ferriss, J.; Wethington, S.; Beavis, A. The gynecologic oncologist as the HPV champion: Missed opportunities for cancer prevention. Gynecol. Oncol. 2021, 162, S292–S293. [Google Scholar] [CrossRef]
- Yetik, I.; Tanoglu, F.B.; Pasin, O.; Cetin, C.; Ozcan, P. Knowledge levels and community guidance of doctors working in family health centers on HPV screening and HPV vaccination. J. Obstet. Gynaecol. Res. 2023, 49, 2519–2527. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, H.; Mao, Y.; Wang, F.; Li, J.; Zheng, P. Analysis on the influencing factors of medical workers’ willingness to recommend HPV vaccine. Fudan Univ. J. Med. Sci. 2023, 50, 40–47. [Google Scholar]
- Lin, M.; Zhang, T. Current status of knowledge, attitude and practice on human papillomavirus and its vaccine among medical professionals in Beijing. Pract. Prev. Med. 2020, 27, 734–736. [Google Scholar]
- Liang, Q.; Qin, C.; Zhou, Y.; Shen, W.; Yang, S.; Zhao, Y.; Jiang, S.; Zhang, H. Healthcare workers’ willingness to receive and recommend human papillomavirus vaccination: A cross-sectional study in Guilin city in 2023. Chin. J. Vaccines Immun. 2024, 30, 149–154. [Google Scholar]
- Ding, M.; Xu, Z.; Lei, Q.; Wang, M.; Zhang, B.; Yang, J.; Cai, Z.; Qiao, Y.; Wang, Y. HPV vaccination knowledge and recommendation behavior among healthcare workers and teachers in Ordos city: A across-sectional analysis. Chin. J. Public. Health 2024, 40, 625–631. [Google Scholar]
- Li, J.; Li, X.; Zhang, Z.; Zhao, D.; Pan, J.; Lu, L.; Wu, J. HPV vaccine-related recommendation and vaccination behavior among medical staff and attendees of immunization clinics in Beijing city. Chin. J. Public Health 2021, 37, 1737–1741. [Google Scholar]
- Chen, S.; Mei, C.; Huang, W.; Liu, P.; Wang, H.; Lin, W.; Yuan, S.; Wang, Y. Human papillomavirus vaccination related knowledge, and recommendations among healthcare providers in Southern China: A cross-sectional survey. BMC Women’s Health 2022, 22, 169. [Google Scholar] [CrossRef]
- Ren, W.; Gao, J.; Hu, Q. Influencing factors of the willingness of male nurses to HPV vaccination. Chin. J. Viral Dis. 2020, 10, 204–208. [Google Scholar]
- Tang, G.; Zhao, B.; Hu, X.; Zhao, Q.; Li, X.; Wan, Y. Knowledge, attitude and practice about human papillomavirus vaccine among female nurses working in a tertiary hospital in Hengyang city. Chin. J. Public Health 2018, 34, 1413–1416. [Google Scholar]
- Li, L.; Lv, Z.; Zhang, J.; Wang, W. Survey on awareness of HPV related knowledge and vaccine among medical workers, Hekou district, Dongying city, 2019. Prev. Med. Trib. 2019, 25, 506–509+513. [Google Scholar]
- Song, D.; Liu, P.; Wu, D.; Zhao, F.; Wang, Y.; Zhang, Y. Knowledge and Attitudes towards Human Papillomavirus Vaccination (HPV) among Healthcare Providers Involved in the Governmental Free HPV Vaccination Program in Shenzhen, Southern China. Vaccines 2023, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Wang, Z. Facilitators and barriers for healthcare providers to recommend HPV vaccination to attendees of public sexually transmitted diseases clinics in Hong Kong, China. PLoS ONE 2019, 14, e0209942. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, X.; Wang, W.; Zhang, R.; Du, M.; Shan, L.; Li, Y.; Wang, X.; Liu, Y.; Zhang, W.; et al. Knowledge of HPV, its vaccines, and attitudes toward HPV vaccines among obstetrician-gynecologists, pediatricians and immunization services providers in Western China. Hum. Vaccines Immunother. 2022, 18, 1–7. [Google Scholar] [CrossRef]
- Kassymbekova, F.; Rommel, A.; Kaidarova, D.; Auyezova, A.; Nukusheva, S.; Dunenova, G.; Bolatbekova, R.; Zhetpisbayeva, I.; Abdushukurova, G.; Glushkova, N. Developing HPV Vaccination Communication Strategies: Assessing Knowledge, Attitudes, and Barriers Among Healthcare Professionals in Kazakhstan. Vaccines 2024, 12, 1225. [Google Scholar] [CrossRef]
- Ayres, S.; Gee, A.; Kim, S.; Hashibe, M.; Praag, A.; Kaiser, D.; Chang, C.P.; Brandt, H.M.; Kepka, D. Human Papillomavirus Vaccination Knowledge, Barriers, and Recommendations Among Healthcare Provider Groups in the Western United States. J. Cancer Educ. Off. J. Am. Assoc. Cancer Education 2022, 37, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.Y.; Huang, Q.; Thompson, P.; Grabert, B.K.; Brewer, N.T.; Gilkey, M.B. Recommending Human Papillomavirus Vaccination at Age 9: A National Survey of Primary Care Professionals. Acad. Pediatr. 2022, 22, 573–580. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Comprehensive Cervical Cancer Control. Available online: https://www.who.int/publications/i/item/9789241548953 (accessed on 9 April 2025).
- World Health Organization. WHO/UNICEF HPV Vaccine Coverage Estimates. Available online: https://www.who.int/publications/m/item/who-unicef-hpv-vaccine-coverage-estimates (accessed on 17 July 2024).
- Llavona-Ortiz, J.Y.; Spanos, K.E.; Kraschnewski, J.L.; D’Souza, G.; Myrick, J.G.; Sznajder, K.K.; Calo, W.A. Associations Between Human Papillomavirus Vaccine Decisions and Exposure to Vaccine Information in Social Media. Cancer Control 2022, 29, 10732748221138404. [Google Scholar] [CrossRef]
- United Nations Children’s Fund. Human Papillomavirus (HPV) Vaccine: Supply and Demand Update. Available online: https://www.unicef.org/supply/reports/human-papillomavirus-hpv-vaccine-supply-and-demand-update (accessed on 9 April 2025).
- National Institution of Health. President’s Cancer Panel Annual Report 2012–2013, Accelerating Hpv Vaccine Uptake: Urgency for Action to Prevent Cancer. Available online: https://deainfo.nci.nih.gov/advisory/pcp/annualReports/HPV/index.htm (accessed on 9 April 2025).
- LaMontagne, D.S.; Bloem, P.J.N.; Brotherton, J.M.L.; Gallagher, K.E.; Badiane, O.; Ndiaye, C. Progress in HPV vaccination in low- and lower-middle-income countries. Int. J. Gynecol. Obstet. 2017, 138, 7–14. [Google Scholar] [CrossRef]
- Chidebe, R.C.W.; Osayi, A.; Torode, J.S. The Global Fund, Cervical Cancer, and HPV infections: What can low- and middle-income countries do to accelerate progress by 2030? eClinicalMedicine 2025, 81, 103127. [Google Scholar] [CrossRef]
- World Health Organization. Cervial Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 9 April 2025).
- Buskwofie, A.; David-West, G.; Clare, C.A. A Review of Cervical Cancer: Incidence and Disparities. J. Natl. Med. Assoc. 2020, 112, 229–232. [Google Scholar] [CrossRef]
- World Health Organization. WHO HPV Vaccine Global Market Study, April 2022. Available online: https://www.who.int/publications/m/item/who-hpv-vaccine-global-market-study-april-2022 (accessed on 9 April 2025).
- Zou, Z.; Fairley, C.K.; Ong, J.J.; Hocking, J.; Canfell, K.; Ma, X.; Chow, E.P.F.; Xu, X.; Zhang, L.; Zhuang, G. Domestic HPV vaccine price and economic returns for cervical cancer prevention in China: A cost-effectiveness analysis. Lancet Glob. Health 2020, 8, e1335–e1344. [Google Scholar] [CrossRef] [PubMed]
- Merck. Merck’s Gardasil® Has Become the First and Currently the Only HPV Vaccine Approved for Males Within China. Available online: https://www.msdchina.com.cn/2025/01/08/company_news_2025-1-8/ (accessed on 8 January 2025).
- Li, J.; Kang, J.; Mao, Y.; Zheng, P.; Abdullah, A.S.; Wu, G.; Wang, F. Investigating HPV- and HPV Vaccine-Related Knowledge, Perceptions, and Information Sources among Health Care Providers in Three Big Cities in China. Vaccines 2020, 8, 499. [Google Scholar] [CrossRef]
- Warner, E.L.; Ding, Q.; Pappas, L.; Bodson, J.; Fowler, B.; Mooney, R.; Kirchhoff, A.C.; Kepka, D. Health Care Providers’ Knowledge of HPV Vaccination, Barriers, and Strategies in a State With Low HPV Vaccine Receipt: Mixed-Methods Study. JMIR Cancer 2017, 3, e12. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, L. The one-dose schedule opens the door to rapid scale-up of HPV vaccination. BMC Med. 2023, 21, 387. [Google Scholar] [CrossRef]
- Jeyachelvi, K.; Juwita, S.; Norwati, D. Human Papillomavirus Infection and its Vaccines: Knowledge and Attitudes of Primary Health Clinic Nurses in Kelantan, Malaysia. Asian Pac. J. Cancer Prev. 2016, 17, 3983–3988. [Google Scholar] [PubMed]
- Nilsen, K.; Aasland, O.G.; Klouman, E. The HPV vaccine: Knowledge and attitudes among public health nurses and general practitioners in Northern Norway after introduction of the vaccine in the school-based vaccination programme. Scand. J. Prim. Health Care 2017, 35, 387–395. [Google Scholar] [CrossRef] [PubMed]

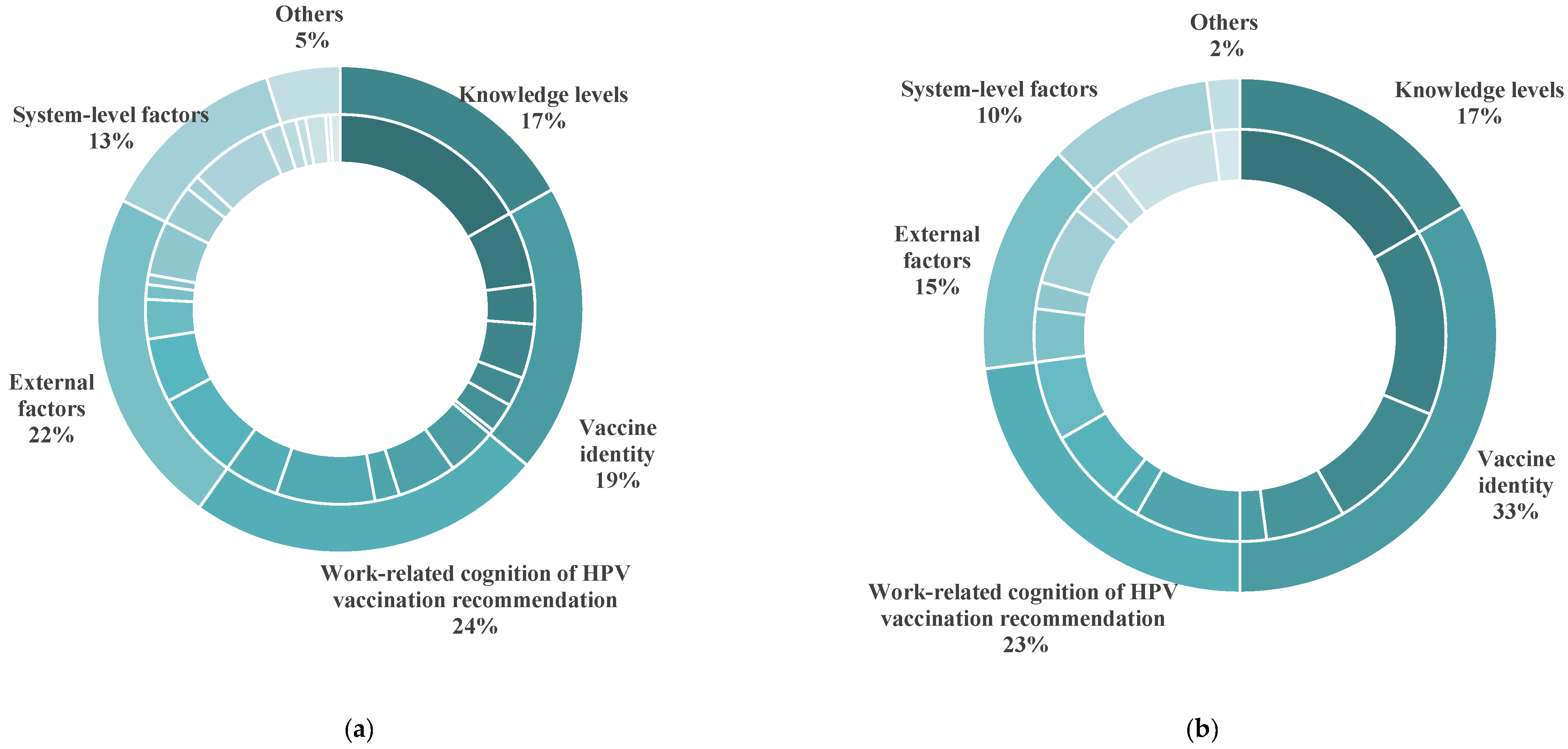
| Categories of Recommendation Strategies | Specific Recommendation Strategies | Number of Reported Studies | Number of Reported Studies Conducted in HICs | Number of Reported Studies Conducted in LMICs |
|---|---|---|---|---|
| Present HPV vaccination as an option rather than forcing, persuading, or leaving individuals to make decisions |
| 17 | 14 | 3 |
| 7 | 7 | / | |
| Persuade individuals to get vaccinated by offering focused information and preference for vaccination |
| 17 | 16 | 1 |
| 2 | 2 | / | |
| 1 | 1 | / | |
| 1 | 1 | / | |
| 2 | 2 | / | |
| 1 | 1 | / | |
| Leverage the continuity of healthcare and the long-term doctor–patient relationship |
| 7 | 7 | / |
| 6 | 6 | / | |
| 3 | 3 | / | |
| Avoid highlighting the specific characteristics of the HPV vaccine |
| 7 | 7 | / |
| 6 | 6 | / | |
| 2 | 2 | / | |
| Tailored recommendation strategies for vaccine-hesitant individuals |
| 4 | 4 | / |
| 4 | 4 | / | |
| 1 | 1 | / | |
| 1 | 1 | / | |
| 1 | 1 | / | |
| 1 | 1 | / | |
| Inform individuals of vaccination based on authority rather than offering them a decision |
| 3 | 3 | / |
| 1 | 1 | / | |
| 1 | / | 1 |
| Categories of Factors | Specific Factors | Number of International Studies Reported the Specific Factor | Number of Studies Conducted in HICs Reported the Specific Factor | Number of Studies Conducted in LMICs Reported the Specific Factor | Number of Studies Conducted in China Reported the Specific Factor |
|---|---|---|---|---|---|
| Perceptions and of recommendation for HPV vaccination |
| 20 | 16 | 4 | 3 |
| 12 | 9 | 3 | 3 | |
| 11 | 8 | 3 | 3 | |
| 10 | 8 | 2 | 4 | |
| 5 | 3 | 2 | / | |
| External factors |
| 18 | 15 | 3 | 2 |
| 13 | 13 | / | 1 | |
| 11 | 6 | 5 | 1 | |
| 8 | 5 | 3 | 3 | |
| 3 | 2 | 1 | / | |
| 2 | 2 | / | / | |
| Vaccine identity |
| 15 | 9 | 6 | 7 |
| 11 | 9 | 2 | 3 | |
| 6 | 4 | 2 | 1 | |
| 6 | 6 | / | / | |
| 8 | 6 | 2 | 5 | |
| 1 | 1 | / | / | |
| Knowledge level | Knowledge level | 41 | 31 | 10 | 8 |
| System-level factors |
| 16 | 9 | 7 | 4 |
| 8 | 2 | 6 | 1 | |
| 4 | 4 | / | / | |
| 3 | 3 | / | / | |
| Others |
| 4 | 4 | / | / |
| 3 | 3 | / | / | |
| 2 | 2 | / | / | |
| 1 | 1 | / | / | |
| 2 | 2 | / | / |
| Categories of Barriers | Specific Barriers |
|---|---|
| Perceptions of recommendation for HPV vaccination |
|
| |
| |
| |
| External factors |
|
| |
| |
| |
| Vaccine identity |
|
| |
| |
| |
| |
| Knowledge level | Insufficient knowledge of HPV infection, HPV vaccine, and communication strategies to recommend HPV vaccination |
| System-level factors |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; He, Y.; Wang, Z.; Sun, J. Strategies, Barriers, and Facilitators for Healthcare Professionals to Recommend HPV Vaccination: A Systematic Review. Vaccines 2025, 13, 402. https://doi.org/10.3390/vaccines13040402
Fu Y, He Y, Wang Z, Sun J. Strategies, Barriers, and Facilitators for Healthcare Professionals to Recommend HPV Vaccination: A Systematic Review. Vaccines. 2025; 13(4):402. https://doi.org/10.3390/vaccines13040402
Chicago/Turabian StyleFu, Yihan, Yinqi He, Zhitao Wang, and Jing Sun. 2025. "Strategies, Barriers, and Facilitators for Healthcare Professionals to Recommend HPV Vaccination: A Systematic Review" Vaccines 13, no. 4: 402. https://doi.org/10.3390/vaccines13040402
APA StyleFu, Y., He, Y., Wang, Z., & Sun, J. (2025). Strategies, Barriers, and Facilitators for Healthcare Professionals to Recommend HPV Vaccination: A Systematic Review. Vaccines, 13(4), 402. https://doi.org/10.3390/vaccines13040402







