Estimating the Public Health and Economic Impact of Annual mRNA COVID-19 Vaccination for Adults Aged 50 and Older in South Korea’s Endemic Era
Abstract
1. Background
2. Methods
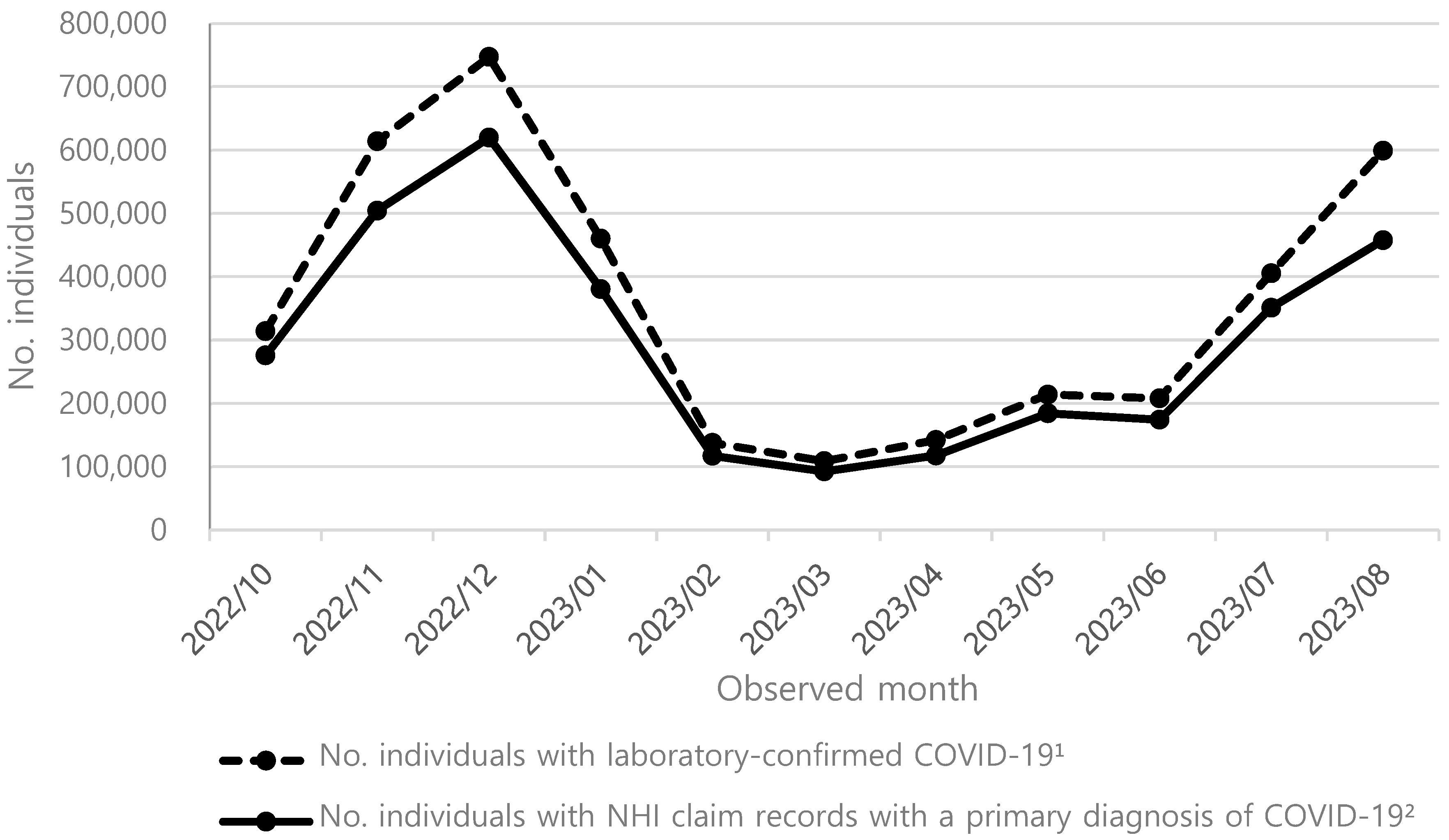
2.1. COVID-19 Symptomatic Infection Incidence Rates Among the Unvaccinated
2.2. COVID-19 Hospitalization Rates in the Unvaccinated
2.3. mRNA COVID-19 Vaccine Uptake Rate
2.4. Vaccine Effectiveness of mRNA-1273 Against COVID-19 Hospitalization
2.5. Comparative Effectiveness of mRNA-1273 over BNT162b2
2.6. Median Cost of COVID-19 Hospitalization
2.7. Sensitivity Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Calculation Steps for COVID-19 Symptomatic Infection Incidence Rate Among the Unvaccinated
Appendix B. Calculation Steps for Average Annual Vaccine Effectiveness of mRNA-1273 Vaccine
Appendix C. Calculation Steps for Average Annual Vaccine Effectiveness Rates of BNT161b2 Vaccine
References
- Korea Disease Control and Prevention Agency. COVID-19 Vaccination Starts from October 11. Available online: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=726216&cg_code=&act=view&nPage=4&newsField= (accessed on 13 January 2025).
- Korea Disease Control and Prevention Agency. COVID-19 Vaccination. Available online: https://dportal.kdca.go.kr/pot/www/CVID19/PRVNTN/VCNTN.jsp?seq=TAB1_1 (accessed on 29 March 2024).
- Korean Statistical Information Service. Trend of Influenza Vaccination Rate. Available online: https://kosis.kr/statHtml/statHtml.do?sso=ok&returnurl=https%3A%2F%2Fkosis.kr%3A443%2FstatHtml%2FstatHtml.do%3Flist_id%3D117_11702_A01_077%26obj_var_id%3D%26seqNo%3D%26tblId%3DDT_11702_N083%26vw_cd%3DMT_OTITLE%26orgId%3D177%26path%3D%252Fcommon%252Fmeta_ (accessed on 13 January 2025).
- Hong, S.; Son, Y.; Lee, M.; Lee, J.H.; Park, J.; Lee, H.; Dragioti, E.; Fond, G.; Boyer, L.; López Sánchez, G.F. Association between sociodemographic factors and vaccine acceptance for influenza and SARS-CoV-2 in South Korea: Nationwide Cross-Sectional Study. JMIR Public Health Surveill. 2024, 10, e56989. [Google Scholar] [PubMed]
- Kopel, H.; Araujo, A.B.; Bogdanov, A.; Zeng, N.; Winer, I.; Winer-Jones, J.P.; Lu, T.; Marks, M.A.; Bonafede, M.; Nguyen, V.H. Effectiveness of the 2023–2024 Omicron XBB. 1.5-containing mRNA COVID-19 vaccine (mRNA-1273.815) in preventing COVID-19–related hospitalizations and medical encounters among adults in the United States. Open Forum Infect. Dis. 2024, 11, ofae695. [Google Scholar] [PubMed]
- Ma, K.C.; Surie, D.; Lauring, A.S.; Martin, E.T.; Leis, A.M.; Papalambros, L.; Gaglani, M.; Columbus, C.; Gottlieb, R.L.; Ghamande, S.; et al. Effectiveness of updated 2023–2024 (Monovalent XBB.1.5) COVID-19 vaccination against SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 lineage hospitalization and a comparison of clinical severity-IVY Network, 26 hospitals, October 18, 2023-March 9, 2024. Clin. Infect. Dis. 2024. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. Announcement of the Status and Schedule of Large-Scale COVID-19 Seroprevalence Survey. Available online: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=720181&cg_code=&act=view&nPage=3&newsField=202207 (accessed on 4 February 2025).
- Korea Statistical Information Service. Life Changes Caused by COVID-19. Available online: https://kosis.kr/statHtml/statHtml.do?sso=ok&returnurl=https%3A%2F%2Fkosis.kr%3A443%2FstatHtml%2FstatHtml.do%3Fconn_path%3DMT_ZTITLE%26list_id%3DB_13_001%26obj_var_id%3D%26seqNo%3D%26tblId%3DDT_154021_22AA006300%26vw_cd%3DMT_ZTITLE%26itm_id%3D%26language%3Dkor%26lang_mode%3Dko%26orgId%3D154%26 (accessed on 4 February 2025).
- Korea Statistical Information Service. Status of Subscribers by Standard Monthly Income Bracket and Age Group. Available online: https://kosis.kr/statHtml/statHtml.do?sso=ok&returnurl=https%3A%2F%2Fkosis.kr%3A443%2FstatHtml%2FstatHtml.do%3Fconn_path%3DMT_ZTITLE%26list_id%3D322_32202_02%26obj_var_id%3D%26seqNo%3D%26tblId%3DDT_32202_B019_1%26vw_cd%3DMT_ZTITLE%26itm_id%3D%26language%3Dkor%26lang_mode%3Dko%26orgId%3D322%26 (accessed on 4 February 2025).
- Wang, F.; Wang, J.-D. Estimating US earnings loss associated with COVID-19 based on human capital calculation. Int. J. Environ. Res. Public Health 2022, 19, 1015. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Lee, H.R.; Choi, Y.H.; Kim, G. Immunization program against influenza in Korea; The 2018–2019 influenza season. Public Health Wkly. Rep. 2019, 12, 9. [Google Scholar]
- Higdon, M.M.; Baidya, A.; Walter, K.K.; Patel, M.K.; Issa, H.; Espié, E.; Feikin, D.R.; Knoll, M.D. Duration of effectiveness of vaccination against COVID-19 caused by the omicron variant. Lancet Infect. Dis. 2022, 22, 1114–1116. [Google Scholar] [PubMed]
- Danza, P.; Koo, T.H.; Haddix, M.; Fisher, R.; Traub, E.; OYong, K.; Balter, S. SARS-CoV-2 infection and hospitalization among adults aged >/=18 years, by vaccination status, before and during SARS-CoV-2 B.1.1.529 (Omicron) variant predominance—Los Angeles County, California, November 7, 2021-January 8, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Olson, S.M.; Self, W.H.; Talbot, H.K.; Lindsell, C.J.; Steingrub, J.S.; Shapiro, N.I.; Ginde, A.A.; Douin, D.J.; Prekker, M.E.; et al. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged >/=65 Years—United States, January-March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 674–679. [Google Scholar] [PubMed]
- Kavikondala, S.; Haeussler, K.; Wang, X.; Bausch-Jurken, M.T.; Nassim, M.; Mishra, N.K.; Malmenäs, M.; Sharma, P.; Van de Velde, N.; Green, N. Comparative effectiveness of mRNA-1273 and BNT162b2 COVID-19 vaccines among older adults: Systematic literature review and meta-analysis using the GRADE Framework. Infect. Dis. Ther. 2024, 13, 779–811. [Google Scholar] [PubMed]
- Xu, K.; Wang, Z.; Qin, M.; Gao, Y.; Luo, N.; Xie, W.; Zou, Y.; Wang, J.; Ma, X. A systematic review and meta-analysis of the effectiveness and safety of COVID-19 vaccination in older adults. Front. Immunol. 2023, 14, 1113156. [Google Scholar]
- Kwon, S.L.; Kim, S.-Y.; Song, M.; Lee, H.-M.; Ban, S.-H.; Lee, M.-S.; Jeong, H. Assessing the determinants of influenza and COVID-19 vaccine co-administration decisions in the elderly. Hum. Vaccin. Immunother. 2024, 20, 2346966. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, L.; Quan, K.; Baber, J.A.; Ho, A.W.; Zhang, Y.; Xu, X.; Lu, C.; Cooper, D.; Koury, K.; Lockhart, S.P. Safety and immunogenicity of the BNT162b2 vaccine co-administered with seasonal inactivated influenza vaccine in adults. Infect. Dis. Ther. 2023, 12, 2241–2258. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tian, X.; Zhang, X.; Yao, H.; Wu, N. Immune interference in effectiveness of influenza and COVID-19 vaccination. Front. Immunol. 2023, 14, 1167214. [Google Scholar]
- Shenyu, W.; Xiaoqian, D.; Bo, C.; Xuan, D.; Zeng, W.; Hangjie, Z.; Qianhui, Z.; Zhenzhen, L.; Chuanfu, Y.; Juan, Y. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine (CoronaVac) co-administered with an inactivated quadrivalent influenza vaccine: A randomized, open-label, controlled study in healthy adults aged 18 to 59 years in China. Vaccine 2022, 40, 5356–5365. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chan, Y.-C.; Niu, R.; Wong, E.W.; van Wyk, M.A. Modeling the impact of vaccination on COVID-19 and its Delta and Omicron variants. Viruses 2022, 14, 1482. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kang, M.; Shin, D.H.; Jung, J.; Choi, S.J.; Kim, N.-H.; Moon, S.M.; Song, K.-H.; Kim, E.S.; Jung, J. Antibiotic prescription in patients with coronavirus disease 2019: Analysis of national health insurance system data in the Republic of Korea. J. Korean Med. Sci. 2023, 38, e189. [Google Scholar] [PubMed]
| Age, Years | No. Population a (A) | No. Individuals with NHI Claim Records with a Primary Diagnosis of COVID-19 b, (B) | % COVID-19 Patients Utilizing HCS c, (C) | Estimated No. Individuals Infected with COVID-19 (D = BC) | Overall Incidence Rate, d % (E = D/A) |
|---|---|---|---|---|---|
| 50–54 | 4,525,612 | 411,837 | 85.24 | 483,165 | 10.68 |
| 55–59 | 4,070,751 | 352,401 | 85.24 | 413,435 | 10.16 |
| 60–64 | 4,258,205 | 415,619 | 83.44 | 498,125 | 11.70 |
| 65–69 | 3,274,183 | 369,504 | 83.44 | 442,856 | 13.53 |
| 70–74 | 2,234,677 | 275,075 | 82.83 | 332,108 | 14.86 |
| 75–79 | 1,637,098 | 204,626 | 82.83 | 247,052 | 15.09 |
| 80+ | 2,289,858 | 283,133 | 75.15 | 376,751 | 16.45 |
| Total | 22,290,384 | 2,793,492 |
| Age, Years | No. Population (A) | Incidence in UV, % (B) | COVID-19 Hospz. in UV, % (C) | VR (If Increase to VR of IV), % (D) | VE by mRNA-1273, % (E) | VE by BNT162b2, % (F) | No. Hospz. Prevented by mRNA-1273 a | No. Hospz. Prevented by BNT162b2 b | Difference in No. Hospz. Prevented: mRNA-1273 vs. BNT162b2 |
|---|---|---|---|---|---|---|---|---|---|
| 50–54 | 4,525,612 | 10.76 | 2.72 | 3.56 (35.10) | 55.62 | 35.79 | 262 [2581] | 168 [1660] | 93 [920] |
| 55–59 | 4,070,751 | 10.23 | 3.49 | 3.56 (35.10) | 55.62 | 35.79 | 288 [2842] | 185 [1828] | 103 [1013] |
| 60–64 | 4,258,205 | 12.21 | 3.93 | 19.62 (59.10) | 55.62 | 35.79 | 2228 [6712] | 1434 [4318] | 795 [2394] |
| 65–69 | 3,274,183 | 14.11 | 4.67 | 19.62 (59.10) | 55.62 | 35.79 | 2353 [7087] | 1514 [4560] | 839 [2527] |
| 70–74 | 2,234,677 | 16.39 | 6.09 | 44.03 (85.90) | 55.62 | 35.79 | 5467 [10,665] | 3517 [6862] | 1949 [3803] |
| 75–79 | 1,637,098 | 16.65 | 9.01 | 44.03 (85.90) | 55.62 | 35.79 | 6015 [11,736] | 3870 [7551] | 2145 [4185] |
| 80+ | 2,289,858 | 18.24 | 19.12 | 46.29 (85.90) | 55.62 | 35.79 | 20,565 [38,163] | 13,231 [24,554] | 7334 [13,610] |
| Total | 22,290,384 | 37,178 [79,785] | 23,920 [51,332] | 13,258 [28,453] |
| Type of Hospitalization, % | Median Costs from Payer’s Perspective a | Median Costs from Societal Perspective b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, Years | General | ICU | Ventilator Use | General | ICU | Ventilator Use | Weighted Median c | General | ICU | Ventilator Use | Weighted Median c |
| 50–54 | 97.41 | 1.61 | 0.98 | 1646 | 5569 | 11,738 | 1808 | 3417 | 8206 | 16,879 | 3626 |
| 55–59 | 96.75 | 2.08 | 1.17 | 1646 | 5569 | 11,738 | 1845 | 3386 | 8170 | 16,813 | 3643 |
| 60–64 | 96.69 | 2.07 | 1.24 | 1646 | 5569 | 11,738 | 1852 | 3265 | 8032 | 16,194 | 3525 |
| 65–69 | 95.87 | 2.44 | 1.68 | 1828 | 5899 | 9818 | 2061 | 3199 | 8248 | 13,194 | 3490 |
| 70–74 | 95.21 | 2.88 | 1.68 | 1828 | 5899 | 9818 | 2097 | 3110 | 8134 | 13,048 | 3444 |
| 75–79 | 94.39 | 3.36 | 2.25 | 1828 | 5899 | 9818 | 2144 | 2978 | 7964 | 12,831 | 3367 |
| 80+ | 95.08 | 3.46 | 1.46 | 1828 | 5899 | 9818 | 2085 | 2978 | 7964 | 12,831 | 3294 |
| Payer’s Perspective a | Societal Perspective b | |||||
|---|---|---|---|---|---|---|
| Age, Years | Costs of Hospz. Prevented by mRNA-1273 | Costs of Hospz. Prevented by BNT162b2 | Difference: mRNA-1273 vs. BNT162b2 | Costs of Hospz. Prevented by mRNA-1273 | Costs of Hospz. Prevented by BNT162b2 | Difference: mRNA-1273 vs. BNT162b2 |
| 50–54 | 473,138 [4,762,543] | 304,409 [3,001,340] | 168,729 [1,761,204] | 949,066 [9,357,363] | 610,614 [6,020,375] | 338,452 [3,336,988] |
| 55–59 | 531,875 [5,244,048] | 342,200 [3,373,935] | 189,675 [1,870,113] | 1,049,921 [10,351,751] | 675,502 [6,660,148] | 374,419 [3,691,603] |
| 60–64 | 4,127,709 [12,433,618] | 2,655,700 [7,999,587] | 1,472,008 [4,434,031] | 7,853,485 [23,656,521] | 5,052,804 [15,220,220] | 2,800,681 [8,436,302] |
| 65–69 | 4,850,015 [14,609,372] | 3,120,420 [9,399,432] | 1,729,595 [5,209,941] | 8,211,422 [24,734,711] | 5,283,095 [15,913,909] | 2,928,327 [8,820,802] |
| 70–74 | 11,465,281 [22,368,104] | 7,376,574 [14,391,273] | 4,088,706 [7,976,831] | 18,829,227 [36,734,741] | 12,114,418 [23,634,533] | 6,714,810 [13,100,208] |
| 75–79 | 12,896,622 [25,160,568] | 8,297,476 [16,187,899] | 4,599,146 [8,972,669] | 20,253,431 [39,513,281] | 13,030,727 [25,422,200] | 7,222,704 [14,091,081] |
| 80+ | 42,883,131 [79,577,899] | 27,590,306 [51,199,121] | 15,292,825 [28,378,778] | 67,752,428 [125,727,664] | 43,590,806 [80,891,126] | 24,161,622 [44,836,537] |
| Total | 77,227,770 [164,156,153] | 49,687,087 [105,552,586] | 27,540,684 [58,603,567] | 124,898,980 [270,076,031] | 80,357,965 [173,762,510] | 44,541,015 [96,313,522] |
| Age, Years | Applying 95% CI of VE of mRNA-1273 | Applying 95% CI of rVE of mRNA-1273 vs. BNT162b2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Lower Bond | Upper Bound | Lower Bound | Upper Bound | |||||
| No. Hospz. Prevented by mRNA-1273 | Hospz. Costs Prevented by mRNA-1273 (Societal Perspective) | No. Hospz. Prevented by mRNA-1273 | Hospz. Costs Prevented by mRNA-1273 (Societal Perspective) | Difference in No. Hospz. Prevented: mRNA-1273 vs. BNT162b2 | Difference in Hospz. Costs Prevented: mRNA-1273 vs. BNT162b2 (Societal Perspective) | Difference in No. Hospz. Prevented: mRNA-1273 vs. BNT162b2 | Difference in Hospz. Costs Prevented: mRNA-1273 vs. BNT162b2 (Societal Perspective) | |
| 50–54 | 230 [2264] | 832,572 [8,208,785] | 287 [2828] | 1,040,119 [10,255,108] | 46 [454] | 167,123 [1,647,757] | 154 [1516] | 557,546 [5,497,156] |
| 55–59 | 253 [2493] | 921,048 [9,081,116] | 316 [3114] | 1,150,651 [11,344,897] | 51 [500] | 184,883 [1,822,861] | 169 [1669] | 616,796 [6,081,328] |
| 60–64 | 1950 [5873] | 6,871,528 [20,698,638] | 2358 [7103] | 8,311,501 [25,036,173] | 392 [1182] | 1,382,936 [4,165,726] | 1309 [3943] | 4,613,675 [13,897,462] |
| 65–69 | 2059 [6201] | 7,184,710 [21,642,016] | 2490 [7501] | 8,690,313 [26,177,242] | 414 [1248] | 1,445,966 [4,355,586] | 1382 [4164] | 4,823,952 [14,530,864] |
| 70–74 | 4762 [9291] | 16,403,718 [32,002,710] | 5473 [10,677] | 18,850,709 [36,776,651] | 963 [1878] | 3,315,677 [6,468,696] | 3211 [6265] | 11,061,579 [21,580,504] |
| 75–79 | 5240 [10,224] | 17,644,461 [34,423,329] | 6022 [11,749] | 20,276,538 [39,558,360] | 1059 [2067] | 3,566,468 [6,957,975] | 3534 [6894] | 11,898,254 [23,212,809] |
| 80+ | 17,909 [33,233] | 58,999,726 [109,485,342] | 20,480 [38,005] | 67,471,048 [125,205,509] | 3621 [6720] | 11,930,665 [22,139,644] | 12,082 [22,420] | 39,802,420 [73,861,047] |
| Total | 32,402 [69,579] | 108,857,762 [235,541,937] | 37,426 [80,977] | 125,790,879 [274,353,940] | 6547 [14,050] | 21,993,719 [47,558,245] | 21,841 [46,871] | 73,374,221 [158,661,171] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, J.; Lee, D.; Yang, H.-D.; Kim, A.-Y.; Lee, H.; Kang, M.; Beck, E.; Joshi, K.; Kang, Y.; Kang, H.-Y. Estimating the Public Health and Economic Impact of Annual mRNA COVID-19 Vaccination for Adults Aged 50 and Older in South Korea’s Endemic Era. Vaccines 2025, 13, 386. https://doi.org/10.3390/vaccines13040386
Jung J, Lee D, Yang H-D, Kim A-Y, Lee H, Kang M, Beck E, Joshi K, Kang Y, Kang H-Y. Estimating the Public Health and Economic Impact of Annual mRNA COVID-19 Vaccination for Adults Aged 50 and Older in South Korea’s Endemic Era. Vaccines. 2025; 13(4):386. https://doi.org/10.3390/vaccines13040386
Chicago/Turabian StyleJung, Jaehee, Dain Lee, Hee-Do Yang, Ah-Young Kim, Haeun Lee, Minkyoung Kang, Ekkehard Beck, Keya Joshi, Youngju Kang, and Hye-Young Kang. 2025. "Estimating the Public Health and Economic Impact of Annual mRNA COVID-19 Vaccination for Adults Aged 50 and Older in South Korea’s Endemic Era" Vaccines 13, no. 4: 386. https://doi.org/10.3390/vaccines13040386
APA StyleJung, J., Lee, D., Yang, H.-D., Kim, A.-Y., Lee, H., Kang, M., Beck, E., Joshi, K., Kang, Y., & Kang, H.-Y. (2025). Estimating the Public Health and Economic Impact of Annual mRNA COVID-19 Vaccination for Adults Aged 50 and Older in South Korea’s Endemic Era. Vaccines, 13(4), 386. https://doi.org/10.3390/vaccines13040386






