Bacillus Calmette–Guérin Vaccination Promotes Efficient and Comprehensive Immune Modulation in Guinea Pig Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Bacterial Culture
2.3. Bacterial Strains and Vaccines
2.4. Infection and Survival Studies
2.5. Hematoxylin and Eosin (H&E) Staining
2.6. Immunohistochemical (IHC) Staining
2.7. Statistical Analysis
3. Results
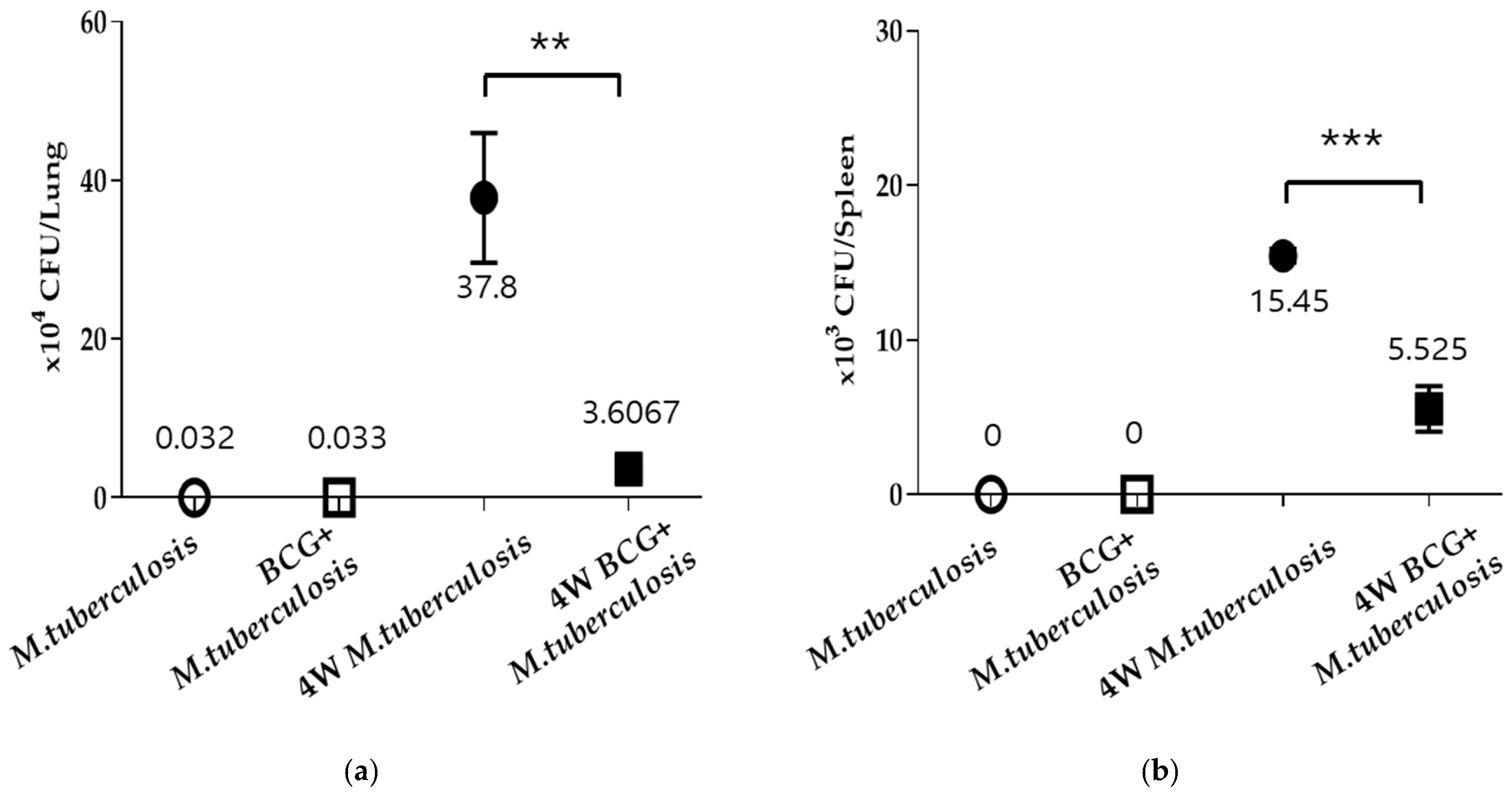
3.1. Vaccination Results in a Confirmed Inhibition of M. tuberculosis Growth
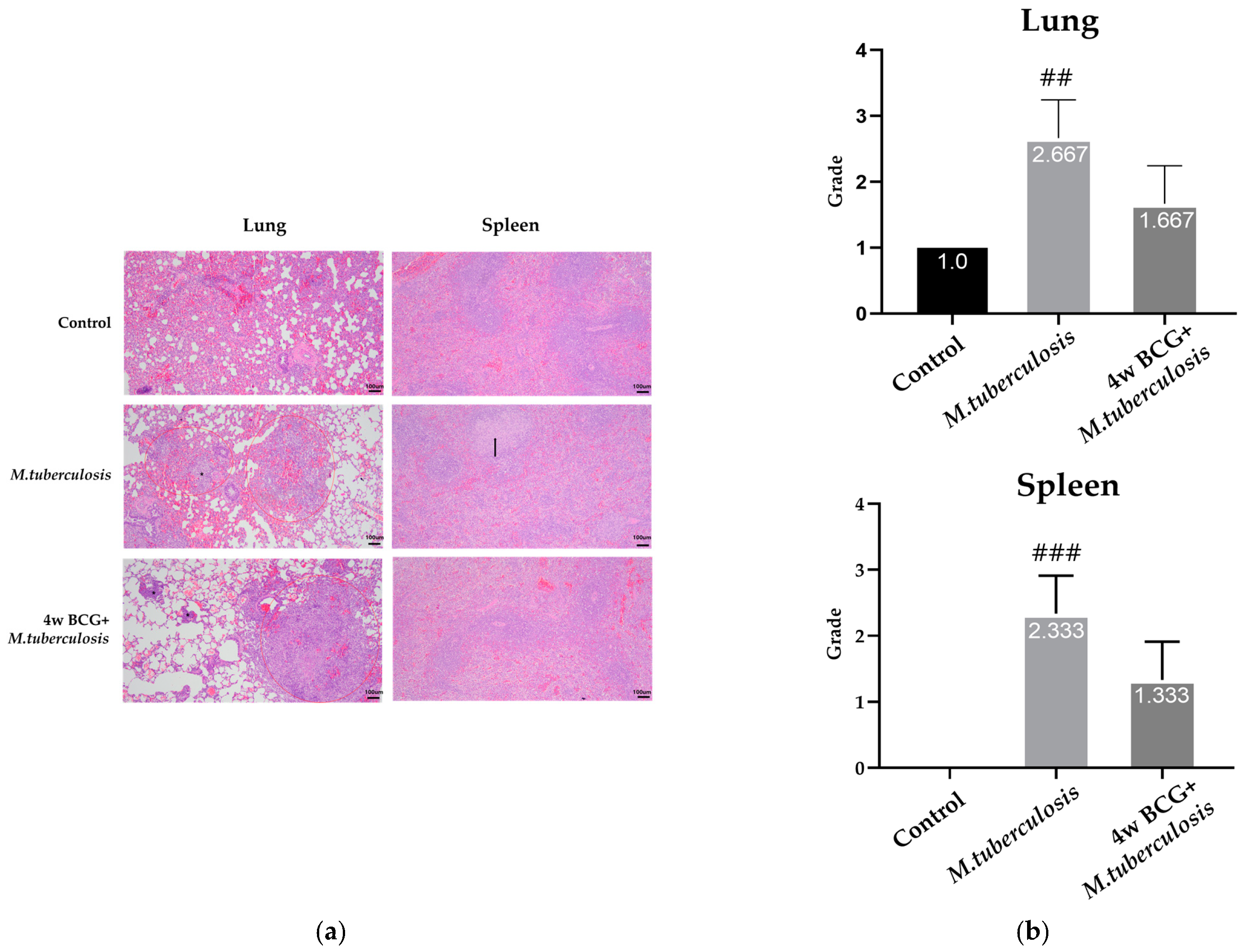
3.2. Histopathological Changes Following BCG Vaccination in Lung and Spleen of Guinea Pig
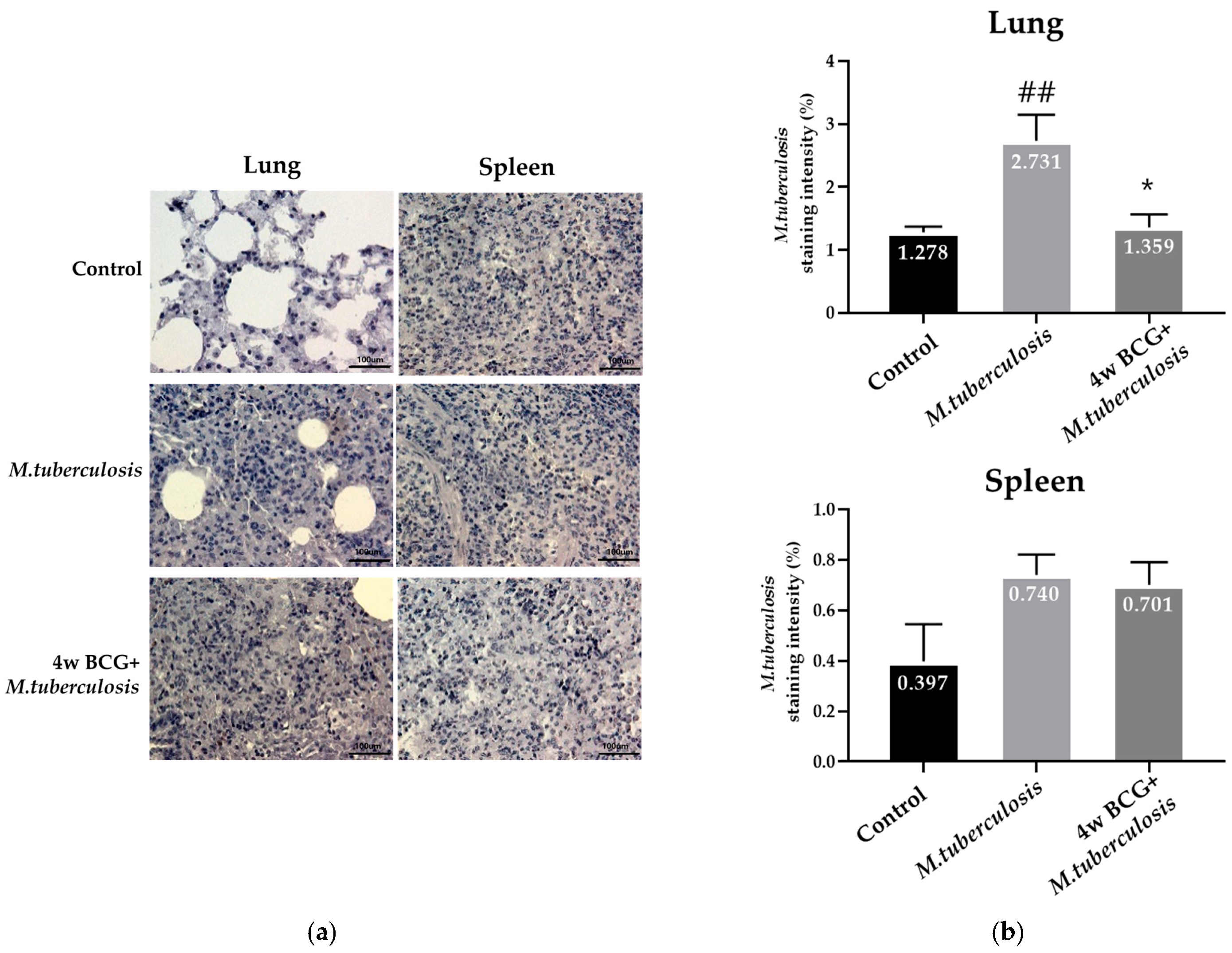
3.3. Analysis of the Infection Level of M. tuberculosis Using Immunohistochemical Staining in Guinea Pig Lung and Spleen
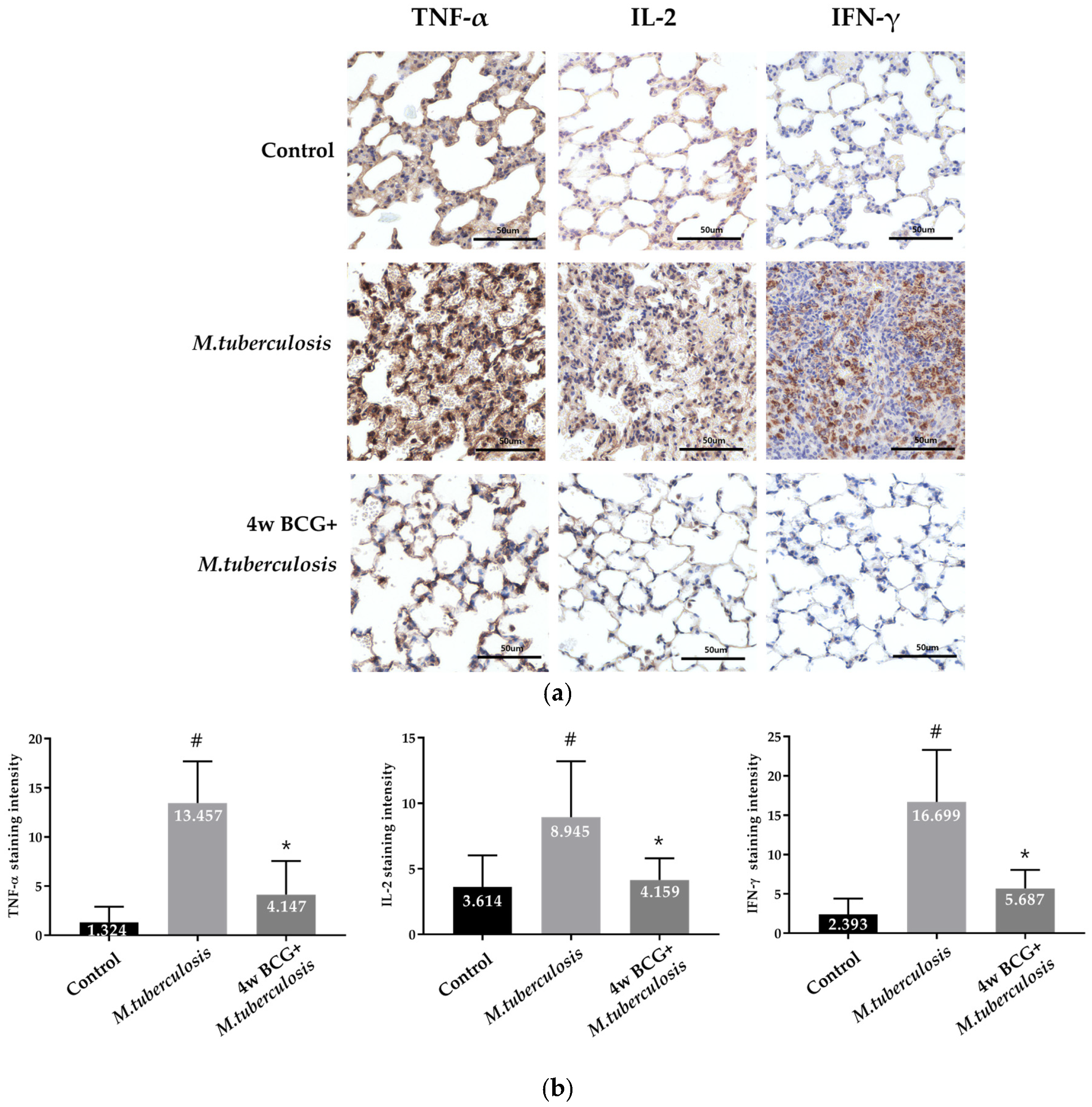
3.4. IHC Analysis of TNF-α, IL-2 and IFN-γ Positive Cells in Guinea Pig Lung
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2021: Supplementary Material; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Kyu, H.H.; Maddison, E.R.; Henry, N.J.; Mumford, J.E.; Barber, R.; Shields, C.; Brown, J.C.; Nguyen, G.; Carter, A.; Wolock, T.M.; et al. The global burden of tuberculosis: Results from the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2018, 18, 261–284. [Google Scholar] [CrossRef]
- Shah, Y.; Paudel, S.; Pandey, K.; Gupta, G.P.; Solo, E.S.; Joshi, J.; Pant, D.K.; Pandey, B.D. Insights into transmission dynamics of Mycobacterium tuberculosis complex in Nepal. Trop. Med. Health 2022, 50, 8. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. BCG vaccine: WHO position paper, February 2018—Recommendations. Vaccine 2018, 36, 3408–3410. [Google Scholar] [CrossRef]
- Fine, P. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet 1995, 346, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Nunn, P.P. The need for new drugs against tuberculosis. Obstacles, opportunities, and next steps. Am. J. Respir. Crit. Care Med. 2001, 163, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Ramon-Luing, L.A.; Carranza, C.; Tellez-Navarrete, N.A.; Medina-Quero, K.; Gonzalez, Y.; Torres, M.; Chavez-Galan, L. Mycobacterium tuberculosis H37Rv Strain Increases the Frequency of CD3+TCR+ Macrophages and Affects Their Phenotype, but Not Their Migration Ability. Int. J. Mol. Sci. 2021, 23, 329. [Google Scholar] [CrossRef]
- O’Garra, A.; Redford, P.S.; McNab, F.W.; Bloom, C.I.; Wilkinson, R.J.; Berry, M.P. The immune response in tuberculosis. Annu. Rev. Immunol. 2013, 31, 475–527. [Google Scholar] [CrossRef]
- Prezzemolo, T.; Guggino, G.; La Manna, M.P.; Di Liberto, D.; Dieli, F.; Caccamo, N. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front. Immunol. 2014, 5, 180. [Google Scholar] [CrossRef]
- Islam, M.N.; Mishra, V.K.; Munalisa, R.; Parveen, F.; Ali, S.F.; Akter, K.; Ahmed, T.; Ho, T.-J.; Huang, C.-Y. Mechanistic insight of mitochondrial dysfunctions in cardiovascular diseases with potential biomarkers. Mol. Cell. Toxicol. 2024, 20, 441–463. [Google Scholar] [CrossRef]
- Li, J.; Lu, J.; Wang, G.; Zhao, A.; Xu, M. Past, present and future of Bacillus Calmette-Guérin vaccine use in China. Vaccines 2022, 10, 1157. [Google Scholar] [CrossRef]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.M.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Kolloli, A.; Kumar, R.; Venketaraman, V.; Subbian, S. Immunopathology of Pulmonary Mycobacterium tuberculosis Infection in a Humanized Mouse Model. Int. J. Mol. Sci. 2024, 25, 1656. [Google Scholar] [CrossRef] [PubMed]
- ISO 9001:2015; Quality Management Systems. ISO: Geneva, Switzerland, 2015.
- Larenas-Munoz, F.; Ruedas-Torres, I.; Hunter, L.; Bird, A.; Agullo-Ros, I.; Winsbury, R.; Clark, S.; Rayner, E.; Salguero, F.J. Characterisation and development of histopathological lesions in a guinea pig model of Mycobacterium tuberculosis infection. Front. Vet. Sci. 2023, 10, 1264200. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Wood, C.; Kwon, D.; Park, K.P.; Fejer, G.; Delorme, V. Mycobacterium tuberculosis Infection and Innate Responses in a New Model of Lung Alveolar Macrophages. Front. Immunol. 2018, 9, 438. [Google Scholar] [CrossRef]
- Mai, D.; Jahn, A.; Murray, T.; Morikubo, M.; Lim, P.N.; Cervantes, M.M.; Pham, L.K.; Nemeth, J.; Urdahl, K.; Diercks, A.H.; et al. Exposure to Mycobacterium remodels alveolar macrophages and the early innate response to Mycobacterium tuberculosis infection. PLoS Pathog. 2024, 20, e1011871. [Google Scholar] [CrossRef]
- Gallegos, A.M.; van Heijst, J.W.J.; Samstein, M.; Su, X.; Pamer, E.G.; Glickman, M.S. A Gamma Interferon Independent Mechanism of CD4 T Cell Mediated Control of M. tuberculosis Infection in vivo. PLoS Pathog. 2011, 7, e1002052. [Google Scholar] [CrossRef]
- Hu, Z.; Shi, L.; Xie, J.; Fan, X.Y. Editorial: Innate and adaptive immunity against tuberculosis infection: Diagnostics, vaccines, and therapeutics. Front. Immunol. 2024, 15, 1366976. [Google Scholar] [CrossRef]
- Park, S.-Y. Chemically induced bacterial ghosts: A novel approach for advancing biomedical applications. Mol. Cell. Toxicol. 2023, 19, 657–665. [Google Scholar] [CrossRef]
- Gong, W.; Wu, X. Differential Diagnosis of Latent Tuberculosis Infection and Active Tuberculosis: A Key to a Successful Tuberculosis Control Strategy. Front. Microbiol. 2021, 12, 745592. [Google Scholar] [CrossRef]
- Okafor, C.N.; Rewane, A.; Momodu, I.I. Bacillus Calmette Guerin. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538185/ (accessed on 31 January 2025).
- Tanner, R.; Villarreal-Ramos, B.; Vordermeier, H.M.; McShane, H. The Humoral Immune Response to BCG Vaccination. Front. Immunol. 2019, 10, 1317. [Google Scholar] [CrossRef]
- Singh, S.; Saavedra-Avila, N.A.; Tiwari, S.; Porcelli, S.A. A century of BCG vaccination: Immune mechanisms, animal models, non-traditional routes and implications for COVID-19. Front. Immunol. 2022, 13, 959656. [Google Scholar] [CrossRef]
- Dunn, C.; Brettle, D.; Cockroft, M.; Keating, E.; Revie, C.; Treanor, D. Quantitative assessment of H&E staining for pathology: Development and clinical evaluation of a novel system. Diagn. Pathol. 2024, 19, 42. [Google Scholar] [CrossRef]
- Basaraba, R.J.; Hunter, R.L. Pathology of Tuberculosis: How the Pathology of Human Tuberculosis Informs and Directs Animal Models. Tuberc. Tuber. Bacillus 2017, 5, 117–129. [Google Scholar] [CrossRef]
- Sasindran, S.J.; Torrelles, J.B. Mycobacterium tuberculosis Infection and Inflammation: What is Beneficial for the Host and for the Bacterium? Front. Microbiol. 2011, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Lai, R.P.; Wilkinson, R.J.; Ottenhoff, T.H.M. The In Vivo Transcriptomic Blueprint of Mycobacterium tuberculosis in the Lung. Front. Immunol. 2021, 12, 763364. [Google Scholar] [CrossRef]
- de Martino, M.; Lodi, L.; Galli, L.; Chiappini, E. Immune Response to Mycobacterium tuberculosis: A Narrative Review. Front. Pediatr. 2019, 7, 350. [Google Scholar] [CrossRef]
- Morrison, H.; McShane, H. Local Pulmonary Immunological Biomarkers in Tuberculosis. Front. Immunol. 2021, 12, 640916. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kang, J.; Jiang, H. Intranasal Immunization with a Recombinant Adenovirus Encoding Multi-Stage Antigens of Mycobacterium tuberculosis Preferentially Elicited CD8+ T Cell Immunity and Conferred a Superior Protection in the Lungs of Mice than Bacillus Calmette-Guerin. Vaccines 2024, 12, 1022. [Google Scholar] [CrossRef]
- Ehlers, S.; Schaible, U.E. The granuloma in tuberculosis: Dynamics of a host-pathogen collusion. Front. Immunol. 2012, 3, 411. [Google Scholar] [CrossRef]
- Yang, J.D.; Mott, D.; Sutiwisesak, R.; Lu, Y.-J.; Raso, F.; Stowell, B.; Babunovic, G.H.; Lee, J.; Carpenter, S.M.; Way, S.S.; et al. Mycobacterium tuberculosis-specific CD4+ and CD8+ T cells differ in their capacity to recognize infected macrophages. PLoS Pathog. 2018, 14, e1007060. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.K.; Thandi, R.S.; Tripathi, D.; Paidipally, P.; McAllister, M.K.; Mulik, S.; Samten, B.; Vankayalapati, R. BCG vaccination reduces the mortality of Mycobacterium tuberculosis-infected type 2 diabetes mellitus mice. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Cyktor, J.C.; Carruthers, B.; Kominsky, R.A.; Beamer, G.L.; Stromberg, P.; Turner, J. IL-10 Inhibits Mature Fibrotic Granuloma Formation during Mycobacterium tuberculosis Infection. J. Immunol. 2013, 190, 2778–2790. [Google Scholar] [CrossRef]
| Steps | Function | Time |
|---|---|---|
| 1 | Pre-heating | 15 min |
| 2 | Nebulizer | 30 min |
| 3 | Cloud decay | 30 min |
| 4 | UV | 15 min |
| 5 | Cool down | 10 min |
| Total Times | 1 h 40 min | |
| Grade | |
|---|---|
| 0 | Normal(No changes in the cells or tissue structure. No increased apoptosis or macrophage aggregation) |
| 1 | Minimal (Slight cell increase, but barely noticeable. Rare apoptotic cells and small macrophage clusters, with no impact on structure) |
| 2 | Slight (Mild cell increase, structure intact. Mild apoptosis, occasional cell clusters, and mild macrophage aggregation) |
| 3 | Moderate(Increase in cellularity at the margins, and a rise in macrophage aggregation) |
| 4 | Severe (Large cell clusters, showing significant tissue damage. Extensive apoptosis, lymphocyte loss, and marked cellular infiltration and structural disruption) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouh, I.-O.; Kim, M.J.; Kim, K.; Lim, H.; Yang, Y.J.; Heo, J.W.; Choi, H.N.; Kim, H.H.; Lee, H.-J.; Koh, P.-O.; et al. Bacillus Calmette–Guérin Vaccination Promotes Efficient and Comprehensive Immune Modulation in Guinea Pig Models. Vaccines 2025, 13, 305. https://doi.org/10.3390/vaccines13030305
Ouh I-O, Kim MJ, Kim K, Lim H, Yang YJ, Heo JW, Choi HN, Kim HH, Lee H-J, Koh P-O, et al. Bacillus Calmette–Guérin Vaccination Promotes Efficient and Comprehensive Immune Modulation in Guinea Pig Models. Vaccines. 2025; 13(3):305. https://doi.org/10.3390/vaccines13030305
Chicago/Turabian StyleOuh, In-Ohk, Min Jung Kim, Kwangwook Kim, Heeji Lim, Ye Jin Yang, Ji Woong Heo, Han Nim Choi, Hun Hwan Kim, Hu-Jang Lee, Phil-Ok Koh, and et al. 2025. "Bacillus Calmette–Guérin Vaccination Promotes Efficient and Comprehensive Immune Modulation in Guinea Pig Models" Vaccines 13, no. 3: 305. https://doi.org/10.3390/vaccines13030305
APA StyleOuh, I.-O., Kim, M. J., Kim, K., Lim, H., Yang, Y. J., Heo, J. W., Choi, H. N., Kim, H. H., Lee, H.-J., Koh, P.-O., Moon, S. Y., Choi, E. B., Lee, Y.-K., & Park, K. I. (2025). Bacillus Calmette–Guérin Vaccination Promotes Efficient and Comprehensive Immune Modulation in Guinea Pig Models. Vaccines, 13(3), 305. https://doi.org/10.3390/vaccines13030305







