SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Ethics Statement and Study Population
2.3. Virus
2.4. Spike Protein Flow Cytometry-Based (SFB) Assay
2.5. Neutralization Assay
2.6. Statistical Analysis
3. Results
3.1. SARS-CoV-2 mRNA Vaccine Can Generate Cross-Reactive Antibody Against Human Seasonal Coronavirus NL63

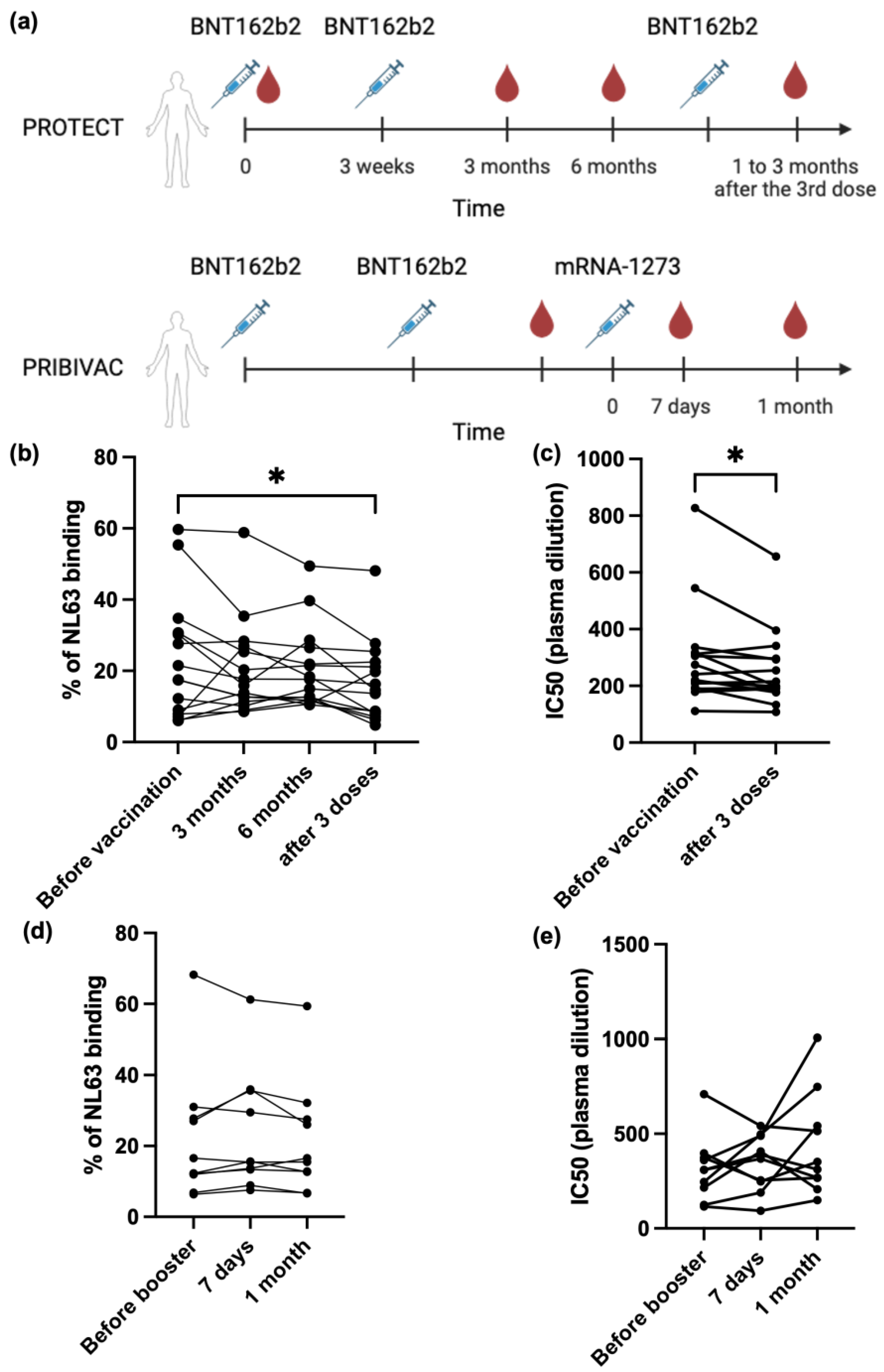
3.2. SARS-CoV-2 mRNA Vaccine Cannot Boost Pre-Existing NL63 Antibody Responses
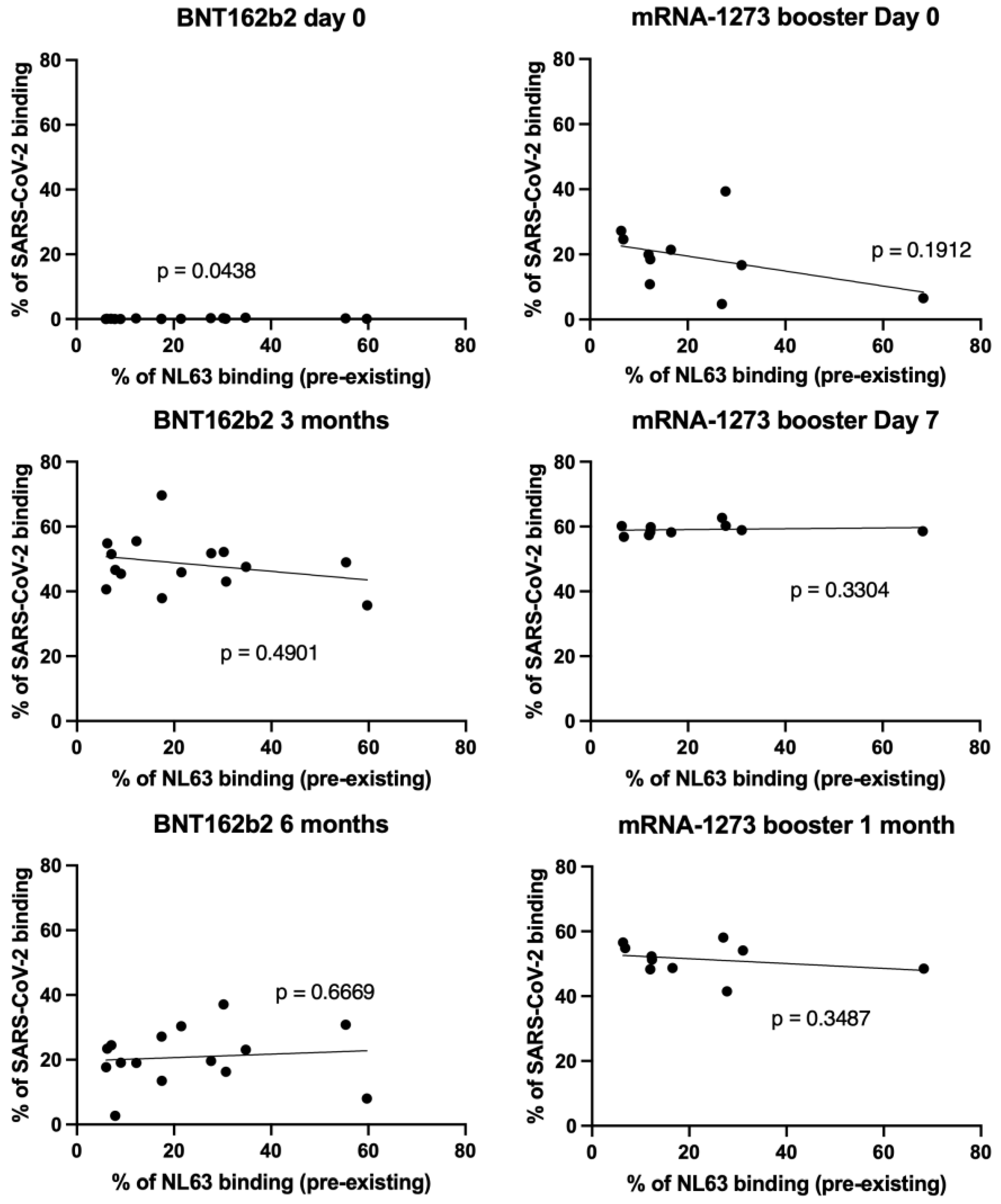
3.3. NL63 Pre-Existing Immune Response Did Not Affect Anti SARS-CoV-2 Antibody Response Imduced by Vaccination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Dijkman, R.; Jebbink, M.F.; El Idrissi, N.B.; Pyrc, K.; Muller, M.A.; Kuijpers, T.W.; Zaaijer, H.L.; van der Hoek, L. Human coronavirus NL63 and 229E seroconversion in children. J. Clin. Microbiol. 2008, 46, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Pyrc, K.; Berkhout, B.; Van Der Hoek, L. Antiviral strategies against human coronaviruses. Infect. Disord. Drug Targets 2007, 7, 59–66. [Google Scholar] [CrossRef]
- Milewska, A.; Nowak, P.; Owczarek, K.; Szczepanski, A.; Zarebski, M.; Hoang, A.; Berniak, K.; Wojarski, J.; Zeglen, S.; Baster, Z.; et al. Entry of human coronavirus NL63 into the cell. J. Virol. 2018, 92, e01933-17. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Clark, J.; Carreño, J.M.; Strohmeier, S.; Yellin, T.; Meade, P.S.; Bhavsar, D.; Muramatsu, H.; Sun, W.; Coughlan, L.; et al. Immunity to seasonal coronavirus spike proteins does not protect from SARS-CoV-2 challenge in a mouse model but has no detrimental effect on protection mediated by COVID-19 mRNA Vaccination. J. Virol. 2023, 97, e0166422. [Google Scholar] [CrossRef]
- Woldemeskel, B.A.; Garliss, C.C.; Blankson, J.N. SARS-CoV-2 mRNA vaccines induce broad CD4+ T Cell Responses that recognize SARS-CoV-2 variants and HCoV-NL63. J. Clin. Investig. 2021, 131, e149335. [Google Scholar] [CrossRef]
- Hu, C.; Wang, Z.; Ren, L.; Hao, Y.; Zhu, M.; Jiang, H.; Wang, S.; Li, D.; Shao, Y. Pre-Existing Anti-HCoV-OC43 immunity influences the durability and cross-reactivity of humoral response to SARS-CoV-2 vaccination. Front. Cell Infect. Microbiol. 2022, 12, 978440. [Google Scholar] [CrossRef]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The Impact of pre-Existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat. Rev. Immunol. 2023, 23, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Adami, F.L.; de Castro, M.V.; Almeida, B.d.S.; Daher, I.P.; Yamamoto, M.M.; Souza Santos, K.; Zatz, M.; Naslavsky, M.S.; Rosa, D.S.; Cunha-Neto, E.; et al. Anti-RBD IgG antibodies from endemic coronaviruses do not protect against the acquisition of SARS-CoV-2 infection among exposed uninfected individuals. Front. Immunol. 2024, 15, 1396603. [Google Scholar] [CrossRef]
- Shrwani, K.; Sharma, R.; Krishnan, M.; Jones, T.; Mayora-Neto, M.; Cantoni, D.; Temperton, N.J.; Dobson, S.L.; Subramaniam, K.; McNamara, P.S.; et al. Detection of serum cross-reactive antibodies and memory response to SARS-CoV-2 in prepandemic and post–COVID-19 convalescent samples. J. Infect. Dis. 2021, 224, 1305–1315. [Google Scholar] [CrossRef]
- Renia, L.; Goh, Y.S.; Rouers, A.; Le Bert, N.; Chia, W.N.; Chavatte, J.-M.; Fong, S.-W.; Chang, Z.W.; Zhuo, N.Z.; Tay, M.Z.; et al. Lower vaccine-acquired immunity in the elderly population following two-dose BNT162b2 vaccination is alleviated by a third vaccine dose. Nat. Commun. 2022, 13, 4615. [Google Scholar] [CrossRef]
- Poh, X.Y.; Tan, C.W.; Lee, I.R.; Chavatte, J.-M.; Fong, S.-W.; Prince, T.; Hartley, C.; Yeoh, A.Y.Y.; Rao, S.; Chia, P.Y.; et al. Antibody response of heterologous vs homologous messenger RNA vaccine boosters against the severe acute respiratory syndrome coronavirus 2 omicron variant: Interim results from the PRIBIVAC study, a randomized clinical trial. Clin. Inf. Dis. 2022, 75, 2088–2096. [Google Scholar] [CrossRef]
- Poh, X.Y.; Lee, I.R.; Lim, C.; Teo, J.; Rao, S.; Chia, P.Y.; Ong, S.W.X.; Lee, T.H.; Lin, R.J.H.; Ng, L.F.P.; et al. Evaluation of the safety and immunogenicity of different COVID-19 vaccine combinations in healthy individuals: Study protocol for a randomized, subject-blinded, controlled phase 3 trial [PRIBIVAC]. Trials 2022, 23, 498. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.S.; Ng, L.F.P.; Renia, L. A Flow cytometry-based assay for serological detection of anti-spike antibodies in COVID-19 patients. STAR Protocol. 2021, 2, 100671. [Google Scholar] [CrossRef]
- Goh, Y.S.; Chavatte, J.M.; Lim, J.A.; Lee, B.; Hor, P.X.; Amrun, S.N.; Lee, C.Y.; Chee, R.S.; Wang, B.; Lee, C.Y.; et al. Sensitive detection of total anti-spike antibodies and isotype switching in asymptomatic and symptomatic individuals with COVID-19. Cell Rep. Med. 2021, 16, 100193. [Google Scholar] [CrossRef]
- Singh, G.; Abbad, A.; Kleiner, G.; Srivastava, K.; Gleason, C.; Group, P.S.; Carreño, J.M.; Simon, V.; Krammer, F. The Post-COVID-19 population has a high prevalence of cross-reactive antibodies to spikes from all orthocoronavirinae genera. mBio 2024, 15, e02250-23. [Google Scholar] [CrossRef] [PubMed]
- Grobben, M.; van der Straten, K.; Brouwer, P.J.; Brinkkemper, M.; Maisonnasse, P.; Dereuddre-Bosquet, N.; Appelman, B.; Lavell, A.A.; van Vught, L.A.; Burger, J.A.; et al. Cross-reactive antibodies after SARS-CoV-2 infection and vaccination. eLife 2021, 10, e70330. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Matsuoka, M.; Tabata, S.; Kitagawa, Y.; Nagura-Ikeda, M.; Kubota, K.; Fukada, A.; Takada, T.; Sato, M.; Noguchi, S.; et al. Cross-reactive humoral immune responses against seasonal human coronaviruses in COVID-19 patients with different disease severities. Int. J. Infect. Dis. 2021, 111, 68–75. [Google Scholar] [CrossRef]
- Denninger, V.; Xu, C.K.; Meisl, G.; Morgunov, A.S.; Fiedler, S.; Ilsley, A.; Emmenegger, M.; Malik, A.Y.; Piziorska, M.A.; Schneider, M.M.; et al. Microfluidic antibody affinity profiling reveals the role of memory reactivation and cross-reactivity in the defense against SARS-CoV-2. ACS Infect. Dis. 2022, 8, 790–799. [Google Scholar] [CrossRef]
- Pattinson, D.; Jester, P.; Guan, L.; Yamayoshi, S.; Chiba, S.; Presler, R.; Rao, H.; Iwatsuki-Horimoto, K.; Ikeda, N.; Hagihara, M.; et al. A novel method to reduce ELISA serial dilution assay workload applied to SARS-CoV-2 and seasonal HCoVs. Viruses 2022, 14, 562. [Google Scholar] [CrossRef]
- Adams, O.; Andrée, M.; Rabl, D.; Ostermann, P.N.; Schaal, H.; Lehnert, E.; Ackerstaff, S.; Müller, L.; Fischer, J.C. Humoral response to SARS-CoV-2 and seasonal coronaviruses in COVID-19 patients. J. Med. Virol. 2022, 94, 1096–1103. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Y.; Kang, L.; Hu, Y.; Wang, L.; Zhong, J.; Chen, H.; Ren, L.; Gu, X.; Wang, G.; et al. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: A retrospective study. Emerg. Microb. Infect. 2021, 10, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020, 5, eabe0367. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.A.; Cantoni, D.; Mayora-Neto, M.; Genova, C.D.; Sampson, A.; Ferrari, M.; Carnell, G.; Nadesalingam, A.; Smith, P.; Chan, A.; et al. Human seasonal coronavirus neutralization and COVID-19 severity. J. Med. Virol. 2022, 94, 4820–4829. [Google Scholar] [CrossRef] [PubMed]
- Camerini, D.; Randall, A.Z.; Trappl-Kimmons, K.; Oberai, A.; Hung, C.; Edgar, J.; Shandling, A.; Huynh, V.; Teng, A.A.; Hermanson, G.; et al. Mapping SARS-CoV-2 antibody epitopes in COVID-19 patients with a multi-coronavirus protein microarray. Microbiol. Spectr. 2021, 9, e01416-21. [Google Scholar] [CrossRef]
- Struck, F.; Schreiner, P.; Staschik, E.; Wochinz-Richter, K.; Schulz, S.; Soutschek, E.; Motz, M.; Bauer, G. Incomplete IgG avidity maturation after seasonal coronavirus infections. J. Med. Virol. 2022, 94, 186–196. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Y.; Hu, Y.; Chang, F.; Wu, Q.; Yang, J.; Chen, J.; Teng, S.; Zhang, J.; He, R.; et al. Monoclonal Antibodies constructed from COVID-19 convalescent memory B cells exhibit potent binding activity to MERS-CoV spike S2 subunit and other human coronaviruses. Front. Immunol. 2022, 13, 1056272. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wolf, J.; Brice, D.C.; Sun, Y.; Locke, M.; Cherry, S.; Castellaw, A.H.; Wehenkel, M.; Crawford, J.C.; Zarnitsyna, V.I.; et al. Pre-Existing Humoral Immunity to human common cold coronaviruses negatively impacts the protective SARS-CoV-2 antibody response. Cell Host Microbe 2022, 30, 83–96.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Zhou, Q.; Wiltse, A.; Zand, M.S. Antibody mediated immunity to SARS-CoV-2 and human coronaviruses: Multiplex beads assay and volumetric absorptive microsampling to generate immune repertoire cartography. Front. Immunol. 2021, 12, 696370. [Google Scholar] [CrossRef]
- Stanley, A.M.; Aksyuk, A.A.; Wilkins, D.; Green, J.A.; Lan, D.; Shoemaker, K.; Tieu, H.-V.; Sobieszczyk, M.E.; Falsey, A.R.; Kelly, E.J. Seasonal human coronavirus humoral responses in AZD1222 (ChaAdOx1 nCoV-19) COVID-19 vaccinated adults reveal limited cross-immunity. Front. Immunol. 2024, 15, 1401728. [Google Scholar] [CrossRef]
- Kolehmainen, P.; Huttunen, M.; Iakubovskaia, A.; Maljanen, S.; Tauriainen, S.; Yatkin, E.; Pasternack, A.; Naves, R.; Toivonen, L.; Tähtinen, P.A.; et al. Coronavirus spike protein-specific antibodies indicate frequent infections and reinfections in infancy and among BNT162b2-vaccinated healthcare workers. Sci. Rep. 2023, 13, 8416. [Google Scholar] [CrossRef]
- Soni, M.K.; Migliori, E.; Fu, J.; Assal, A.; Chan, H.T.; Pan, J.; Khatiwada, P.; Ciubotariu, R.; May, M.S.; Pereira, M.R.; et al. The prospect of universal coronavirus immunity: Characterization of reciprocal and non-reciprocal T cell responses against SARS-CoV2 and common human coronaviruses. Front. Immunol. 2023, 14, 1212203. [Google Scholar] [CrossRef] [PubMed]
- Lineburg, K.E.; Grant, E.J.; Swaminathan, S.; Chatzileontiadou, D.S.M.; Szeto, C.; Sloane, H.; Panikkar, A.; Raju, J.; Crooks, P.; Rehan, S.; et al. CD8+ T cells specific for an immunodominant SARS-CoV-2 nucleocapsid epitope cross-react with selective seasonal coronaviruses. Immunity 2021, 54, 1055–1065.e5. [Google Scholar] [CrossRef]
- Cantoni, D.; Siracusano, G.; Mayora-Neto, M.; Pastori, C.; Fantoni, T.; Lytras, S.; Di Genova, C.; Hughes, J.; Lopalco, L.; Temperton, N. Analysis of antibody neutralisation activity against SARS-CoV-2 variants and seasonal human coronaviruses NL63, HKU1, and 229E induced by three different COVID-19 vaccine platforms. Vaccines 2022, 11, 58. [Google Scholar] [CrossRef]
- Klompus, S.; Leviatan, S.; Vogl, T.; Mazor, R.D.; Kalka, I.N.; Stoler-Barak, L.; Nathan, N.; Peres, A.; Moss, L.; Godneva, A.; et al. Cross-reactive antibodies against human coronaviruses and the animal coronavirome suggest diagnostics for future zoonotic spillovers. Sci. Immunol. 2021, 6, eabe9950. [Google Scholar] [CrossRef]
- Jacob-Dolan, C.; Feldman, J.; McMahan, K.; Yu, J.; Zahn, R.; Wegmann, F.; Schuitemaker, H.; Schmidt, A.G.; Barouch, D.H. Coronavirus-specific antibody cross reactivity in rhesus macaques following SARS-CoV-2 vaccination and infection. J. Virol. 2021, 95, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Simula, E.R.; Manca, M.A.; Jasemi, S.; Uzzau, S.; Rubino, S.; Manchia, P.; Bitti, A.; Palermo, M.; Sechi, L.A. HCoV-NL63 and SARS-CoV-2 Share Recognized Epitopes by the Humoral Response in Sera of People Collected Pre- and during CoV-2 Pandemic. Microorganisms 2020, 8, 1993. [Google Scholar] [CrossRef] [PubMed]
- Wirsching, S.; Harder, L.; Heymanns, M.; Gröndahl, B.; Hilbert, K.; Kowalzik, F.; Meyer, C.; Gehring, S. Long-Term, CD4+ memory T cell response to SARS-CoV-2. Front. Immunol. 2022, 13, 800070. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Han, Z.; Lang, B.; Li, Y.; Mai, G.; Chen, H.; Feng, L.; Chen, Y.; Luo, H.; Xiong, Y.; et al. Effect of seasonal coronavirus immune imprinting on the immunogenicity of inactivated COVID-19 vaccination. Front. Immunol. 2023, 14, 1195533. [Google Scholar] [CrossRef]
- Naghibosadat, M.; Babuadze, G.G.; Pei, Y.; Hurst, J.; Salvant, E.; Gaete, K.; Biondi, M.; Moloo, B.; Goldstein, A.; Avery, S.; et al. Vaccination against SARS-CoV-2 provides low-level cross-protection against common cold coronaviruses in mouse and non-human primate animal models. J. Virol. 2025, 99, e01390-24. [Google Scholar] [CrossRef]
- Devaux, C.A.; Fantini, J. Unravelling antigenic cross-reactions toward the world of coronaviruses: Extent of the stability of shared epitopes and SARS-CoV-2 anti-spike cross-neutralizing antibodies. Pathogens 2023, 12, 713. [Google Scholar] [CrossRef]
- Rawat, P.; Jemimah, S.; Ponnuswamy, P.K.; Gromiha, M.M. Why are ACE2 binding coronavirus strains SARS-CoV/SARS-CoV-2 wild and NL63 mild ? Proteins 2021, 89, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Dacon, C.; Tucker, C.; Peng, L.; Lee, C.D.; Lin, T.H.; Yuan, M.; Cong, Y.; Wang, L.; Purser, L.; Williams, J.K.; et al. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science 2022, 377, 728–735. [Google Scholar] [CrossRef]
- Asamoah-Boaheng, M.; Grunau, B.; Karim, M.E.; Jassem, A.N.; Bolster, J.; Marquez, A.C.; Scheuermeyer, F.X.; Goldfarb, D.M. Are higher antibody levels against seasonal human coronaviruses associated with a more robust humoral immune response after SARS-CoV-2 vaccination? Front. Immunol. 2022, 13, 954093. [Google Scholar] [CrossRef]
- da Silva Antunes, R.; Pallikkuth, S.; Williams, E.; Dawen Yu, E.; Mateus, J.; Quiambao, L.; Wang, E.; Rawlings, S.A.; Stadlbauer, D.; Jiang, K.; et al. Differential T-cell reactivity to endemic coronaviruses and SARS-CoV-2 in community and health care workers. J. Infect. Dis. 2021, 224, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Loyal, L.; Braun, J.; Henze, L.; Kruse, B.; Dingeldey, M.; Reimer, U.; Kern, F.; Schwarz, T.; Mangold, M.; Unger, C.; et al. Cross-Reactive CD4+ T Cells enhance SARS-CoV-2 immune responses upon infection and vaccination. Science 2021, 374, eabh1823. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-Specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Swadling, L.; Diniz, M.O.; Schmidt, N.M.; Amin, O.E.; Chandran, A.; Shaw, E.; Pade, C.; Gibbons, J.M.; Le Bert, N.; Tan, A.T.; et al. Pre-existing polymerase-specific t cells expand in abortive seronegative SARS-CoV-2. Nature 2022, 601, 110–117. [Google Scholar] [CrossRef]
- Shimizu, K.; Iyoda, T.; Sanpei, A.; Nakazato, H.; Okada, M.; Ueda, S.; Kato-Murayama, M.; Murayama, K.; Shirouzu, M.; Harada, N.; et al. Identification of TCR repertoires in functionally competent cytotoxic t cells cross-reactive to SARS-CoV-2. Commun. Biol. 2021, 4, 1365. [Google Scholar] [CrossRef]
- Coulon, P.-G.; Prakash, S.; Dhanushkodi, N.R.; Srivastava, R.; Zayou, L.; Tifrea, D.F.; Edwards, R.A.; Figueroa, C.J.; Schubl, S.D.; Hsieh, L.; et al. High frequencies of alpha common cold coronavirus/SARS-CoV-2 cross-reactive functional CD4+ and CD8+ memory T cells are associated with protection from symptomatic and fatal SARS-CoV-2 infections in unvaccinated COVID-19 patients. Front. Immunol. 2024, 15, 1343716. [Google Scholar] [CrossRef]
| Experiment Group | Control Group | Antibody or T Cells? | Tested Antigen on NL63 | Result | Ref. |
|---|---|---|---|---|---|
| PCR-positive | PCR-negative close contacts | IgG antibody | RBD | No difference | [8] |
| PCR-positive | Pre-COVID-19 samples | IgG antibody | RBD | No difference | [9] |
| COVID-19 severe | COVID-19 mild | IgG antibody | S and N | No difference | [17] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | S1 | No difference | [18] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | S and N | No difference | [19] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | N | No difference | [20] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | S | No difference | [21] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | RBD | No difference | [22] |
| COVID-19 patients | Healthy individuals | IgG antibody | Pseudovirus | Higher neutralization | [23] |
| COVID-19 convalescents | Pre-COVID-19 samples | IgG antibody | S2 and N | Higher response | [24] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | N | Higher response | [25] |
| COVID-19 convalescents | COVID-19 admission | IgG antibody | S and N | Higher against N in severe patients | [8] |
| COVID-19 convalescents | Healthy individuals | IgG antibody | S | Higher response | [16] |
| COVID-19 convalescents | Healthy individuals | IgG mAbs | S | 3 strongly cross-reactive mAbs | [26] |
| Healthcare workers with direct/indirect contact to COVID-19 patients | Healthcare workers with no contact to COVID-19 patients | IgG, IgM and IgA antibody | S | Higher IgM between direct and no contact group | [27] |
| COVID-19 convalescents or BNT162b2 vaccination (2 doses) | Pre-COVID-19 samples | IgG antibody | S and N | No difference | [28] |
| AZD1222 primary vaccination and/or booster | Placebo | IgG antibody | S | No difference | [29] |
| After 1-dose vaccination (did not mention what vaccine was used) | Before vaccination (same individuals) | IgG antibody | Pseudovirus | Higher neutralization | [30] |
| BNT162b2 or mRNA-1273 vaccinated | Healthy individuals | IgG antibody | S | No difference | [15] |
| BNT162b2 vaccination (2 and 3 doses) | Before vaccination (same individuals) | IgG antibody | S1 | No difference | [31] |
| BNT162b2 vaccination (2 doses) | Before vaccination (same individuals) | IgG antibody | S | No difference | [32] |
| AZD1222, BNT162b2 or mRNA-1273 vaccinated (2 doses) | AZD1222, BNT162b2 or mRNA-1273 vaccinated (1 dose) | IgG antibody | Pseudovirus | Higher neutralization for mRNA-1273 | [33] |
| BNT162b2 or mRNA-1273 | Healthy individuals | IgG antibody | S | No difference | [34] |
| BNT162b2 or mRNA-1273 (2 doses) | Before vaccination (same individuals) | IgG antibody | S | No difference | [35] |
| BBIBP-CorV vaccinated (2 doses) | Before vaccination (same individuals) | IgG antibody | S | Higher response | [6] |
| Infected, Ad26-vaccinated or DNA-vaccinated + re-infected macaques | Before treatment (same animals) | IgG antibody | S | Higher response | [36] |
| COVID-19 convalescents | Healthy individuals | CD4+ T cells | Pool peptide library S1 and S2 | No difference | [37] |
| COVID-19 exposed individuals | Healthy individuals | CD4+ T cells | Pool peptide library S1, S2, M and NP | Higher against S1, M and NP | [38] |
| BNT162b2 or mRNA-1273 vaccinated | Healthy individuals | CD4+ T cells | Pool peptide library (whole virus) | Higher response | [5] |
| COVID-19 convalescents | Healthy individuals | CD8+ T cells | Homologous N105–113 (PPKVHFYYL) | No difference | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Chang, Z.W.; Goh, Y.S.; Tan, Y.J.; Hor, P.X.; Loh, C.Y.; Lye, D.C.; Young, B.E.; Ng, L.F.P.; Tay, M.Z.; et al. SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses. Vaccines 2025, 13, 268. https://doi.org/10.3390/vaccines13030268
Tang W, Chang ZW, Goh YS, Tan YJ, Hor PX, Loh CY, Lye DC, Young BE, Ng LFP, Tay MZ, et al. SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses. Vaccines. 2025; 13(3):268. https://doi.org/10.3390/vaccines13030268
Chicago/Turabian StyleTang, Weiyi, Zi Wei Chang, Yun Shan Goh, Yong Jie Tan, Pei Xiang Hor, Chiew Yee Loh, David C. Lye, Barnaby E. Young, Lisa F. P. Ng, Matthew Zirui Tay, and et al. 2025. "SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses" Vaccines 13, no. 3: 268. https://doi.org/10.3390/vaccines13030268
APA StyleTang, W., Chang, Z. W., Goh, Y. S., Tan, Y. J., Hor, P. X., Loh, C. Y., Lye, D. C., Young, B. E., Ng, L. F. P., Tay, M. Z., Rénia, L., on behalf of the COVID-19 Cohort Study Group, NCID Study Group, & COVID Clinicians’ Group. (2025). SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses. Vaccines, 13(3), 268. https://doi.org/10.3390/vaccines13030268






