The Impact of Vaccination Frequency on COVID-19 Public Health Outcomes: A Model-Based Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Agent-Based Simulation of Infection and Mortality Burden
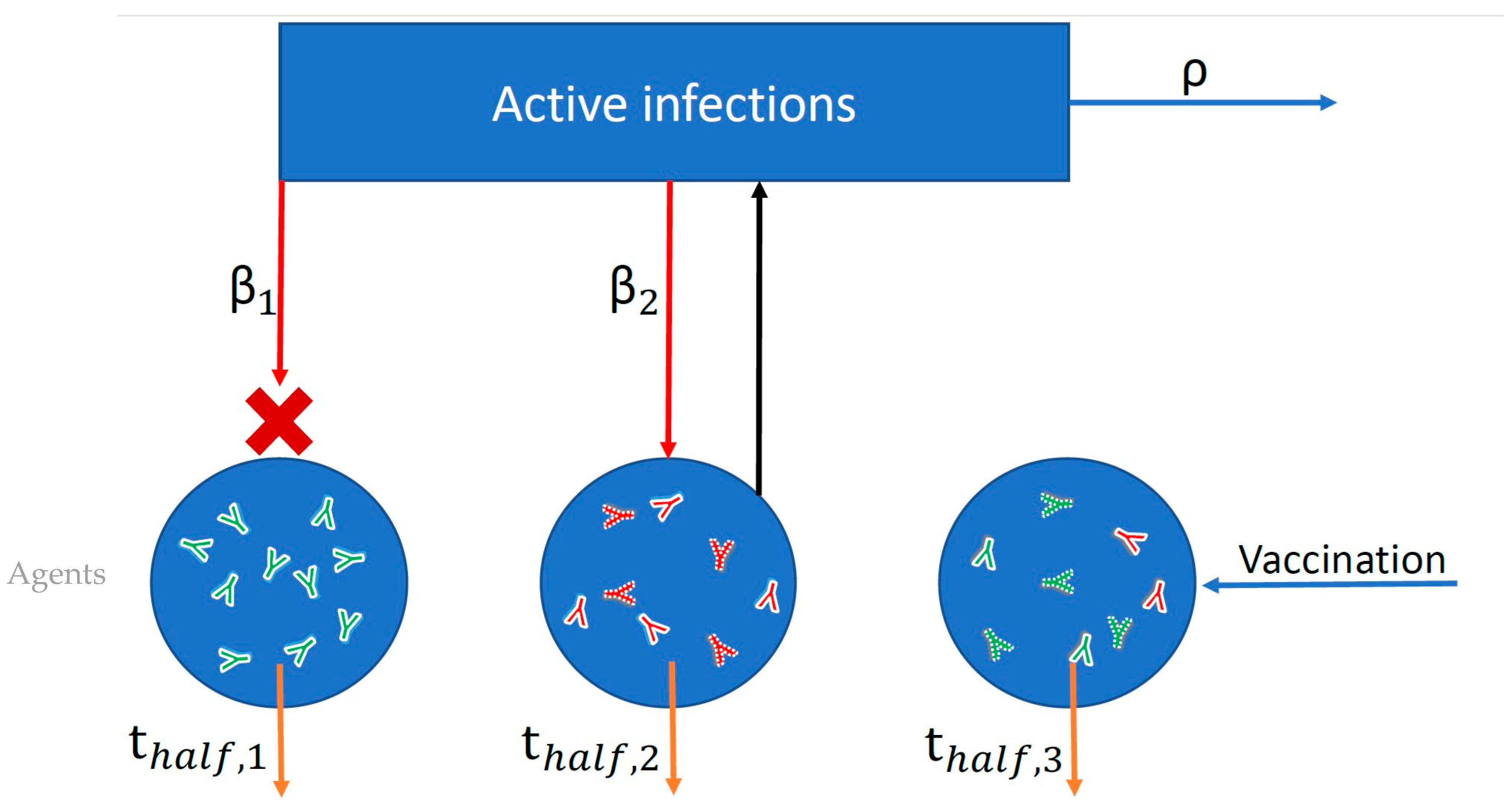
2.1.1. Model Overview
2.1.2. Population Mixed-Effects Model Fit for Neutralization Potency over Time After Infection
2.1.3. Agent-Based Simulation of SARS-CoV-2 Dynamics
2.2. Susceptible–Infectious–Recovered–Susceptible (SIRS) Model of Strain Invasion
3. Results
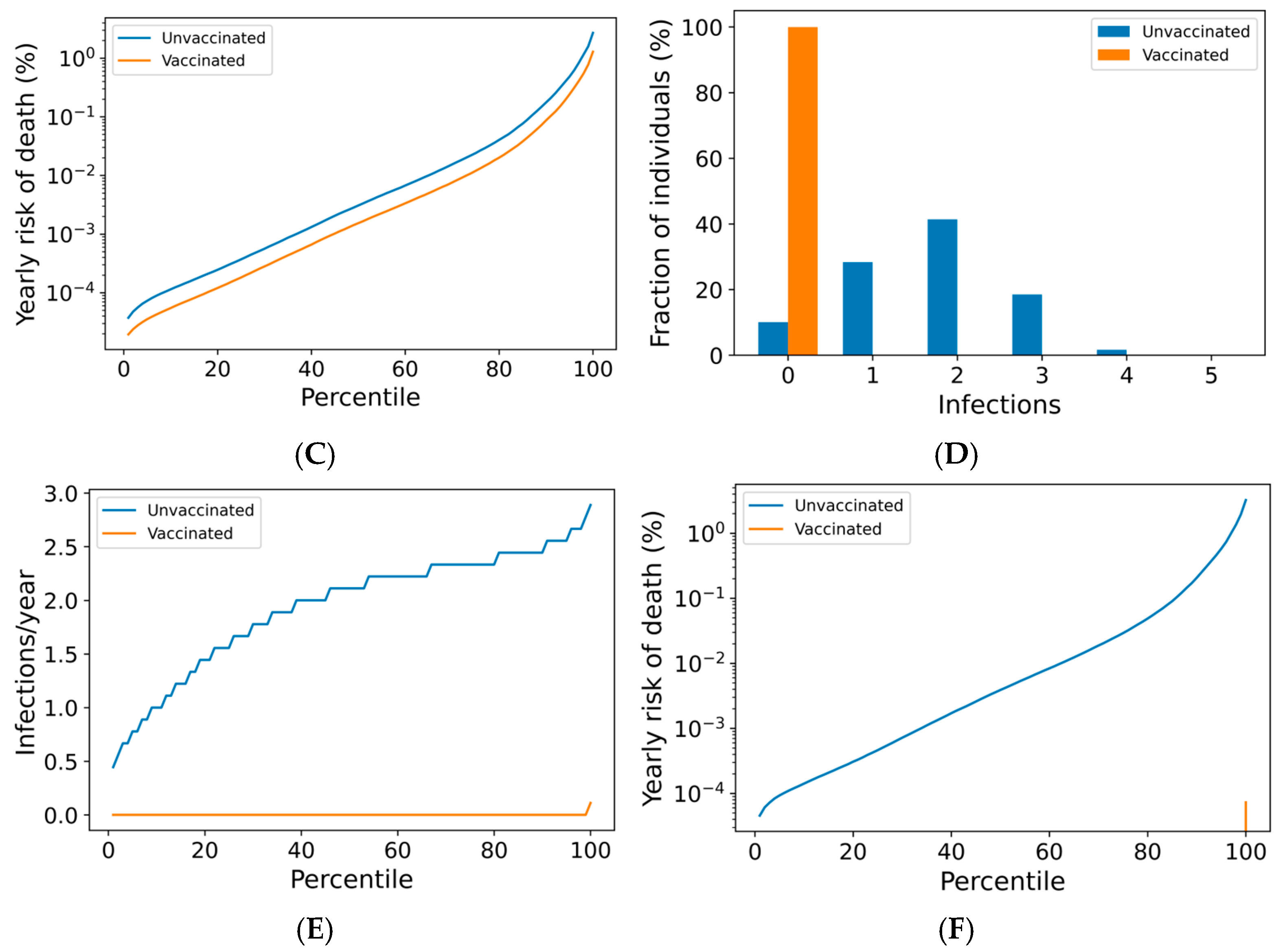
3.1. Boosting Frequency Determines Vaccine Efficacy Throughout the Population
3.2. Breakthrough Infections Under Frequent Boosting Schedules Are Driven by Poor nAb Kinetics
3.3. High Compliance with Frequent Boosting Could Suppress Omicron Spread
3.4. Improved Vaccine Kinetics Improves Booster Regime Efficacy
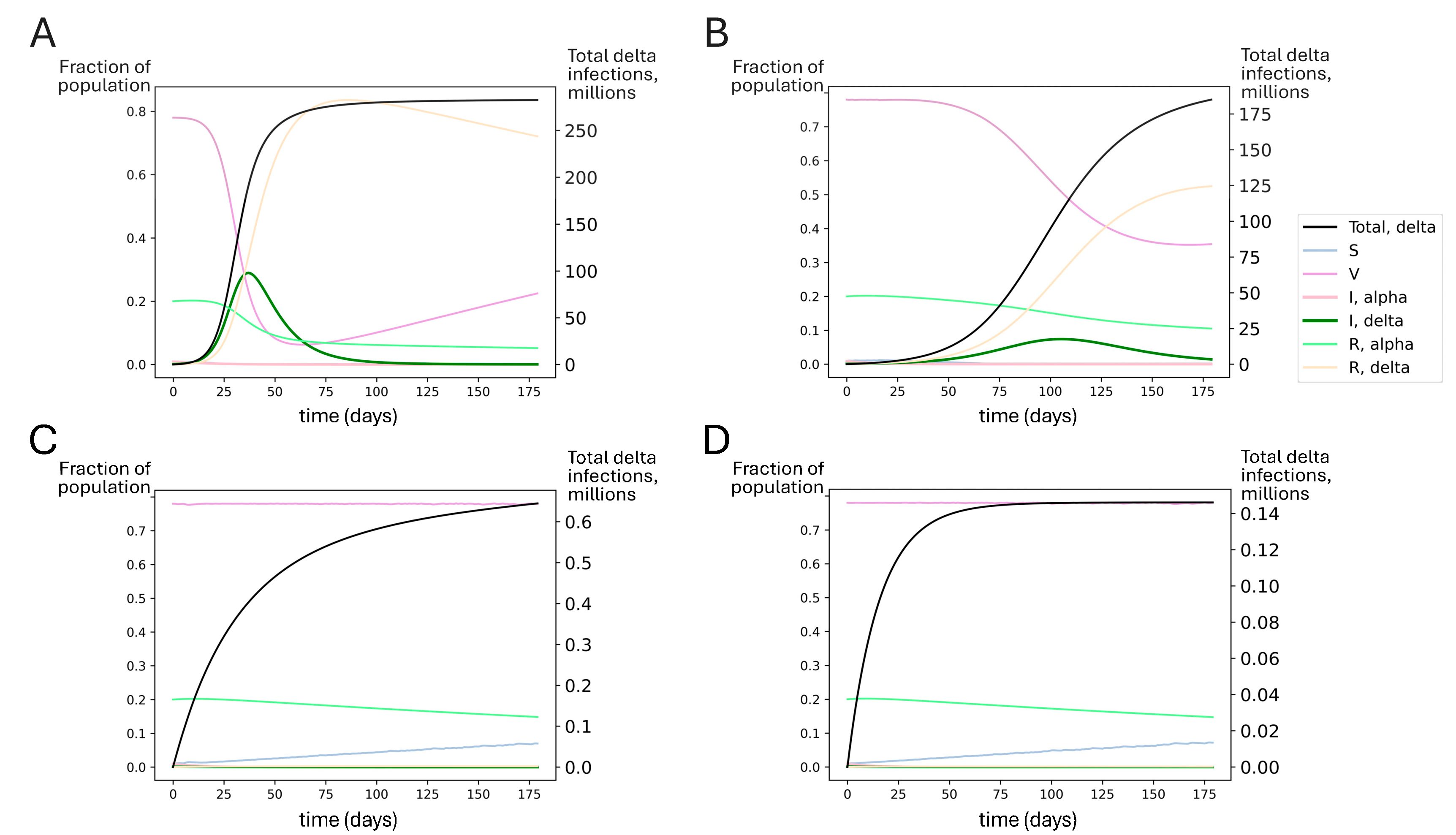
3.5. Boosting Could Have Likely Averted the Delta Wave of Summer 2021
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Eng. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Olson, S.M.; Self, W.H.; Talbot, H.K.; Lindsell, C.J.; Steingrub, J.S.; Shapiro, N.I.; Ginde, A.A.; Douin, D.J.; Prekker, M.E.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years—United States, January–March 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 674–679. [Google Scholar] [CrossRef]
- Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine (accessed on 18 September 2023).
- Tseng, H.F.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta Variants. Nat. Med. 2022, 28, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Prunas, O.; Warren, J.L.; Crawford, F.W.; Gazit, S.; Patalon, T.; Weinberger, D.M.; Pitzer, V.E. Vaccination with BNT162b2 Reduces Transmission of SARS-CoV-2 to Household Contacts in Israel. Science 2022, 375, 1151–1154. [Google Scholar] [CrossRef]
- Pegu, A.; O’Connell, S.E.; Schmidt, S.D.; O’Dell, S.; Talana, C.A.; Lai, L.; Albert, J.; Anderson, E.; Bennett, H.; Corbett, K.S.; et al. Durability of mRNA-1273 Vaccine-Induced Antibodies against SARS-CoV-2 Variants. Science 2021, 373, 1372–1377. [Google Scholar] [CrossRef]
- Ibarrondo, F.J.; Hofmann, C.; Fulcher, J.A.; Goodman-Meza, D.; Mu, W.; Hausner, M.A.; Ali, A.; Balamurugan, A.; Taus, E.; Elliott, J.; et al. Primary, Recall, and Decay Kinetics of SARS-CoV-2 Vaccine Antibody Responses. ACS Nano 2021, 15, 11180–11191. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef]
- Notarte, K.I.; Guerrero-Arguero, I.; Velasco, J.V.; Ver, A.T.; Santos de Oliveira, M.H.; Catahay, J.A.; Khan, M.S.R.; Pastrana, A.; Juszczyk, G.; Torrelles, J.B.; et al. Characterization of the Significant Decline in Humoral Immune Response Six Months Post-SARS-CoV-2 mRNA Vaccination: A Systematic Review. J. Med. Virol. 2022, 94, 2939–2961. [Google Scholar] [CrossRef]
- Weisblum, Y.; Schmidt, F.; Zhang, F.; DaSilva, J.; Poston, D.; Lorenzi, J.C.; Muecksch, F.; Rutkowska, M.; Hoffmann, H.-H.; Michailidis, E.; et al. Escape from Neutralizing Antibodies by SARS-CoV-2 Spike Protein Variants. eLife 2020, 9, e61312. [Google Scholar] [CrossRef]
- Li, Q.; Wu, J.; Nie, J.; Zhang, L.; Hao, H.; Liu, S.; Zhao, C.; Zhang, Q.; Liu, H.; Nie, L.; et al. The Impact of Mutations in SARS-CoV-2 Spike on Viral Infectivity and Antigenicity. Cell 2020, 182, 1284–1294.e9. [Google Scholar] [CrossRef]
- Greaney, A.J.; Loes, A.N.; Crawford, K.H.D.; Starr, T.N.; Malone, K.D.; Chu, H.Y.; Bloom, J.D. Comprehensive Mapping of Mutations in the SARS-CoV-2 Receptor-Binding Domain That Affect Recognition by Polyclonal Human Plasma Antibodies. Cell Host Microbe 2021, 29, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Andreano, E.; Piccini, G.; Licastro, D.; Casalino, L.; Johnson, N.V.; Paciello, I.; Dal Monego, S.; Pantano, E.; Manganaro, N.; Manenti, A.; et al. SARS-CoV-2 Escape from a Highly Neutralizing COVID-19 Convalescent Plasma. Proc. Natl. Acad. Sci. USA 2021, 118, e2103154118. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Björk, J.; Bonander, C.; Moghaddassi, M.; Rasmussen, M.; Malmqvist, U.; Inghammar, M.; Kahn, F. COVID-19 Vaccine Effectiveness against Severe Disease from SARS-CoV-2 Omicron BA.1 and BA.2 Subvariants–Surveillance Results from Southern Sweden, December 2021 to March 2022. Eurosurveillance 2022, 27, 2200322. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Surveillance Report: Week 15. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1069256/Vaccine_surveillance_report_-_week_15.pdf (accessed on 14 May 2022).
- Suah, J.L.; Husin, M.; Tok, P.S.K.; Tng, B.H.; Thevananthan, T.; Low, E.V.; Appannan, M.R.; Zin, F.M.; Zin, S.M.; Yahaya, H.; et al. Waning COVID-19 Vaccine Effectiveness for BNT162b2 and CoronaVac in Malaysia: An Observational Study. Int. J. Infect. Dis. 2022, 119, 69–76. [Google Scholar] [CrossRef]
- Wright, B.J.; Tideman, S.; Diaz, G.A.; French, T.; Parsons, G.T.; Robicsek, A. Comparative Vaccine Effectiveness against Severe COVID-19 over Time in US Hospital Administrative Data: A Case-Control Study. Lancet Respir. Med. 2022, 10, 557–565. [Google Scholar] [CrossRef]
- Immunogenicity of BA.5 Bivalent mRNA Vaccine Boosters|NEJM. Available online: https://www.nejm.org/doi/full/10.1056/NEJMc2213948 (accessed on 18 September 2023).
- Link-Gelles, R.; Ciesla, A.A.; Fleming-Dutra, K.E.; Smith, Z.R.; Britton, A.; Wiegand, R.E.; Miller, J.D.; Accorsi, E.K.; Schrag, S.J.; Verani, J.R.; et al. Effectiveness of Bivalent mRNA Vaccines in Preventing Symptomatic SARS-CoV-2 Infection—Increasing Community Access to Testing Program, United States, September-November 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising Antibody Titres as Predictors of Protection against SARS-CoV-2 Variants and the Impact of Boosting: A Meta-Analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- China Eases ‘Zero Covid’ Restrictions in Victory for Protesters. Available online: https://www.nytimes.com/2022/12/07/world/asia/china-zero-covid-protests.html (accessed on 18 September 2023).
- CDC Streamlines COVID-19 Guidance to Help the Public Better Protect Themselves and Understand Their Risk. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/media/releases/2022/p0811-covid-guidance.html (accessed on 18 September 2023).
- Focosi, D. Molnupiravir: From Hope to Epic Fail? Viruses 2022, 14, 2560. [Google Scholar] [CrossRef]
- Pfizer Reports Additional Data on PAXLOVIDTM Supporting Upcoming New Drug Application Submission to U.S. FDA. Available online: https://www.businesswire.com/news/home/20220613005755/en/Pfizer-Reports-Additional-Data-on-PAXLOVID%E2%84%A2-Supporting-Upcoming-New-Drug-Application-Submission-to-U.S.-FDA (accessed on 18 September 2023).
- Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab (accessed on 28 September 2023).
- There Are No Useful Monoclonal Antibody Treatments Left Against New COVID Variants. Available online: https://www.usnews.com/news/health-news/articles/2022-12-05/there-are-no-useful-monoclonal-antibody-treatments-left-against-new-covid-variants (accessed on 18 September 2023).
- Coronavirus (COVID-19) Update: FDA Limits Use of Certain Monoclonal Antibodies to Treat COVID-19 Due to the Omicron Variant. Available online: https://www.medpagetoday.com/infectiousdisease/covid19/113392 (accessed on 18 September 2023).
- Hashem, A.M.; Algaissi, A.; Almahboub, S.A.; Alfaleh, M.A.; Abujamel, T.S.; Alamri, S.S.; Alluhaybi, K.A.; Hobani, H.I.; AlHarbi, R.H.; Alsulaiman, R.M.; et al. Early Humoral Response Correlates with Disease Severity and Outcomes in COVID-19 Patients. Viruses 2020, 12, 1390. [Google Scholar] [CrossRef]
- Maier, H.E.; Balmaseda, A.; Ojeda, S.; Cerpas, C.; Sanchez, N.; Plazaola, M.; van Bakel, H.; Kubale, J.; Lopez, R.; Saborio, S.; et al. An Immune Correlate of SARS-CoV-2 Infection and Severity of Reinfections. medRxiv 2021. [Google Scholar] [CrossRef]
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.-L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020, 58, e02107-20. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of Protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.B.; Montefiori, D.C.; McDermott, A.B.; Fong, Y.; Benkeser, D.; Deng, W.; Zhou, H.; Houchens, C.R.; Martins, K.; Jayashankar, L.; et al. Immune Correlates Analysis of the mRNA-1273 COVID-19 Vaccine Efficacy Clinical Trial. Science 2022, 375, 43–50. [Google Scholar] [CrossRef]
- Cohen, J.; Stuart, R.; Rosenfeld, K.; Lyons, H.; White, M.; Kerr, C.; Klein, D.; Famulare, M. Quantifying the Role of Naturally- and Vaccine-Derived Neutralizing Antibodies as a Correlate of Protection against COVID-19 Variant. medRxiv 2021. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron Extensively but Incompletely Escapes Pfizer BNT162b2 Neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Cromer, D.; Reynaldi, A.; Steain, M.; Triccas, J.A.; Davenport, M.P.; Khoury, D.S. Relating In Vitro Neutralization Level and Protection in the CVnCoV (CUREVAC) Trial. Clin. Infect. Dis. 2022, 75, e878–e879. [Google Scholar] [CrossRef]
- Koutsakos, M.; Lee, W.S.; Reynaldi, A.; Tan, H.-X.; Gare, G.; Kinsella, P.; Liew, K.C.; Taiaroa, G.; Williamson, D.A.; Kent, H.E.; et al. The Magnitude and Timing of Recalled Immunity after Breakthrough Infection Is Shaped by SARS-CoV-2 Variants. Immunity 2022, 55, 1316–1326.e4. [Google Scholar] [CrossRef]
- The T-Cell Covid Cavalry. Available online: https://www.wsj.com/articles/the-t-cell-covid-cavalry-antibodies-vaccines-omicron-11640906490 (accessed on 22 September 2023).
- Leslie, M. T Cells Found in Coronavirus Patients “bode Well” for Long-Term Immunity. Science 2020, 368, 809–810. [Google Scholar] [CrossRef]
- T Cells Protect against COVID-19 in Absence of Antibody Response. Available online: https://www.nih.gov/news-events/nih-research-matters/t-cells-protect-against-covid-19-absence-antibody-response (accessed on 18 September 2023).
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 Vaccination Induces Immunological T Cell Memory Able to Cross-Recognize Variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological Memory to SARS-CoV-2 Assessed for up to 8 Months after Infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Guo, L.; Wang, G.; Wang, Y.; Zhang, Q.; Ren, L.; Gu, X.; Huang, T.; Zhong, J.; Wang, Y.; Wang, X.; et al. SARS-CoV-2-Specific Antibody and T-Cell Responses 1 Year after Infection in People Recovered from COVID-19: A Longitudinal Cohort Study. Lancet Microbe 2022, 3, e348–e356. [Google Scholar] [CrossRef]
- Tenforde, M.W. Early Estimates of Bivalent mRNA Vaccine Effectiveness in Preventing COVID-19–Associated Emergency Department or Urgent Care Encounters and Hospitalizations Among Immunocompetent Adults—VISION Network, Nine States, September–November 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 1616–1624. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 SeroHub. Available online: https://covid19serohub.nih.gov/ (accessed on 18 September 2023).
- EXCLUSIVE WHO Estimates COVID-19 Boosters Needed Yearly for Most Vulnerable. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/exclusive-who-estimates-covid-19-boosters-needed-yearly-most-vulnerable-2021-06-24/ (accessed on 18 September 2023).
- Oliver, S. Evidence to Recommendation Framework; COVID-19 Vaccination Guidance. In Proceedings of the ACIP Meeting, Atlanta, GA, USA, 17 June 2022. [Google Scholar]
- Weekly COVID-19 Vaccination Dashboard|COVIDVaxView|CDC. Available online: https://www.cdc.gov/covidvaxview/weekly-dashboard/ (accessed on 16 August 2024).
- Acko, T. COVID-19 Booster Dose in India: Eligibility & Registration. Available online: https://www.acko.com/health-insurance/covid-19-booster-dose-in-india/ (accessed on 16 August 2024).
- Current Rules and Recommendations. Available online: https://www.krisinformation.se/en/hazards-and-risks/disasters-and-incidents/2020/official-information-on-the-new-coronavirus/current-rules-and-recommendations (accessed on 16 August 2024).
- Who’s Eligible for the 2024 COVID-19 Vaccine, or ‘Autumn Booster’?—UK Health Security Agency. Available online: https://ukhsa.blog.gov.uk/2024/08/02/whos-eligible-for-the-2024-covid-19-vaccine-or-autumn-booster/ (accessed on 16 August 2024).
- Stoddard, M.; Yuan, L.; Sarkar, S.; Mangalaganesh, S.; Nolan, R.P.; Bottino, D.; Hather, G.; Hochberg, N.S.; White, L.F.; Chakravarty, A. Heterogeneity in Vaccinal Immunity to SARS-CoV-2 Can Be Addressed by a Personalized Booster Strategy. Vaccines 2023, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Morrone, M.C.; Patrono, C.; Santoro, M.G.; Schiaffino, S.; Remuzzi, G.; Bussolati, G. COVID-19 Commission of the Accademia Nazionale dei Lincei Long Covid: Where We Stand and Challenges Ahead. Cell Death Differ. 2022, 29, 1891–1900. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after Breakthrough SARS-CoV-2 Infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Stoddard, M.; Yuan, L.; Sarkar, S.; Mazewski, M.; van Egeren, D.; Mangalaganesh, S.; Nolan, R.P.; Rogers, M.S.; Hather, G.; White, L.F.; et al. Shielding under Endemic SARS-CoV-2 Conditions Is Easier Said than Done: A Model-Based Analysis. medRxiv 2023. [Google Scholar] [CrossRef]
- New Data Shows Long Covid Is Keeping as Many as 4 Million People out of Work. Available online: https://www.brookings.edu/articles/new-data-shows-long-covid-is-keeping-as-many-as-4-million-people-out-of-work/ (accessed on 18 September 2023).
- Long-Haulers and Labor Market Outcomes. Available online: https://www.minneapolisfed.org/research/institute-working-papers/long-haulers-and-labor-market-outcomes (accessed on 18 September 2023).
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 Vaccination on the Risk of Developing Long-COVID and on Existing Long-COVID Symptoms: A Systematic Review. EClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association Between BNT162b2 Vaccination and Long COVID After Infections Not Requiring Hospitalization in Health Care Workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12422. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective Effect of COVID-19 Vaccination against Long COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef]
- Man, M.A.; Rosca, D.; Bratosin, F.; Fira-Mladinescu, O.; Ilie, A.C.; Burtic, S.-R.; Fildan, A.P.; Fizedean, C.M.; Jianu, A.M.; Negrean, R.A.; et al. Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccines 2024, 12, 189. [Google Scholar] [CrossRef]
- Kim, S.; Kang, H. Is the Vaccine for COVID-19 Effective in Preventing and Treating Long COVID? J. Stud. Res. 2022, 11. [Google Scholar] [CrossRef]
- Wang, K.; Long, Q.-X.; Deng, H.-J.; Hu, J.; Gao, Q.-Z.; Zhang, G.-J.; He, C.-L.; Huang, L.-Y.; Hu, J.-L.; Chen, J.; et al. Longitudinal Dynamics of the Neutralizing Antibody Response to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Clin. Infect. Dis. 2021, 73, e531–e539. [Google Scholar] [CrossRef]
- Leung, K.; Jit, M.; Lau, E.H.Y.; Wu, J.T. Social Contact Patterns Relevant to the Spread of Respiratory Infectious Diseases in Hong Kong. Sci. Rep. 2017, 7, 7974. [Google Scholar] [CrossRef]
- United States Demographic Statistics. Available online: https://www.infoplease.com/us/census/demographic-statistics (accessed on 18 September 2023).
- SARS-CoV-2 Infection Rates of Antibody-Positive Compared with Antibody-Negative Health-Care Workers in England: A Large, Multicentre, Prospective Cohort Study (SIREN)—The Lancet. Available online: https://www.thelancet.com/article/S0140-6736(21)00675-9/fulltext (accessed on 22 January 2023).
- Rocklöv, J.; Sjödin, H.; Wilder-Smith, A. COVID-19 Outbreak on the Diamond Princess Cruise Ship: Estimating the Epidemic Potential and Effectiveness of Public Health Countermeasures. J. Travel Med. 2020, 27, taaa030. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of Covid-19 mRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef]
- Khan, K.; Karim, F.; Cele, S.; Reedoy, K.; San, J.E.; Lustig, G.; Tegally, H.; Rosenberg, Y.; Bernstein, M.; Jule, Z.; et al. Omicron Infection Enhances Delta Antibody Immunity in Vaccinated Persons. Nature 2022, 607, 356–359. [Google Scholar] [CrossRef]
- Levin, A.T.; Hanage, W.P.; Owusu-Boaitey, N.; Cochran, K.B.; Walsh, S.P.; Meyerowitz-Katz, G. Assessing the Age Specificity of Infection Fatality Rates for COVID-19: Systematic Review, Meta-Analysis, and Public Policy Implications. Eur. J. Epidemiol. 2020, 35, 1123–1138. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Y.; Zhao, Y.; He, D. Reduction in the Infection Fatality Rate of Omicron Variant Compared with Previous Variants in South Africa. Int. J. Infect. Dis. 2022, 120, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Van Egeren, D.; Stoddard, M.; Novokhodko, A.; Rogers, M.S.; Joseph-McCarthy, D.; Zetter, B.; Chakravarty, A. Rapid Relaxation of Pandemic Restrictions after Vaccine Rollout Favors Growth of SARS-CoV-2 Variants: A Model-Based Analysis. PLoS ONE 2021, 16, e0258997. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rocklöv, J. The Effective Reproductive Number of the Omicron Variant of SARS-CoV-2 Is Several Times Relative to Delta. J. Travel Med. 2022, 29, taac037. [Google Scholar] [CrossRef]
- Du, Z.; Hong, H.; Wang, S.; Ma, L.; Liu, C.; Bai, Y.; Adam, D.C.; Tian, L.; Wang, L.; Lau, E.H.Y.; et al. Reproduction Number of the Omicron Variant Triples That of the Delta Variant. Viruses 2022, 14, 821. [Google Scholar] [CrossRef]
- Cao, Y.; Jian, F.; Wang, J.; Yu, Y.; Song, W.; Yisimayi, A.; Wang, J.; An, R.; Chen, X.; Zhang, N.; et al. Imprinted SARS-CoV-2 Humoral Immunity Induces Convergent Omicron RBD Evolution. Nature 2022, 614, 521–529. [Google Scholar] [CrossRef]
- Khan, K.; Karim, F.; Cele, S.; San, J.E.; Lustig, G.; Bernstein, M.; Ganga, Y.; Jule, Z.; Reedoy, K.; Ngcobo, N.; et al. 2 Omicron Infection Enhances Neutralizing Immunity against the Delta Variant. medRxiv 2022. [Google Scholar] [CrossRef]
- Hein, S.; Mhedhbi, I.; Zahn, T.; Sabino, C.; Benz, N.I.; Husria, Y.; Renelt, P.M.; Braun, F.; Oberle, D.; Maier, T.J.; et al. Quantitative and Qualitative Difference in Antibody Response against Omicron and Ancestral SARS-CoV-2 after Third and Fourth Vaccination. Vaccines 2022, 10, 796. [Google Scholar] [CrossRef]
- Chu, L.; Vrbicky, K.; Montefiori, D.; Huang, W.; Nestorova, B.; Chang, Y.; Carfi, A.; Edwards, D.K.; Oestreicher, J.; Legault, H.; et al. Immune Response to SARS-CoV-2 after a Booster of mRNA-1273: An Open-Label Phase 2 Trial. Nat. Med. 2022, 28, 1042–1049. [Google Scholar] [CrossRef]
- Joseph, A. White House Signals Most People Will Only Need Annual Covid Booster. STAT, 6 September.
- CDC COVID Data Tracker Weekly Review. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/past-reports/index.html (accessed on 17 January 2023).
- Fisman, D.N.; Tuite, A.R. Evaluation of the Relative Virulence of Novel SARS-CoV-2 Variants: A Retrospective Cohort Study in Ontario, Canada. CMAJ 2021, 193, E1619–E1625. [Google Scholar] [CrossRef]
- Davies, N.G.; Abbott, S.; Barnard, R.C.; Jarvis, C.I.; Kucharski, A.J.; Munday, J.D.; Pearson, C.A.B.; Russell, T.W.; Tully, D.C.; Washburne, A.D.; et al. Estimated Transmissibility and Impact of SARS-CoV-2 Lineage B.1.1.7 in England. Science 2021, 372, eabg3055. [Google Scholar] [CrossRef] [PubMed]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a Third Dose of the BNT162b2 mRNA COVID-19 Vaccine for Preventing Severe Outcomes in Israel: An Observational Study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of Effectiveness of Vaccines against SARS-CoV-2 Infection and COVID-19 Disease: Results of a Systematic Review and Meta-Regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Helfand, M.; Fiordalisi, C.; Wiedrick, J.; Ramsey, K.L.; Armstrong, C.; Gean, E.; Winchell, K.; Arkhipova-Jenkins, I. Risk for Reinfection After SARS-CoV-2: A Living, Rapid Review for American College of Physicians Practice Points on the Role of the Antibody Response in Conferring Immunity Following SARS-CoV-2 Infection. Ann. Intern. Med. 2022, 175, 547–555. [Google Scholar] [CrossRef]
- SARS-CoV-2 Variants of Concern and Variants under Investigation in England—Technical Briefing 28. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1033101/Technical_Briefing_28_12_Nov_2021.pdf (accessed on 20 September 2023).
- Ogunwole, S.U.; Rabe, M.A.; Roberts, A.W.; Caplan, Z. Adult Population Grew Faster Than Total Population From 2010 to 2020. Available online: https://www.census.gov/library/stories/2021/08/united-states-adult-population-grew-faster-than-nations-total-population-from-2010-to-2020.html (accessed on 25 January 2023).
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; et al. Coronavirus Pandemic (COVID-19). Our World in Data. 2020. Available online: https://ourworldindata.org/coronavirus (accessed on 15 January 2023).
- Stoddard, M.; Novokhodko, A.; Sarkar, S.; Van Egeren, D.; White, L.F.; Hochberg, N.S.; Rogers, M.S.; Zetter, B.; Joseph-McCarthy, D.; Chakravarty, A. Endemicity Is Not a Victory: The Unmitigated Downside Risks of Widespread SARS-CoV-2 Transmission. COVID 2022, 2, 1689–1709. [Google Scholar] [CrossRef]
- Bottino, D.; Hather, G.; Yuan, L.; Stoddard, M.; White, L.; Chakravarty, A. Using Mixed-Effects Modeling to Estimate Decay Kinetics of Response to SARS-CoV-2 Infection. Antib. Ther. 2021, 4, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Delta Variant Already Dominant in, U.S. CDC Estimates Show. Available online: https://www.reuters.com/world/us/delta-variant-already-dominant-us-cdc-estimates-show-2021-07-07/ (accessed on 18 September 2023).
- COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 18 September 2023).
- United States COVID—Coronavirus Statistics. Available online: https://www.worldometers.info/coronavirus/country/us/ (accessed on 18 September 2023).
- Cases, Data, and Surveillance. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/past-reports/03182022.html (accessed on 18 September 2023).
- Government of Canada, S.C. Experiences of Canadians with Long-Term Symptoms Following COVID-19. Available online: https://www150.statcan.gc.ca/n1/pub/75-006-x/2023001/article/00015-eng.htm (accessed on 14 March 2025).
- Van Egeren, D.; Novokhodko, A.; Stoddard, M.; Tran, U.; Zetter, B.; Rogers, M.; Pentelute, B.L.; Carlson, J.M.; Hixon, M.; Joseph-McCarthy, D.; et al. Risk of Rapid Evolutionary Escape from Biomedical Interventions Targeting SARS-CoV-2 Spike Protein. PLoS ONE 2021, 16, e0250780. [Google Scholar] [CrossRef]
- Stoddard, M.; Sarkar, S.; Yuan, L.; Nolan, R.P.; White, D.E.; White, L.F.; Hochberg, N.S.; Chakravarty, A. Beyond the New Normal: Assessing the Feasibility of Vaccine-Based Suppression of SARS-CoV-2. PLoS ONE 2021, 16, e0254734. [Google Scholar] [CrossRef]
- Egeren, D.V.; Novokhodko, A.; Stoddard, M.; Tran, U.; Zetter, B.; Rogers, M.; Pentelute, B.L.; Carlson, J.M.; Hixon, M.; Joseph-McCarthy, D.; et al. Risk of Evolutionary Escape from Neutralizing Antibodies Targeting SARS-CoV-2 Spike Protein. medRxiv 2020. [Google Scholar] [CrossRef]
- Stoddard, M.; Van Egeren, D.; Johnson, K.E.; Rao, S.; Furgeson, J.; White, D.E.; Nolan, R.P.; Hochberg, N.; Chakravarty, A. Individually Optimal Choices Can Be Collectively Disastrous in COVID-19 Disease Control. BMC Public Health 2021, 21, 832. [Google Scholar] [CrossRef]
- Alejo, J.L.; Mitchell, J.; Chiang, T.P.-Y.; Abedon, A.T.; Boyarsky, B.J.; Avery, R.K.; Tobian, A.A.R.; Levan, M.L.; Massie, A.B.; Garonzik-Wang, J.M.; et al. Antibody Response to a Fourth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Transplantation 2021, 105, e280–e281. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Thaunat, O.; Benotmane, I.; Masset, C.; Blancho, G. Antibody Response to a Fourth Messenger RNA COVID-19 Vaccine Dose in Kidney Transplant Recipients: A Case Series. Ann. Intern. Med. 2022, 175, 455–456. [Google Scholar] [CrossRef]
- Abedon, A.T.; Teles, M.S.; Alejo, J.L.; Kim, J.D.; Mitchell, J.; Chiang, T.P.Y.; Avery, R.K.; Tobian, A.A.R.; Levan, M.L.; Warren, D.S.; et al. Improved Antibody Response After a Fifth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Transplantation 2022, 106, e262–e263. [Google Scholar] [CrossRef]
- Parisi, S.G.; Mengoli, C.; Basso, M.; Vicenti, I.; Gatti, F.; Scaggiante, R.; Fiaschi, L.; Giammarino, F.; Iannetta, M.; Malagnino, V.; et al. Long-Term Longitudinal Analysis of Neutralizing Antibody Response to Three Vaccine Doses in a Real-Life Setting of Previously SARS-CoV-2 Infected Healthcare Workers: A Model for Predicting Response to Further Vaccine Doses. Vaccines 2022, 10, 1237. [Google Scholar] [CrossRef]
- Teles, M.; Connolly, C.M.; Frey, S.; Chiang, T.P.-Y.; Alejo, J.J.; Boyarsky, B.J.; Shah, A.A.; Albayda, J.; Christopher-Stine, L.; Werbel, W.A.; et al. Attenuated Response to Fourth Dose SARS-CoV-2 Vaccination in Patients with Autoimmune Disease: A Case Series. Ann. Rheum. Dis. 2022, 81, 738–740. [Google Scholar] [CrossRef]
- Gao, F.-X.; Wu, R.-X.; Shen, M.-Y.; Huang, J.-J.; Li, T.-T.; Hu, C.; Luo, F.-Y.; Song, S.-Y.; Mu, S.; Hao, Y.-N.; et al. Extended SARS-CoV-2 RBD Booster Vaccination Induces Humoral and Cellular Immune Tolerance in Mice. IScience 2022, 25, 105479. [Google Scholar] [CrossRef] [PubMed]
- Sellers, R.S. Translating Mouse Models: Immune Variation and Efficacy Testing. Toxicol. Pathol. 2017, 45, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Chemaitelly, H.; Ayoub, H.H.; Tang, P.; Hasan, M.R.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; Al-Kanaani, Z.; Al-Kuwari, E.; et al. Immune Imprinting and Protection against Repeat Reinfection with SARS-CoV-2. N. Engl. J. Med. 2022, 387, 1716–1718. [Google Scholar] [CrossRef]
- Collier, A.Y.; Miller, J.; Hachmann, N.P.; McMahan, K.; Liu, J.; Bondzie, E.A.; Gallup, L.; Rowe, M.; Schonberg, E.; Thai, S.; et al. Immunogenicity of BA.5 Bivalent mRNA Vaccine Boosters. N. Engl. J. Med. 2023, 388, 565–567. [Google Scholar] [CrossRef]
- Qu, P.; Faraone, J.N.; Evans, J.P.; Zheng, Y.-M.; Carlin, C.; Anghelina, M.; Stevens, P.; Fernandez, S.; Jones, D.; Panchal, A.; et al. Extraordinary Evasion of Neutralizing Antibody Response by Omicron XBB.1.5, CH.1.1 and CA.3.1 Variants. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gilboa, M.; Regev-Yochay, G.; Mandelboim, M.; Indenbaum, V.; Asraf, K.; Fluss, R.; Amit, S.; Mendelson, E.; Doolman, R.; Afek, A.; et al. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Netw. Open 2022, 5, e2231778. [Google Scholar] [CrossRef] [PubMed]
- Bellusci, L.; Grubbs, G.; Zahra, F.T.; Forgacs, D.; Golding, H.; Ross, T.M.; Khurana, S. Antibody Affinity and Cross-Variant Neutralization of SARS-CoV-2 Omicron BA.1, BA.2 and BA.3 Following Third mRNA Vaccination. Nat. Commun. 2022, 13, 4617. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.S.; Oh, M.L.H.; Phua, S.K.; Liang, Y.-L.; Aw, T.C. 210-Day Kinetics of Total, IgG, and Neutralizing Spike Antibodies across a Course of 3 Doses of BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 1703. [Google Scholar] [CrossRef]
- Liang, X.-M.; Xu, Q.-Y.; Jia, Z.-J.; Wu, M.-J.; Liu, Y.-Y.; Lin, L.-R.; Liu, L.-L.; Yang, T.-C. A Third Dose of an Inactivated Vaccine Dramatically Increased the Levels and Decay Times of Anti-SARS-CoV-2 Antibodies, but Disappointingly Declined Again: A Prospective, Longitudinal, Cohort Study at 18 Serial Time Points Over 368 Days. Front. Immunol. 2022, 13, 876037. [Google Scholar] [CrossRef]
- Dapporto, F.; Marchi, S.; Leonardi, M.; Piu, P.; Lovreglio, P.; Decaro, N.; Buonvino, N.; Stufano, A.; Lorusso, E.; Bombardieri, E.; et al. Antibody Avidity and Neutralizing Response against SARS-CoV-2 Omicron Variant after Infection or Vaccination. J. Immunol. Res. 2022, 2022, 4813199. [Google Scholar] [CrossRef]
- Plotkin, S.A. Correlates of Protection Induced by Vaccination. Clin. Vaccine Immunol. 2010, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Bruel, T.; Pinaud, L.; Tondeur, L.; Planas, D.; Staropoli, I.; Porrot, F.; Guivel-Benhassine, F.; Attia, M.; Pelleau, S.; Woudenberg, T.; et al. Neutralising Antibody Responses to SARS-CoV-2 Omicron among Elderly Nursing Home Residents Following a Booster Dose of BNT162b2 Vaccine: A Community-Based, Prospective, Longitudinal Cohort Study. EClinicalMedicine 2022, 51, 101576. [Google Scholar] [CrossRef]
- Sparks, J.A.; Wallace, Z.S.; Seet, A.M.; Gianfrancesco, M.A.; Izadi, Z.; Hyrich, K.L.; Strangfeld, A.; Gossec, L.; Carmona, L.; Mateus, E.F.; et al. Associations of Baseline Use of Biologic or Targeted Synthetic DMARDs with COVID-19 Severity in Rheumatoid Arthritis: Results from the COVID-19 Global Rheumatology Alliance Physician Registry. Ann. Rheum. Dis. 2021, 80, 1137–1146. [Google Scholar] [CrossRef]
- Bitoun, S.; Henry, J.; Desjardins, D.; Vauloup-Fellous, C.; Dib, N.; Belkhir, R.; Mouna, L.; Joly, C.; Bitu, M.; Ly, B.; et al. Rituximab Impairs B Cell Response But Not T Cell Response to COVID-19 Vaccine in Autoimmune Diseases. Arthritis Rheumatol. 2022, 74, 927–933. [Google Scholar] [CrossRef]
- Andersen, K.M.; Bates, B.A.; Rashidi, E.S.; Olex, A.L.; Mannon, R.B.; Patel, R.C.; Singh, J.; Sun, J.; Auwaerter, P.G.; Ng, D.K.; et al. Long-Term Use of Immunosuppressive Medicines and in-Hospital COVID-19 Outcomes: A Retrospective Cohort Study Using Data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022, 4, e33–e41. [Google Scholar] [CrossRef]
- Ekin, A.; Coskun, B.N.; Dalkilic, E.; Pehlivan, Y. The Effects of COVID-19 Infection on the Mortality of Patients Receiving Rituximab Therapy. Ir. J. Med. Sci. 2023, 192, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- Strangfeld, A.; Schäfer, M.; Gianfrancesco, M.A.; Lawson-Tovey, S.; Liew, J.W.; Ljung, L.; Mateus, E.F.; Richez, C.; Santos, M.J.; Schmajuk, G.; et al. Factors Associated with COVID-19-Related Death in People with Rheumatic Diseases: Results from the COVID-19 Global Rheumatology Alliance Physician-Reported Registry. Ann. Rheum. Dis. 2021, 80, 930–942. [Google Scholar] [CrossRef]
- Avouac, J.; Drumez, E.; Hachulla, E.; Seror, R.; Georgin-Lavialle, S.; El Mahou, S.; Pertuiset, E.; Pham, T.; Marotte, H.; Servettaz, A.; et al. COVID-19 Outcomes in Patients with Inflammatory Rheumatic and Musculoskeletal Diseases Treated with Rituximab: A Cohort Study. Lancet Rheumatol. 2021, 3, e419–e426. [Google Scholar] [CrossRef] [PubMed]
- Sormani, M.P.; De Rossi, N.; Schiavetti, I.; Carmisciano, L.; Cordioli, C.; Moiola, L.; Radaelli, M.; Immovilli, P.; Capobianco, M.; Trojano, M.; et al. Disease-Modifying Therapies and Coronavirus Disease 2019 Severity in Multiple Sclerosis. Ann. Neurol. 2021, 89, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Bsteh, G.; Dürauer, S.; Assar, H.; Hegen, H.; Heschl, B.; Leutmezer, F.; Pauli, F.D.; Gradl, C.; Traxler, G.; Zulehner, G.; et al. Humoral Immune Response after COVID-19 in Multiple Sclerosis: A Nation-Wide Austrian Study. Mult. Scler. J. 2021, 27, 2209–2218. [Google Scholar] [CrossRef]
- Gadani, S.P.; Reyes-Mantilla, M.; Jank, L.; Harris, S.; Douglas, M.; Smith, M.D.; Calabresi, P.A.; Mowry, E.M.; Fitzgerald, K.C.; Bhargava, P. Discordant Humoral and T Cell Immune Responses to SARS-CoV-2 Vaccination in People with Multiple Sclerosis on Anti-CD20 Therapy. medRxiv 2021. [Google Scholar] [CrossRef]
- Hada, M.; Mosholder, A.D.; Leishear, K.; Perez-Vilar, S. Systematic Review of Risk of SARS-CoV-2 Infection and Severity of COVID-19 with Therapies Approved to Treat Multiple Sclerosis. Neurol. Sci. 2022, 43, 1557–1567. [Google Scholar] [CrossRef]
- Mohanraj, D.; Baldwin, S.; Singh, S.; Gordon, A.; Whitelegg, A. Cellular and Humoral Responses to SARS-CoV-2 Vaccination in Immunosuppressed Patients. Cell Immunol. 2022, 373, 104501. [Google Scholar] [CrossRef]
- Calabrese, C.M.; Kirchner, E.; Husni, E.M.; Moss, B.P.; Fernandez, A.P.; Jin, Y.; Calabrese, L.H. Breakthrough SARS-CoV-2 Infections in Patients With Immune-Mediated Disease Undergoing B Cell-Depleting Therapy: A Retrospective Cohort Analysis. Arthritis Rheumatol. 2022, 74, 1906–1915. [Google Scholar] [CrossRef]
- Di Fusco, M.; Lin, J.; Vaghela, S.; Lingohr-Smith, M.; Nguyen, J.L.; Scassellati Sforzolini, T.; Judy, J.; Cane, A.; Moran, M.M. COVID-19 Vaccine Effectiveness among Immunocompromised Populations: A Targeted Literature Review of Real-World Studies. Expert Rev. Vaccines 2022, 21, 435–451. [Google Scholar] [CrossRef]
- Mues, K.E.; Kirk, B.; Patel, D.A.; Gelman, A.; Chavers, L.S.; Talarico, C.A.; Esposito, D.B.; Martin, D.; Mansi, J.; Chen, X.; et al. Real-World Comparative Effectiveness of mRNA-1273 and BNT162b2 Vaccines among Immunocompromised Adults Identified in Administrative Claims Data in the United States. Vaccine 2022, 40, 6730. [Google Scholar] [CrossRef] [PubMed]
- Hoff, L.S.; Ravichandran, N.; Shinjo, S.K.; Day, J.; Sen, P.; Junior, J.G.; Lilleker, J.B.; Joshi, M.; Agarwal, V.; Kardes, S.; et al. COVID-19 Severity and Vaccine Breakthrough Infections in Idiopathic Inflammatory Myopathies, Other Systemic Autoimmune and Inflammatory Diseases, and Healthy Controls: A Multicenter Cross-Sectional Study from the COVID-19 Vaccination in Autoimmune Diseases (COVAD) Survey. Rheumatol. Int. 2023, 43, 47–58. [Google Scholar] [CrossRef]
- Galmiche, S.; Luong Nguyen, L.B.; Tartour, E.; de Lamballerie, X.; Wittkop, L.; Loubet, P.; Launay, O. Immunological and Clinical Efficacy of COVID-19 Vaccines in Immunocompromised Populations: A Systematic Review. Clin. Microbiol. Infect. 2022, 28, 163–177. [Google Scholar] [CrossRef]
- Oberhardt, V.; Luxenburger, H.; Kemming, J.; Schulien, I.; Ciminski, K.; Giese, S.; Csernalabics, B.; Lang-Meli, J.; Janowska, I.; Staniek, J.; et al. Rapid and Stable Mobilization of CD8+ T Cells by SARS-CoV-2 mRNA Vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Wu, X.; Krist, M.P.; Hsiang, T.-Y.; Campbell, V.L.; McClurkan, C.L.; Favors, S.M.; Hemingway, L.A.; Godornes, C.; Tong, D.Q.; et al. T Cell Response to Intact SARS-CoV-2 Includes Coronavirus Cross-Reactive and Variant-Specific Components. JCI Insight 2022, 7, e158126. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T Cell Immune Response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA Vaccines Induce Durable Immune Memory to SARS-CoV-2 and Variants of Concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Rydyznski Moderbacher, C.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-Dose mRNA-1273 COVID-19 Vaccine Generates Durable Memory Enhanced by Cross-Reactive T Cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef]
- Riou, C.; Keeton, R.; Moyo-Gwete, T.; Hermanus, T.; Kgagudi, P.; Baguma, R.; Valley-Omar, Z.; Smith, M.; Tegally, H.; Doolabh, D.; et al. Escape from Recognition of SARS-CoV-2 Variant Spike Epitopes but Overall Preservation of T Cell Immunity. Sci. Transl. Med. 2022, 14, eabj6824. [Google Scholar] [CrossRef]
- Grifoni, A.; Sidney, J.; Vita, R.; Peters, B.; Crotty, S.; Weiskopf, D.; Sette, A. SARS-CoV-2 Human T Cell Epitopes: Adaptive Immune Response against COVID-19. Cell Host Microbe 2021, 29, 1076–1092. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 Variants on the Total CD4+ and CD8+ T Cell Reactivity in Infected or Vaccinated Individuals. Cell Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef] [PubMed]
- Call in the T-Cell Cavalry to Fight COVID in the Immunocompromised. Available online: https://www.medpagetoday.com/opinion/second-opinions/93805 (accessed on 19 September 2023).
- The Mystery That Could Explain Why COVID Vaccines Work so Well. Available online: https://www.smh.com.au/national/the-mystery-that-could-explain-why-covid-vaccines-work-so-well-20210427-p57mq0.html (accessed on 19 September 2023).
- Zhang, H.; Deng, S.; Ren, L.; Zheng, P.; Hu, X.; Jin, T.; Tan, X. Profiling CD8+ T Cell Epitopes of COVID-19 Convalescents Reveals Reduced Cellular Immune Responses to SARS-CoV-2 Variants. Cell Rep. 2021, 36, 109708. [Google Scholar] [CrossRef]
- Agerer, B.; Koblischke, M.; Gudipati, V.; Montaño-Gutierrez, L.F.; Smyth, M.; Popa, A.; Genger, J.-W.; Endler, L.; Florian, D.M.; Mühlgrabner, V.; et al. SARS-CoV-2 Mutations in MHC-I-Restricted Epitopes Evade CD8+ T Cell Responses. Sci. Immunol. 2021, 6, eabg6461. [Google Scholar] [CrossRef]
- de Silva, T.I.; Liu, G.; Lindsey, B.B.; Dong, D.; Moore, S.C.; Hsu, N.S.; Shah, D.; Wellington, D.; Mentzer, A.J.; Angyal, A.; et al. The Impact of Viral Mutations on Recognition by SARS-CoV-2 Specific T Cells. IScience 2021, 24, 103353. [Google Scholar] [CrossRef] [PubMed]
- Pretti, M.A.M.; Galvani, R.G.; Scherer, N.M.; Farias, A.S.; Boroni, M. In Silico Analysis of Mutant Epitopes in New SARS-CoV-2 Lineages Suggest Global Enhanced CD8+ T Cell Reactivity and Also Signs of Immune Response Escape. Infect. Genet. Evol. 2022, 99, 105236. [Google Scholar] [CrossRef] [PubMed]
- Tye, E.X.C.; Jinks, E.; Haigh, T.A.; Kaul, B.; Patel, P.; Parry, H.M.; Newby, M.L.; Crispin, M.; Kaur, N.; Moss, P.; et al. Mutations in SARS-CoV-2 Spike Protein Impair Epitope-Specific CD4+ T Cell Recognition. Nat. Immunol. 2022, 23, 1726–1734. [Google Scholar] [CrossRef]
- Dolton, G.; Rius, C.; Hasan, M.S.; Wall, A.; Szomolay, B.; Behiry, E.; Whalley, T.; Southgate, J.; Fuller, A.; COVID-19 Genomics UK (COG-UK) consortium; et al. Emergence of Immune Escape at Dominant SARS-CoV-2 Killer T Cell Epitope. Cell 2022, 185, 2936–2951.e19. [Google Scholar] [CrossRef]
- Pastorio, C.; Zech, F.; Noettger, S.; Jung, C.; Jacob, T.; Sanderson, T.; Sparrer, K.M.J.; Kirchhoff, F. Determinants of Spike Infectivity, Processing, and Neutralization in SARS-CoV-2 Omicron Subvariants BA.1 and BA.2. Cell Host Microbe 2022, 30, 1255–1268.e5. [Google Scholar] [CrossRef]
- Kombe Kombe, A.J.; Biteghe, F.A.N.; Ndoutoume, Z.N.; Jin, T. CD8+ T-Cell Immune Escape by SARS-CoV-2 Variants of Concern. Front. Immunol. 2022, 13, 962079. [Google Scholar] [CrossRef]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal Analysis Shows Durable and Broad Immune Memory after SARS-CoV-2 Infection with Persisting Antibody Responses and Memory B and T Cells. medRxiv 2021. [Google Scholar] [CrossRef]
- May, D.H.; Rubin, B.E.R.; Dalai, S.C.; Patel, K.; Shafiani, S.; Elyanow, R.; Noakes, M.T.; Snyder, T.M.; Robins, H.S. Immunosequencing and Epitope Mapping Reveal Substantial Preservation of the T Cell Immune Response to Omicron Generated by SARS-CoV-2 Vaccines. medRxiv 2021. [Google Scholar] [CrossRef]
- Detect and Analyze Variants of SARS-CoV-2. Available online: https://cov-spectrum.org (accessed on 19 September 2023).
- Ahmed, S.F.; Quadeer, A.A.; McKay, M.R. SARS-CoV-2 T Cell Responses Elicited by COVID-19 Vaccines or Infection Are Expected to Remain Robust against Omicron. Viruses 2022, 14, 79. [Google Scholar] [CrossRef]
- De Marco, L.; D’Orso, S.; Pirronello, M.; Verdiani, A.; Termine, A.; Fabrizio, C.; Capone, A.; Sabatini, A.; Guerrera, G.; Placido, R.; et al. Assessment of T-Cell Reactivity to the SARS-CoV-2 Omicron Variant by Immunized Individuals. JAMA Netw. Open 2022, 5, e2210871. [Google Scholar] [CrossRef]
- Patalon, T.; Saciuk, Y.; Peretz, A.; Perez, G.; Lurie, Y.; Maor, Y.; Gazit, S. Waning Effectiveness of the Third Dose of the BNT162b2 mRNA COVID-19 Vaccine. Nat. Commun. 2022, 13, 3203. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral Loads of Delta-Variant SARS-CoV-2 Breakthrough Infections after Vaccination and Booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, B.; Lotan, R.; Kalkstein, N.; Peretz, A.; Perez, G.; Ben-Tov, A.; Chodick, G.; Gazit, S.; Patalon, T. Correlation of SARS-CoV-2-Breakthrough Infections to Time-from-Vaccine. Nat. Commun. 2021, 12, 6379. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Tang, P.; Hasan, M.R.; AlMukdad, S.; Yassine, H.M.; Benslimane, F.M.; Al Khatib, H.A.; Coyle, P.; Ayoub, H.H.; Al Kanaani, Z.; et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N. Engl. J. Med. 2021, 385, e83. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity of, and Effectiveness of mRNA Vaccines against, Covid-19 from Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: Prospective Observational Study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef]
- Dowell, A.C.; Ireland, G.; Zuo, J.; Moss, P.; Ladhani, S.; sKIDs Investigation Team. Association of Spike-Specific T Cells With Relative Protection From Subsequent SARS-CoV-2 Omicron Infection in Young Children. JAMA Pediatr. 2023, 177, 96–97. [Google Scholar] [CrossRef]
- Hajian-Tilaki, K. Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J. Intern. Med. 2013, 4, 627–635. [Google Scholar] [PubMed]
- Pardieck, I.N.; van der Sluis, T.C.; van der Gracht, E.T.I.; Veerkamp, D.M.B.; Behr, F.M.; van Duikeren, S.; Beyrend, G.; Rip, J.; Nadafi, R.; Beyranvand Nejad, E.; et al. A Third Vaccination with a Single T Cell Epitope Confers Protection in a Murine Model of SARS-CoV-2 Infection. Nat. Commun. 2022, 13, 3966. [Google Scholar] [CrossRef]
- Bilich, T.; Roerden, M.; Maringer, Y.; Nelde, A.; Heitmann, J.S.; Dubbelaar, M.L.; Peter, A.; Hörber, S.; Bauer, J.; Rieth, J.; et al. Preexisting and Post-COVID-19 Immune Responses to SARS-CoV-2 in Patients with Cancer. Cancer Discov. 2021, 11, 1982–1995. [Google Scholar] [CrossRef]
- Bange, E.M.; Han, N.A.; Wileyto, P.; Kim, J.Y.; Gouma, S.; Robinson, J.; Greenplate, A.R.; Hwee, M.A.; Porterfield, F.; Owoyemi, O.; et al. CD8+ T Cells Contribute to Survival in Patients with COVID-19 and Hematologic Cancer. Nat. Med. 2021, 27, 1280–1289. [Google Scholar] [CrossRef]
- Kundu, R.; Narean, J.S.; Wang, L.; Fenn, J.; Pillay, T.; Fernandez, N.D.; Conibear, E.; Koycheva, A.; Davies, M.; Tolosa-Wright, M.; et al. Cross-Reactive Memory T Cells Associate with Protection against SARS-CoV-2 Infection in COVID-19 Contacts. Nat. Commun. 2022, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological Dysfunction Persists for 8 Months Following Initial Mild-to-Moderate SARS-CoV-2 Infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; McConnell, S.; Casadevall, A.; Cappello, E.; Valdiserra, G.; Tuccori, M. Monoclonal Antibody Therapies against SARS-CoV-2. Lancet Infect. Dis. 2022, 22, e311–e326. [Google Scholar] [CrossRef]
- Dagotto, G.; Ventura, J.D.; Martinez, D.R.; Anioke, T.; Chung, B.S.; Siamatu, M.; Barrett, J.; Miller, J.; Schäfer, A.; Yu, J.; et al. Immunogenicity and Protective Efficacy of a Rhesus Adenoviral Vaccine Targeting Conserved COVID-19 Replication Transcription Complex. npj Vaccines 2022, 7, 125. [Google Scholar] [CrossRef]
- Thieme, C.J.; Anft, M.; Paniskaki, K.; Blazquez-Navarro, A.; Doevelaar, A.; Seibert, F.S.; Hoelzer, B.; Konik, M.J.; Berger, M.M.; Brenner, T.; et al. Robust T Cell Response Toward Spike, Membrane, and Nucleocapsid SARS-CoV-2 Proteins Is Not Associated with Recovery in Critical COVID-19 Patients. Cell Rep. Med. 2020, 1, 100092. [Google Scholar] [CrossRef]
- Govender, M.; Hopkins, F.R.; Göransson, R.; Svanberg, C.; Shankar, E.M.; Hjorth, M.; Nilsdotter-Augustinsson, Å.; Sjöwall, J.; Nyström, S.; Larsson, M. T Cell Perturbations Persist for at Least 6 Months Following Hospitalization for COVID-19. Front. Immunol. 2022, 13, 931039. [Google Scholar] [CrossRef]
- Flacco, M.E.; Acuti Martellucci, C.; Baccolini, V.; De Vito, C.; Renzi, E.; Villari, P.; Manzoli, L. Risk of Reinfection and Disease after SARS-CoV-2 Primary Infection: Meta-Analysis. Eur. J. Clin. Investig. 2022, 52, e13845. [Google Scholar] [CrossRef]
- Bacher, P.; Rosati, E.; Esser, D.; Martini, G.R.; Saggau, C.; Schiminsky, E.; Dargvainiene, J.; Schröder, I.; Wieters, I.; Khodamoradi, Y.; et al. Low-Avidity CD4+ T Cell Responses to SARS-CoV-2 in Unexposed Individuals and Humans with Severe COVID-19. Immunity 2020, 53, 1258–1271.e5. [Google Scholar] [CrossRef]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The Impact of Pre-Existing Cross-Reactive Immunity on SARS-CoV-2 Infection and Vaccine Responses. Nat. Rev. Immunol. 2023, 23, 304–316. [Google Scholar] [CrossRef]
- Forni, D.; Cagliani, R.; Pontremoli, C.; Mozzi, A.; Pozzoli, U.; Clerici, M.; Sironi, M. Antigenic Variation of SARS-CoV-2 in Response to Immune Pressure. Mol. Ecol. 2020, 30, 3548–3559. [Google Scholar] [CrossRef]
- Nelde, A.; Bilich, T.; Heitmann, J.S.; Maringer, Y.; Salih, H.R.; Roerden, M.; Lübke, M.; Bauer, J.; Rieth, J.; Wacker, M.; et al. SARS-CoV-2-Derived Peptides Define Heterologous and COVID-19-Induced T Cell Recognition. Nat. Immunol. 2021, 22, 74–85. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T Cell Immunity in Cases of COVID-19 and SARS, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; Nafady-Hego, H.; Rashad, A.; El-Badawy, O.; Nasif, K.A.; Mostafa, A.T.; Osman, H.A.; Dongol, E.M.; Hashim, A.A.; Abdelrazek, G.M.; et al. Increased Percentage of Apoptotic and CTLA-4 (CD152) Expressing Cells in CD4+/CD8+ Cells in COVID-19 Patients. Medicine 2022, 101, e30650. [Google Scholar] [CrossRef]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and Immunological Features of Severe and Moderate Coronavirus Disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional Exhaustion of Antiviral Lymphocytes in COVID-19 Patients. Cell Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef]
- Wang, F.; Nie, J.; Wang, H.; Zhao, Q.; Xiong, Y.; Deng, L.; Song, S.; Ma, Z.; Mo, P.; Zhang, Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020, 221, 1762–1769. [Google Scholar] [CrossRef]
- Arshad, N.; Laurent-Rolle, M.; Ahmed, W.S.; Hsu, J.C.-C.; Mitchell, S.M.; Pawlak, J.; Sengupta, D.; Biswas, K.H.; Cresswell, P. SARS-CoV-2 Accessory Proteins ORF7a and ORF3a Use Distinct Mechanisms to down-Regulate MHC-I Surface Expression. Proc. Natl. Acad. Sci. USA 2023, 120, e2208525120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Li, Y.; Huang, F.; Luo, B.; Yuan, Y.; Xia, B.; Ma, X.; Yang, T.; Yu, F.; et al. The ORF8 Protein of SARS-CoV-2 Mediates Immune Evasion through down-Regulating MHC-Ι. Proc. Natl. Acad. Sci. USA 2021, 118, e2024202118. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.K.; Burnham, K.L.; Jones, E.M.; Tan, M.M.H.; Saputil, R.C.; Baillon, L.; Selck, C.; Giang, N.; Argüello, R.; Pillay, C.; et al. Transcriptional Reprogramming from Innate Immune Functions to a Pro-Thrombotic Signature by Monocytes in COVID-19. Nat. Commun. 2022, 13, 7947. [Google Scholar] [CrossRef]
- Shen, X.-R.; Geng, R.; Li, Q.; Chen, Y.; Li, S.-F.; Wang, Q.; Min, J.; Yang, Y.; Li, B.; Jiang, R.-D.; et al. ACE2-Independent Infection of T Lymphocytes by SARS-CoV-2. Signal Transduct. Target. Ther. 2022, 7, 83. [Google Scholar] [CrossRef]
- Brunetti, N.S.; Davanzo, G.G.; de Moraes, D.; Ferrari, A.J.; Souza, G.F.; Muraro, S.P.; Knittel, T.L.; Boldrini, V.O.; Monteiro, L.B.; Virgílio-da-Silva, J.V.; et al. SARS-CoV-2 uses CD4 to infect T helper lymphocytes. Elife 2023, 12, e84790. [Google Scholar] [CrossRef] [PubMed]
- Lymphocytopenia–Hematology and Oncology. Available online: https://www.merckmanuals.com/professional/hematology-and-oncology/leukopenias/lymphocytopenia (accessed on 19 September 2023).
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Zheng, H.-Y.; Zhang, M.; Yang, C.-X.; Zhang, N.; Wang, X.-C.; Yang, X.-P.; Dong, X.-Q.; Zheng, Y.-T. Elevated Exhaustion Levels and Reduced Functional Diversity of T Cells in Peripheral Blood May Predict Severe Progression in COVID-19 Patients. Cell Mol. Immunol. 2020, 17, 541–543. [Google Scholar] [CrossRef]
- Cizmecioglu, A.; Akay Cizmecioglu, H.; Goktepe, M.H.; Emsen, A.; Korkmaz, C.; Esenkaya Tasbent, F.; Colkesen, F.; Artac, H. Apoptosis-Induced T-Cell Lymphopenia Is Related to COVID-19 Severity. J. Med. Virol. 2021, 93, 2867–2874. [Google Scholar] [CrossRef]
- André, S.; Picard, M.; Cezar, R.; Roux-Dalvai, F.; Alleaume-Butaux, A.; Soundaramourty, C.; Cruz, A.S.; Mendes-Frias, A.; Gotti, C.; Leclercq, M.; et al. T Cell Apoptosis Characterizes Severe Covid-19 Disease. Cell Death Differ. 2022, 29, 1486–1499. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.-Q.; Wang, Q.; Miao, H. Lymphopenia Predicts Disease Severity of COVID-19: A Descriptive and Predictive Study. Signal Transduct. Target. Ther. 2020, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Files, J.K.; Boppana, S.; Perez, M.D.; Sarkar, S.; Lowman, K.E.; Qin, K.; Sterrett, S.; Carlin, E.; Bansal, A.; Sabbaj, S.; et al. Sustained Cellular Immune Dysregulation in Individuals Recovering from SARS-CoV-2 Infection. J. Clin. Investig. 2021, 131, e140491. [Google Scholar] [CrossRef]
- Danwang, C.; Noubiap, J.J.; Robert, A.; Yombi, J.C. Outcomes of Patients with HIV and COVID-19 Co-Infection: A Systematic Review and Meta-Analysis. AIDS Res. Ther. 2022, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of Protection against SARS-CoV-2 in Rhesus Macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Hasenkrug, K.J.; Feldmann, F.; Myers, L.; Santiago, M.L.; Guo, K.; Barrett, B.S.; Mickens, K.L.; Carmody, A.; Okumura, A.; Rao, D.; et al. Recovery from Acute SARS-CoV-2 Infection and Development of Anamnestic Immune Responses in T Cell-Depleted Rhesus Macaques. mBio 2021, 12, e0150321. [Google Scholar] [CrossRef]
- Reynolds, J.; Shojania, K.; Marra, C.A. Abatacept: A Novel Treatment for Moderate-to-Severe Rheumatoid Arthritis. Pharmacotherapy 2007, 27, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Chitale, S.; Moots, R. Abatacept: The First T Lymphocyte Co-Stimulation Modulator, for the Treatment of Rheumatoid Arthritis. Expert Opin. Biol. Ther. 2008, 8, 115–122. [Google Scholar] [CrossRef]
- Vincenti, F. Costimulation Blockade in Autoimmunity and Transplantation. J. Allergy Clin. Immunol. 2008, 121, 299–306. [Google Scholar] [CrossRef]
- Ko, E.R.; Anstrom, K.J.; Panettieri, R.A.; Lachiewicz, A.M.; Maillo, M.; O’Halloran, J.A.; Boucher, C.; Smith, P.B.; McCarthy, M.W.; Segura Nunez, P.; et al. Abatacept for Treatment of Adults Hospitalized with Moderate or Severe COVID-19. medRxiv 2022. [Google Scholar] [CrossRef]
- Bristol Myers Squibb Announces Topline Results Showing Treatment with Orencia (Abatacept) Improved Survival in People Hospitalized with COVID-19. Available online: https://news.bms.com/news/details/2022/Bristol-Myers-Squibb-Announces-Topline-Results-Showing-Treatment-with-Orencia-abatacept-Improved-Survival-in-People-Hospitalized-with-COVID-19/default.aspx (accessed on 19 September 2023).
- Hamdy, A.; Leonardi, A. Superantigens and SARS-CoV-2. Pathogens 2022, 11, 390. [Google Scholar] [CrossRef]
- Schreibing, F.; Hannani, M.T.; Kim, H.; Nagai, J.S.; Ticconi, F.; Fewings, E.; Bleckwehl, T.; Begemann, M.; Torow, N.; Kuppe, C.; et al. Dissecting CD8+ T Cell Pathology of Severe SARS-CoV-2 Infection by Single-Cell Immunoprofiling. Front. Immunol. 2022, 13, 1066176. [Google Scholar] [CrossRef] [PubMed]
- Georg, P.; Astaburuaga-García, R.; Bonaguro, L.; Brumhard, S.; Michalick, L.; Lippert, L.J.; Kostevc, T.; Gäbel, C.; Schneider, M.; Streitz, M.; et al. Complement Activation Induces Excessive T Cell Cytotoxicity in Severe COVID-19. Cell 2022, 185, 493–512.e25. [Google Scholar] [CrossRef]
- Kalfaoglu, B.; Almeida-Santos, J.; Tye, C.A.; Satou, Y.; Ono, M. T-Cell Dysregulation in COVID-19. Biochem. Biophys. Res. Commun. 2021, 538, 204–210. [Google Scholar] [CrossRef]
- Zeng, C.; Evans, J.P.; Reisinger, S.; Woyach, J.; Liscynesky, C.; Boghdadly, Z.E.; Rubinstein, M.P.; Chakravarthy, K.; Saif, L.; Oltz, E.M.; et al. Impaired Neutralizing Antibody Response to COVID-19 mRNA Vaccines in Cancer Patients. medRxiv 2021. [Google Scholar] [CrossRef]
- Terpos, E.; Stellas, D.; Rosati, M.; Sergentanis, T.N.; Hu, X.; Politou, M.; Pappa, V.; Ntanasis-Stathopoulos, I.; Karaliota, S.; Bear, J.; et al. SARS-CoV-2 Antibody Kinetics Eight Months from COVID-19 Onset: Persistence of Spike Antibodies but Loss of Neutralizing Antibodies in 24% of Convalescent Plasma Donors. Eur. J. Intern. Med. 2021, 89, 87–96. [Google Scholar] [CrossRef]
- Hartl, D.L.; Clark, A.G. Principles of Population Genetics, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 1997; ISBN 978-0-87893-306-8. [Google Scholar]
- Gillespie, J.H. Population Genetics, 1st ed.; Johns Hopkins University Press: Baltimore, MD, USA; London, UK, 1998. [Google Scholar]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron Escapes the Majority of Existing SARS-CoV-2 Neutralizing Antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- I Think There’s Perhaps Been Some Confusion Regarding Transmissibility vs Immune Escape in Omicron. Available online: https://twitter.com/trvrb/status/1465364300936085506?lang=en (accessed on 19 September 2023).
- The Biden Administration Is Pushing New Covid Boosters. Who’s Listening? Available online: https://www.statnews.com/2022/08/31/the-biden-administration-is-pushing-another-round-of-covid-boosters-whos-listening/ (accessed on 18 September 2023).
- Rubin, R. COVID-19 Vaccine Makers Plan for Annual Boosters, but It’s Not Clear They’ll Be Needed. JAMA 2021, 326, 2247–2249. [Google Scholar] [CrossRef] [PubMed]
- Scientists Said We’d Take Annual COVID Jabs like Flu Shots. Now Fauci Says It Might Be Only Every 5 Years. Available online: https://fortune.com/2022/02/09/scientists-said-wed-take-annual-covid-jabs-like-flu-shots-now-fauci-says-it-might-be-only-every-5-years/ (accessed on 18 September 2023).
- KFF COVID-19 Vaccine Monitor: December 2022–Methodology. Available online: https://www.kff.org/report-section/kff-covid-19-vaccine-monitor-december-2022-methodology/ (accessed on 18 September 2023).
- Canetti, M.; Barda, N.; Gilboa, M.; Indenbaum, V.; Asraf, K.; Gonen, T.; Weiss-Ottolenghi, Y.; Amit, S.; Doolman, R.; Mendelson, E.; et al. Six-Month Follow-up after a Fourth BNT162b2 Vaccine Dose. N. Engl. J. Med. 2022, 387, 2092–2094. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Amir, O.; Freedman, L.; Alroy-Preis, S.; Ash, N.; Huppert, A.; Milo, R. Protection by a Fourth Dose of BNT162b2 against Omicron in Israel. N. Engl. J. Med. 2022, 386, 1712–1720. [Google Scholar] [CrossRef]
- Stay Up to Date with COVID-19 Vaccines. Available online: https://www.cdc.gov/covid/vaccines/stay-up-to-date.html (accessed on 18 September 2023).






| Parameter | Value | Units | Standard Error | Relative Standard Error (%) |
|---|---|---|---|---|
| Fixed effects (median) | ||||
| kp, pop | 38.3 | IC50/days | 5.31 | 13.9 |
| kel, pop | 0.00628 | 1/days | 0.0012 | 19.1 |
| Tin, pop | 26.9 | days | 26.9 | 15.1 |
| Standard deviation of the random effects | ||||
| ωk | 0.731 | IC50/days | 0.156 | 21.3 |
| ωkel | 0.346 | 1/days | 0.141 | 40.8 |
| ωTin | 0.708 | days | 0.181 | 25.6 |
| Correlations | ||||
| corrk,Tin | −0.977 | 0.0255 | 2.61 | |
| Error model parameters | ||||
| a | 121 | 9.11 | 7.51 | |
| Parameter | Value | Source |
|---|---|---|
| h (Hill coefficient) | 1.018 | [32] |
| EC50,infection (CP titer *) | 0.35 | |
| EC50,death (CP titer *) | 0.03 | [32] |
| R0 (individuals) | 8.2 | [76,77] |
| Immune evasion half-life (days) | 73 | [78] |
| Infection duration (days) | 10 | [70] |
| Increase in titer on reinfection (fold) | 14.4 | [79] |
| Increase in titer on revaccination (fold) | 10 | [71,80,81] |
| Revaccination interval (days) | 60–365 | [82] |
| Fraction boosted (%) | 50 | [83] |
| Simulation duration (years) | 1 and 10 | |
| Simulated population size | 100,000 |
| Parameter | Value | Units |
|---|---|---|
| Natural immunity decay rate | 0.002 [57] | 1/days |
| Vaccine immunity decay rate | 0.002 [57] | 1/days |
| Delta R0 | 6.0 [84] | Individuals |
| Alpha R0 | 5.3 [85] | Individuals |
| Vei, delta, recent primary series | 70.2 [5] | % |
| Vei, delta, distant primary series | 40.2 [5] | % |
| Vei, delta, recent first booster | 93 [86] | % |
| Vei, pre-delta, primary series | 90 [87] | % |
| Vei, pre-delta, booster | 95 | % |
| Cross-immunity protection | 81 [88,89] | % |
| Percentage of adults in US population | 78 [90] | % |
| US population vaccinated, summer 2021 | 48 [91] | % |
| Transmission mitigation, summer 2021 | 50 | % |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, L.; Stoddard, M.; Sarkar, S.; Egeren, D.v.; Mangalaganesh, S.; Nolan, R.P.; Rogers, M.S.; Hather, G.; White, L.F.; Chakravarty, A. The Impact of Vaccination Frequency on COVID-19 Public Health Outcomes: A Model-Based Analysis. Vaccines 2025, 13, 368. https://doi.org/10.3390/vaccines13040368
Yuan L, Stoddard M, Sarkar S, Egeren Dv, Mangalaganesh S, Nolan RP, Rogers MS, Hather G, White LF, Chakravarty A. The Impact of Vaccination Frequency on COVID-19 Public Health Outcomes: A Model-Based Analysis. Vaccines. 2025; 13(4):368. https://doi.org/10.3390/vaccines13040368
Chicago/Turabian StyleYuan, Lin, Madison Stoddard, Sharanya Sarkar, Debra van Egeren, Shruthi Mangalaganesh, Ryan P. Nolan, Michael S. Rogers, Greg Hather, Laura F. White, and Arijit Chakravarty. 2025. "The Impact of Vaccination Frequency on COVID-19 Public Health Outcomes: A Model-Based Analysis" Vaccines 13, no. 4: 368. https://doi.org/10.3390/vaccines13040368
APA StyleYuan, L., Stoddard, M., Sarkar, S., Egeren, D. v., Mangalaganesh, S., Nolan, R. P., Rogers, M. S., Hather, G., White, L. F., & Chakravarty, A. (2025). The Impact of Vaccination Frequency on COVID-19 Public Health Outcomes: A Model-Based Analysis. Vaccines, 13(4), 368. https://doi.org/10.3390/vaccines13040368






