Construction of Two Recombinant Pseudorabies Viruses with Deletion of Virulence Genes and Evaluation of Their Immune Protection in Mice and Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses, Cells, and Plasmids
2.2. Primers and Plasmid Construction
2.3. The Extraction of the Complete Full-Length Genome of the Virus
2.4. Construction of Viruses
2.5. In Vitro Growth Properties and Plaque Morphology
2.6. Safety and Protection Assessment in Mice
2.7. Pathogenicity and Immunological Experiments in Pigs
2.8. ELISA Detection of gB- and gD-Specific Antibodies
2.9. Neutralization Tests
2.10. Histopathological Examination
2.11. Statistical Analysis
3. Results
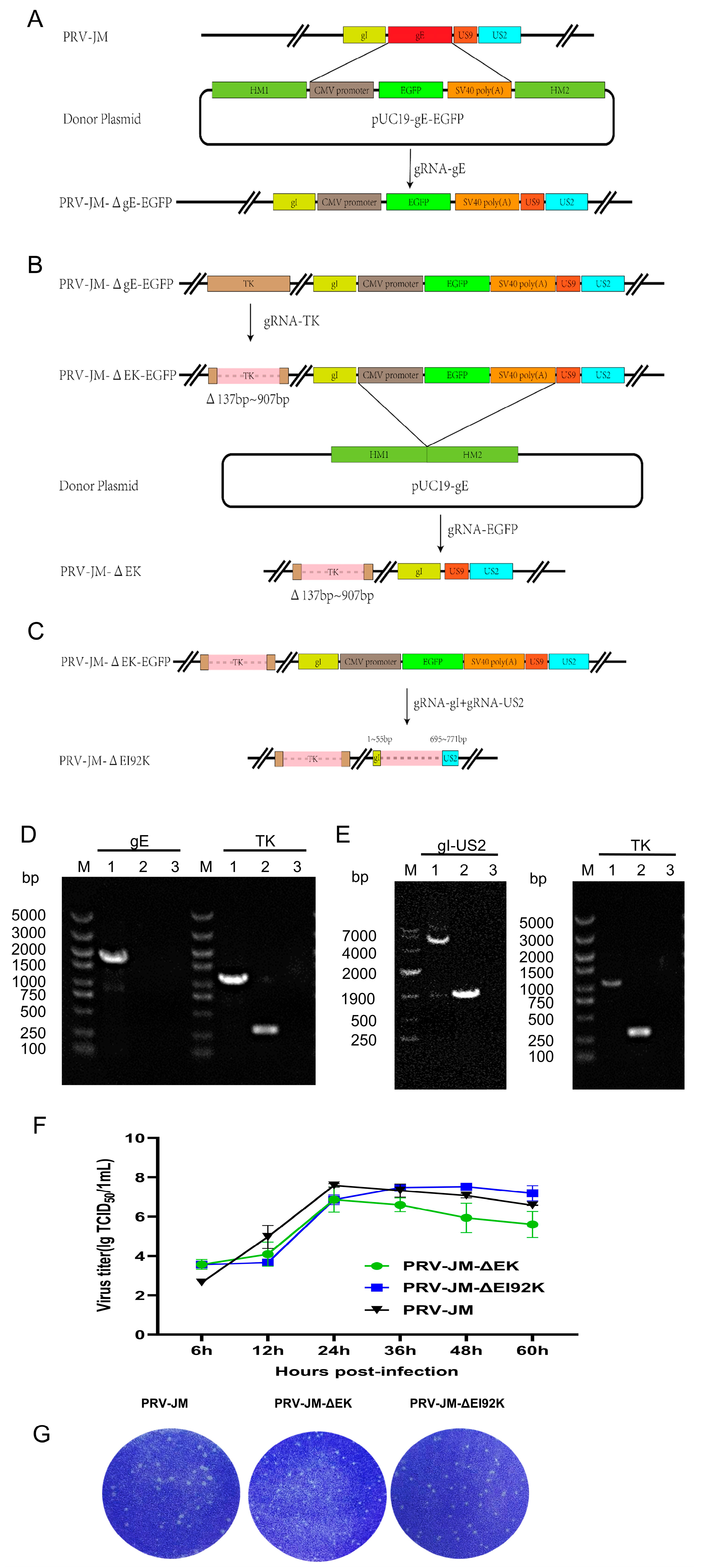
3.1. Construction of Recombinant Viruses
3.2. Growth Characteristics of Recombinant Viruses
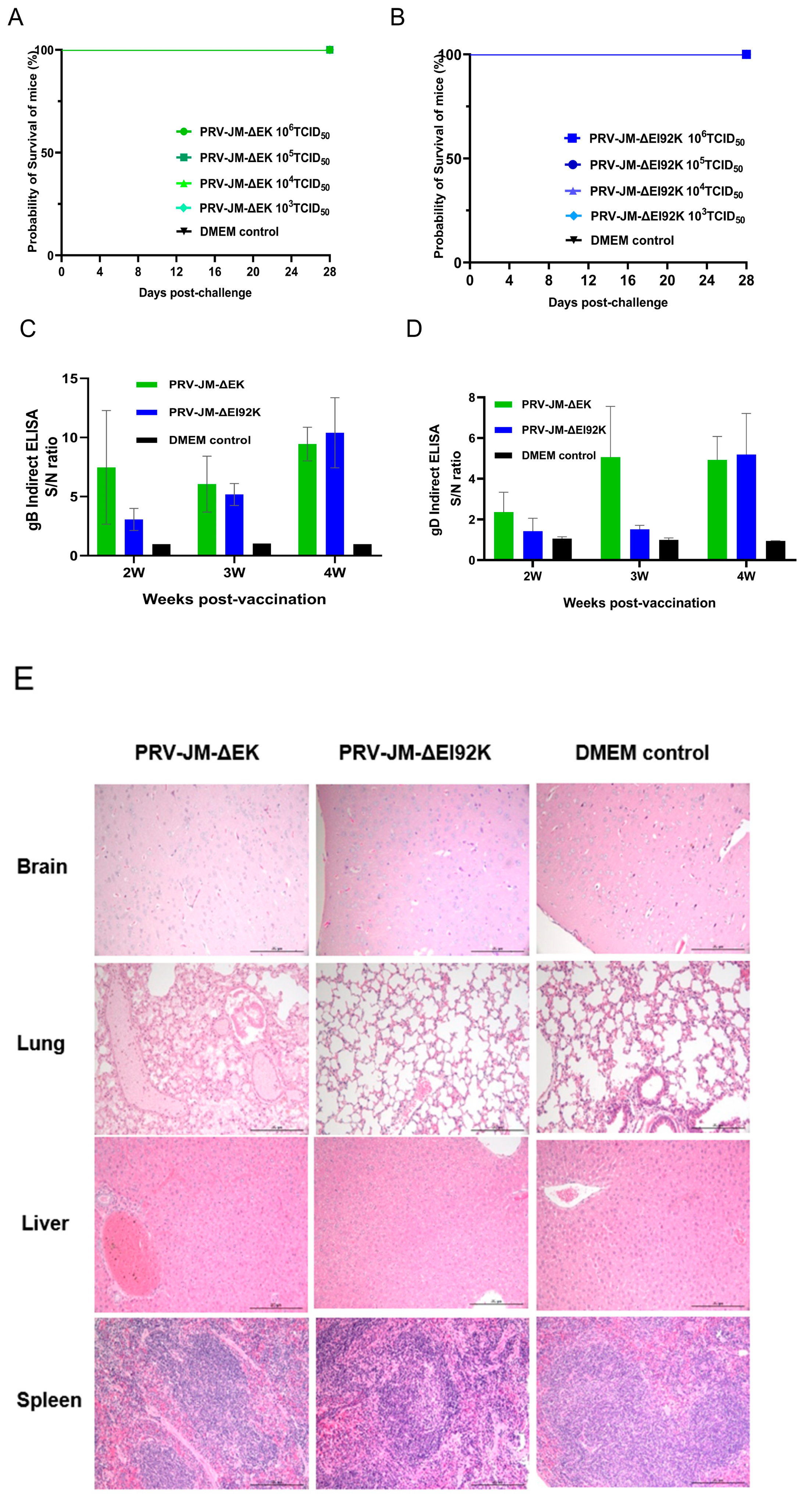
3.3. Safety Experiment of Recombinant Virus in Mice
3.4. Protective Experiment of Recombinant Virus in Mice
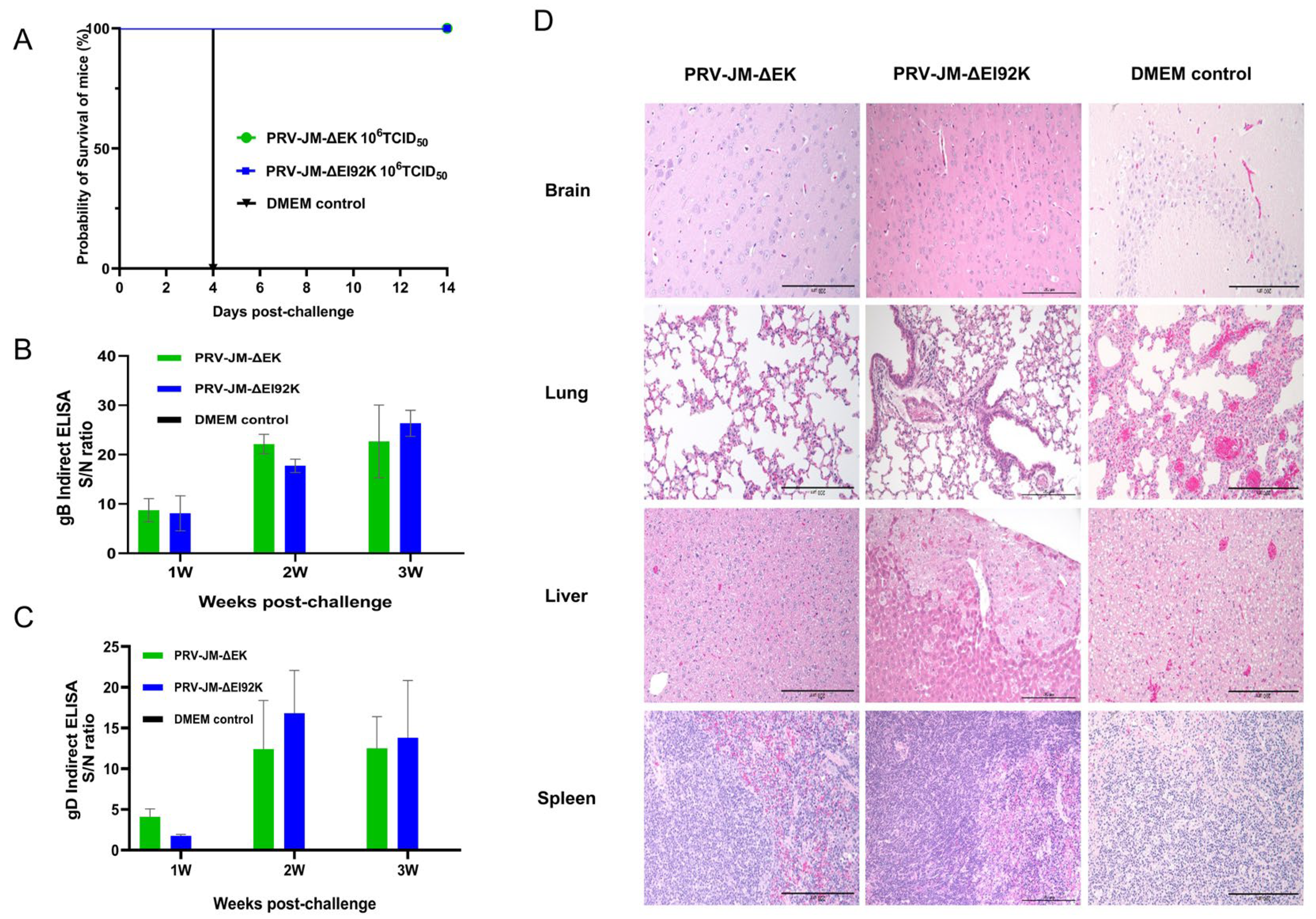
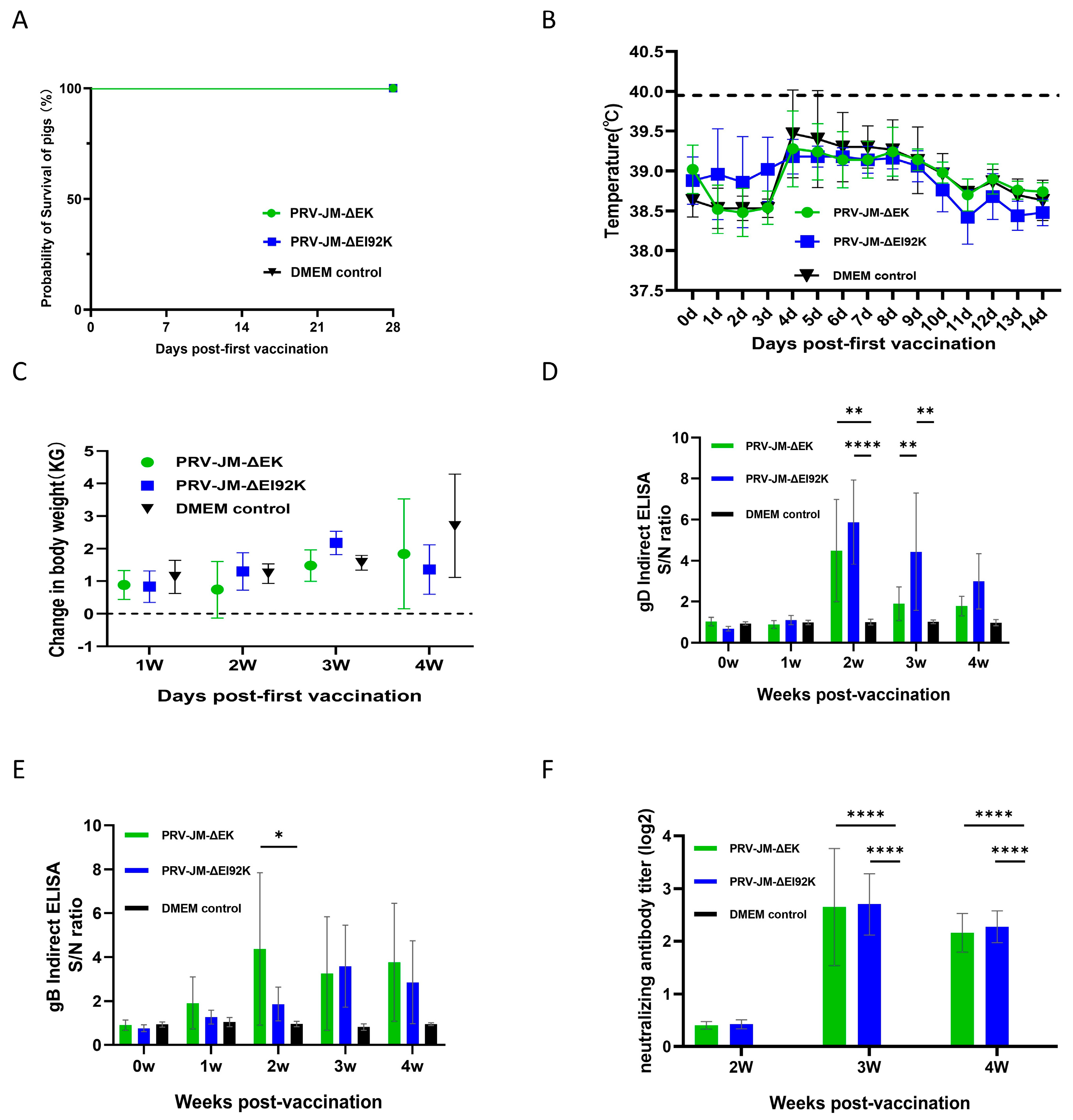
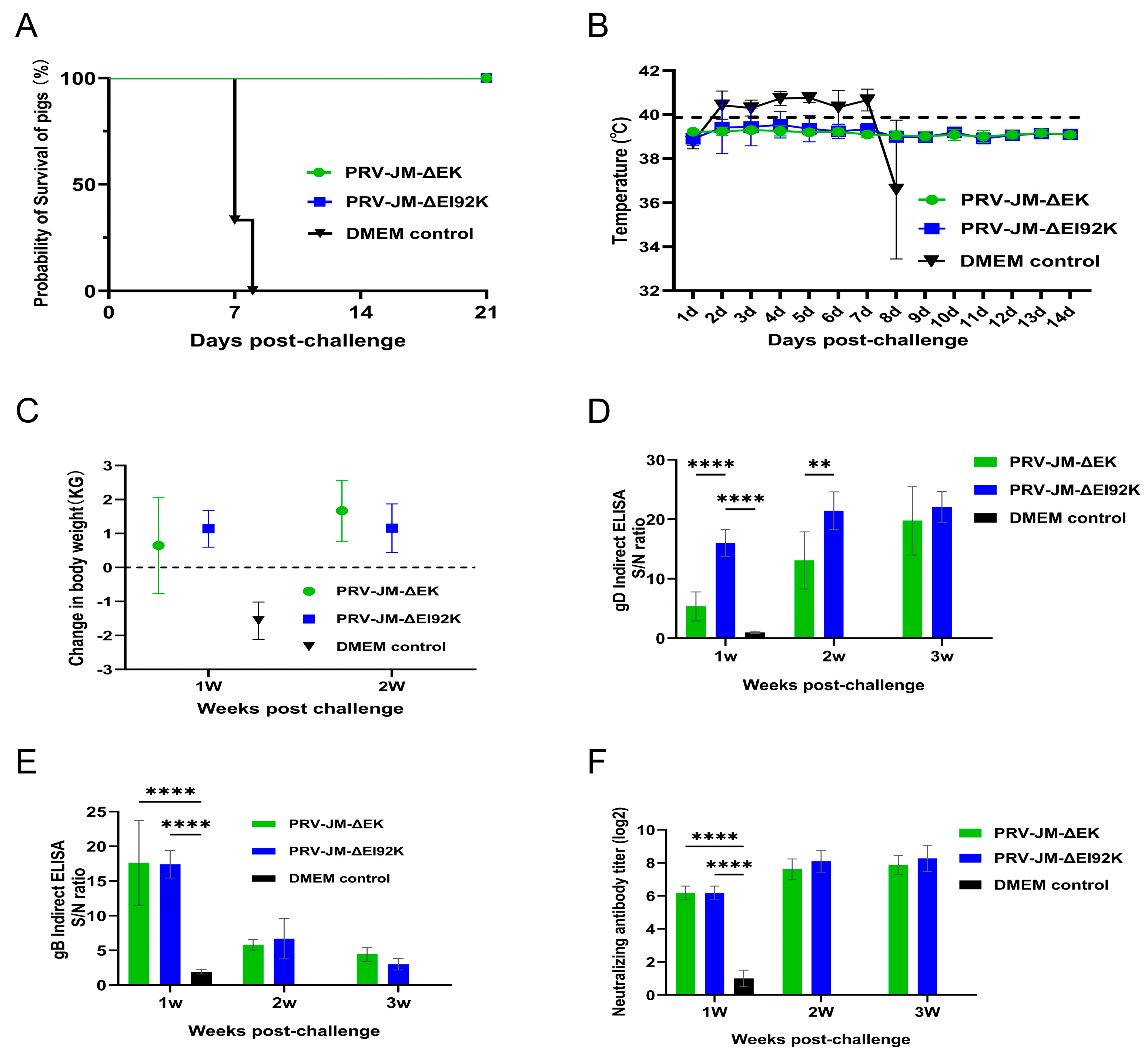
3.5. Protection of Both Recombinant Viruses in Pigs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed]
- McGeoch, D.J.; Dolan, A.; Ralph, A.C. Toward a comprehensive phylogeny for mammalian and avian herpesviruses. J. Virol. 2000, 74, 10401–10406. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, J.; Zhu, D.; Wang, N.; Gao, Q.; Chen, W.; Tang, H.; Wang, J.; Zhang, X.; Liu, H.; et al. Cryo-EM structure of a herpesvirus capsid at 3.1 Å. Science 2018, 360, eaao7283. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Li, X.; Tian, K. Generating Recombinant Pseudorabies Virus for Use as a Vaccine Platform. Methods Mol. Biol. 2017, 1581, 79–96. [Google Scholar] [CrossRef]
- Izzati, U.Z.; Kaneko, Y.; Kaneko, C.; Yoshida, A.; Suwanruengsri, M.; Okabayashi, T.; Hirai, T.; Yamaguchi, R. Distribution of Pseudorabies Virus Antigen in Hunting Dogs with Concurrent Paragonimus westermani Infection. J. Comp. Pathol. 2021, 188, 44–51. [Google Scholar] [CrossRef]
- Laval, K.; Enquist, L.W. The Neuropathic Itch Caused by Pseudorabies Virus. Pathogens 2020, 9, 254. [Google Scholar] [CrossRef]
- Freuling, C.M.; Muller, T.F.; Mettenleiter, T.C. Vaccines against pseudorabies virus (PrV). Vet. Microbiol. 2017, 206, 3–9. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, X.; Liu, G.; Li, Y.; Dong, S.; Lin, J.; Zhang, R.; Cai, X.; Shan, H. Comparative transcriptomic analysis of PK15 cells infected with a PRV variant and the Bartha-K/61 vaccine strain. Front. Microbiol. 2023, 14, 1164170. [Google Scholar] [CrossRef]
- Kong, H.; Zhang, K.; Liu, Y.; Shang, Y.; Wu, B.; Liu, X. Attenuated live vaccine (Bartha-K16) caused pseudorabies (Aujeszky’s disease) in sheep. Vet. Res. Commun. 2013, 37, 329–332. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Pijoan, C. Pneumonia in pigs induced by intranasal challenge exposure with pseudorabies virus and Pasteurella multocida. Am. J. Vet. Res. 1987, 48, 1446–1448. [Google Scholar]
- Zhang, X.; Shu, X.; Bai, H.; Li, W.; Li, X.; Wu, C.; Gao, Y.; Wang, Y.; Yang, K.; Song, C. Effect of porcine circovirus type 2 on the severity of lung and brain damage in piglets infected with porcine pseudorabies virus. Vet. Microbiol. 2019, 237, 108394. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yao, J.; Yang, Y.; Luo, W.; Yuan, X.; Yang, L.; Wang, A. Current Status and Challenge of Pseudorabies Virus Infection in China. Virol. Sin. 2021, 36, 588–607. [Google Scholar] [CrossRef] [PubMed]
- An, T.Q.; Peng, J.M.; Tian, Z.J.; Zhao, H.Y.; Li, N.; Liu, Y.M.; Chen, J.Z.; Leng, C.L.; Sun, Y.; Chang, D.; et al. Pseudorabies virus variant in Bartha-K61-vaccinated pigs, China, 2012. Emerg. Infect. Dis. 2013, 19, 1749–1755. [Google Scholar] [CrossRef]
- Szpara, M.L.; Parsons, L.; Enquist, L.W. Sequence variability in clinical and laboratory isolates of herpes simplex virus 1 reveals new mutations. J. Virol. 2010, 84, 5303–5313. [Google Scholar] [CrossRef]
- Luo, Y.; Li, N.; Cong, X.; Wang, C.H.; Du, M.; Li, L.; Zhao, B.; Yuan, J.; Liu, D.D.; Li, S.; et al. Pathogenicity and genomic characterization of a pseudorabies virus variant isolated from Bartha-K61-vaccinated swine population in China. Vet. Microbiol. 2014, 174, 107–115. [Google Scholar] [CrossRef]
- Hu, R.M.; Zhou, Q.; Song, W.B.; Sun, E.C.; Zhang, M.M.; He, Q.G.; Chen, H.C.; Wu, B.; Liu, Z.F. Novel pseudorabies virus variant with defects in TK, gE and gI protects growing pigs against lethal challenge. Vaccine 2015, 33, 5733–5740. [Google Scholar] [CrossRef]
- Gu, Z.; Hou, C.; Sun, H.; Yang, W.; Dong, J.; Bai, J.; Jiang, P. Emergence of highly virulent pseudorabies virus in southern China. Can. J. Vet. Res. 2015, 79, 221–228. [Google Scholar]
- Tong, W.; Liu, F.; Zheng, H.; Liang, C.; Zhou, Y.J.; Jiang, Y.F.; Shan, T.L.; Gao, F.; Li, G.X.; Tong, G.Z. Emergence of a Pseudorabies virus variant with increased virulence to piglets. Vet. Microbiol. 2015, 181, 236–240. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Xu, Q.; Wu, J.; Zhai, X.; Li, S.; Wang, J.; Ni, J.; Yuan, L.; Song, X.; et al. Investigation on pseudorabies prevalence in Chinese swine breeding farms in 2013–2016. Trop. Anim. Health Prod. 2018, 50, 1279–1285. [Google Scholar] [CrossRef]
- Ye, C.; Guo, J.C.; Gao, J.C.; Wang, T.Y.; Zhao, K.; Chang, X.B.; Wang, Q.; Peng, J.M.; Tian, Z.J.; Cai, X.H.; et al. Genomic analyses reveal that partial sequence of an earlier pseudorabies virus in China is originated from a Bartha-vaccine-like strain. Virology 2016, 491, 56–63. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, Q.Z.; Tian, Z.J.; Zheng, H.; Zhao, K.; Liu, F.; Guo, J.C.; Tong, W.; Jiang, C.G.; Wang, S.J.; et al. Genomic characterization of emergent pseudorabies virus in China reveals marked sequence divergence: Evidence for the existence of two major genotypes. Virology 2015, 483, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Chen, Q.Y.; Chen, R.J.; Che, Y.L.; Wang, L.B.; Wang, C.Y.; Yan, S.; Liu, Y.T.; Xiu, J.S.; Zhou, L.J. Pathogenicity and Whole Genome Sequence Analysis of a Pseudorabies Virus Strain FJ-2012 Isolated from Fujian, Southern China. Can. J. Infect. Dis. Med. Microbiol. 2017, 2017, 9073172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, L.; Liu, H.; Ye, G.; Huang, L.; Weng, C. Isolation and Characterization of Two Pseudorabies Virus and Evaluation of Their Effects on Host Natural Immune Responses and Pathogenicity. Viruses 2022, 14, 712. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.D. Application of CRISPR/Cas9 Technology in Pseudorabies Virus Research. Ph.D. Thesis, Chinese Academy of Agricultural Sciences (CASA), Beijing, China, 2018. [Google Scholar]
- Lavis, L.D. Histochemistry: Live and in color. J. Histochem. Cytochem. 2011, 59, 139–145. [Google Scholar] [CrossRef]
- Lyu, C.; Wang, S.; Sun, M.; Tang, Y.; Peng, J.; Tian, Z.; Cai, X. Deletion of pseudorabies virus US2 gene enhances viral titers in a porcine cerebral cortex primary culture system. Virus Genes 2018, 54, 406–413. [Google Scholar] [CrossRef]
- Ma, Z.; Han, Z.; Liu, Z.; Meng, F.; Wang, H.; Cao, L.; Li, Y.; Jiao, Q.; Liu, S.; Liu, M. Epidemiological investigation of porcine pseudorabies virus and its coinfection rate in Shandong Province in China from 2015 to 2018. J. Vet. Sci. 2020, 21, e36. [Google Scholar] [CrossRef]
- Yu, X.; Sun, Q.; Ku, X.; He, D.; Li, Z.; Ghonaim, A.H.; Fan, S.; He, Q. The epidemiological investigation of co-infection of major respiratory bacteria with pseudorabies virus in intensive pig farms in China. Vet. Med. Sci. 2021, 7, 175–183. [Google Scholar] [CrossRef]
- Kit, S.; Kit, M.; Pirtle, E.C. Attenuated properties of thymidine kinase-negative deletion mutant of pseudorabies virus. Am. J. Vet. Res. 1985, 46, 1359–1367. [Google Scholar] [CrossRef]
- Huanchun, C.; Fuchun, Z.; Liurong, F.; Qigai, H.; Bin, W.; Wenzhou, H. Construction of TK−/gG−/LacZ+ Mutant of Pseudorabies Virus Strain Ea. Chin. J. Virol. 2001, 17, 69–74. [Google Scholar]
- Yao, J.; Juanping, W.; Yichao, H.; Xin, W.; Fan, M.; Zhenhua, F.; Yuping, L.; Wenjun, L. The lmmunity Test of gE Gene Deleted Vaccine of Pseudorabies Virus. China Anim. Husb. Vet. Med. 2011, 38, 184–187. [Google Scholar]
- Kit, S.; Sheppard, M.; Ichimura, H.; Kit, M. Second-generation pseudorabies virus vaccine with deletions in thymidine kinase and glycoprotein genes. Am. J. Vet. Res. 1987, 48, 780–793. [Google Scholar] [PubMed]
- Qigai, H. The Study on the Gene-Deleted Vaccine of Swine Pseudorabies. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2000. [Google Scholar]
- Yuanyan, L.; Yanfen, H.; Xiaorong, Z.; Sujuan, C.; Daxin, P.; Xiufan, L. Construction of a gE and TK Double Genes Deletion Mutant of Pseudorabies Virus by Cre-loxP System. Acta Vet. Zootech. Sin. 2012, 43, 1802–1809. [Google Scholar]
- Tong, W.; Li, G.; Liang, C.; Liu, F.; Tian, Q.; Cao, Y.; Li, L.; Zheng, X.; Zheng, H.; Tong, G. A live, attenuated pseudorabies virus strain JS-2012 deleted for gE/gI protects against both classical and emerging strains. Antivir. Res. 2016, 130, 110–117. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Han, L.; Yu, J.; Ye, G.; Liu, H.; Liu, Y.; Zhou, Q.; Zhang, Z.; Weng, C. Construction of Two Recombinant Pseudorabies Viruses with Deletion of Virulence Genes and Evaluation of Their Immune Protection in Mice and Piglets. Vaccines 2025, 13, 359. https://doi.org/10.3390/vaccines13040359
Wang S, Han L, Yu J, Ye G, Liu H, Liu Y, Zhou Q, Zhang Z, Weng C. Construction of Two Recombinant Pseudorabies Viruses with Deletion of Virulence Genes and Evaluation of Their Immune Protection in Mice and Piglets. Vaccines. 2025; 13(4):359. https://doi.org/10.3390/vaccines13040359
Chicago/Turabian StyleWang, Shanghui, Longfei Han, Jimin Yu, Guangqiang Ye, Hongyang Liu, Yunfei Liu, Qiongqiong Zhou, Zhaoxia Zhang, and Changjiang Weng. 2025. "Construction of Two Recombinant Pseudorabies Viruses with Deletion of Virulence Genes and Evaluation of Their Immune Protection in Mice and Piglets" Vaccines 13, no. 4: 359. https://doi.org/10.3390/vaccines13040359
APA StyleWang, S., Han, L., Yu, J., Ye, G., Liu, H., Liu, Y., Zhou, Q., Zhang, Z., & Weng, C. (2025). Construction of Two Recombinant Pseudorabies Viruses with Deletion of Virulence Genes and Evaluation of Their Immune Protection in Mice and Piglets. Vaccines, 13(4), 359. https://doi.org/10.3390/vaccines13040359





