Discrepancy in SARS-CoV-2 Infection Status Among PCR, Serological, and Cellular Immunity Assays of Nucleocapsids: A Historical Cohort Study
Abstract
1. Introduction
2. Materials and Methods
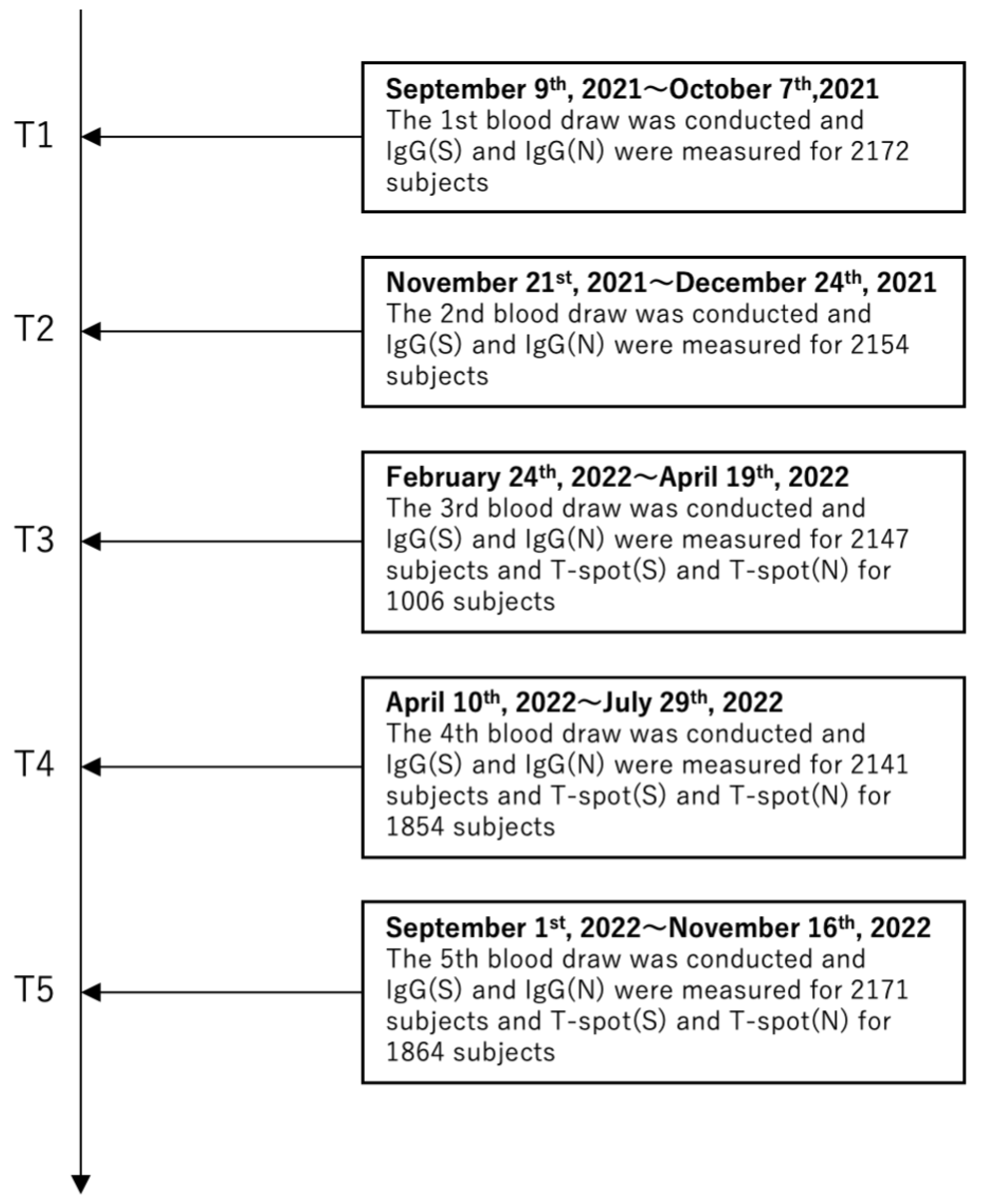
2.1. Study Design and Participants
2.2. Serological Testing
2.3. Cellular Immune Response Measurements
2.4. Primary Outcome
2.5. Statistical Analyses
3. Results
3.1. Participant Characteristics
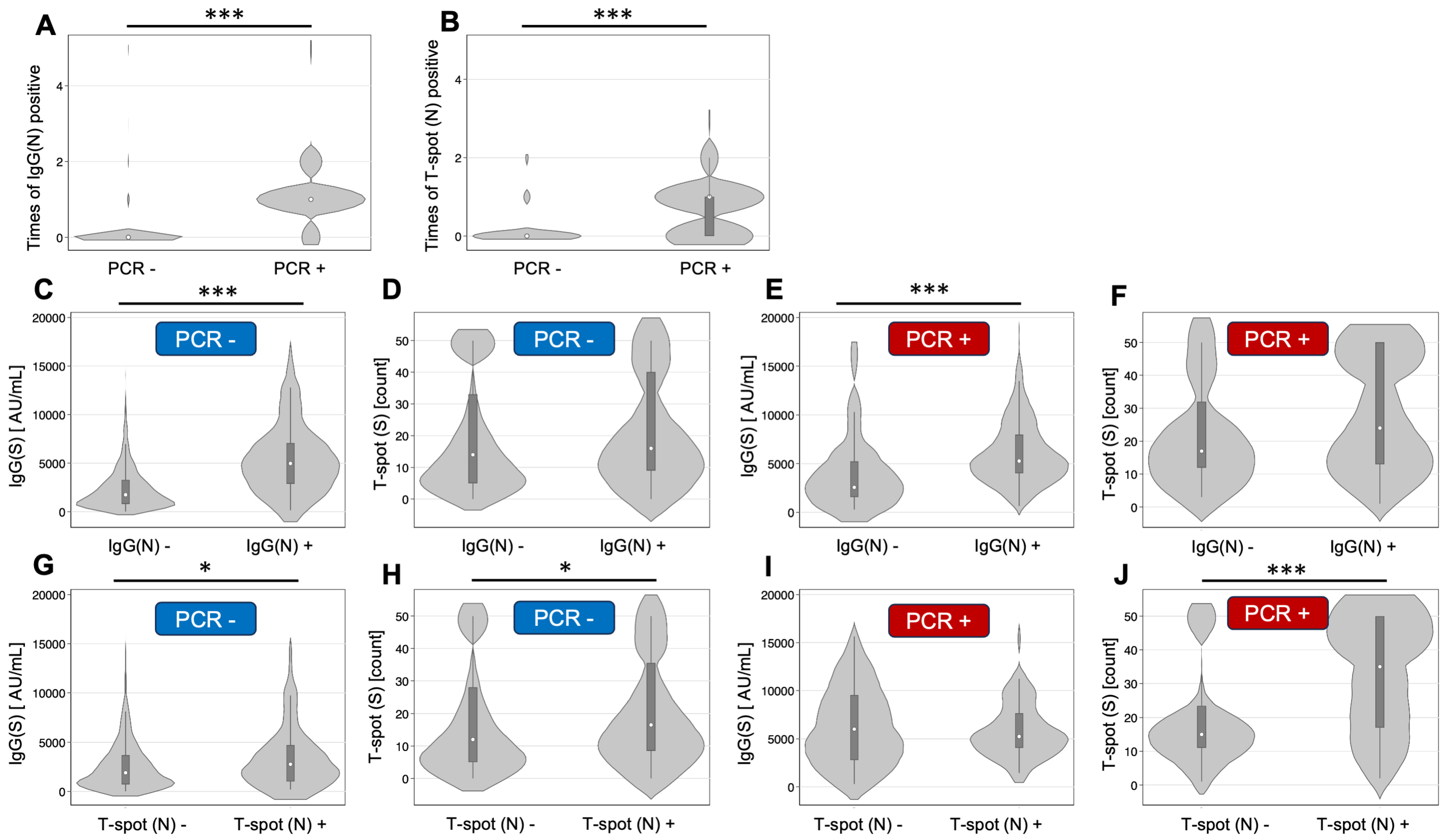
3.2. Relationship Between Infection Status and IgG(N) and T-spot(N)
3.3. Grouping by Infection History and IgG(N) Determination
3.4. Grouping by Infection History and T-spot(N) Status
3.5. Relationships Between Different Assays
3.6. Symptoms Among Infected Participants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IgG(N) | Immunoglobin G for nucleocapsid |
| PCR | Polymerase chain reaction |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| CLIA | Chemiluminescent immunoassay |
References
- Zhou, L.; Ayeh, S.K.; Chidambaram, V.; Karakousis, P.C. Modes of transmission of SARS-CoV-2 and evidence for preventive behavioral interventions. BMC Infect. Dis. 2021, 21, 496. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Li, J.; Cheng, Z.J. Rapid antigen test combine with nucleic acid detection: A better strategy for COVID-19 screening at points of entry. J. Epidemiol. Glob. Health 2022, 12, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, J.U.; Shim, S.R. Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS-CoV-2 infection: A systematic review and meta-analysis. J. Clin. Virol. 2021, 144, 104985. [Google Scholar] [CrossRef] [PubMed]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis—A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection. Ann. Intern. Med. 2020, 173, 362–367. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, X.; Deng, Y.; Tang, Y.; Lu, J. COVID-19: Asymptomatic carrier transmission is an underestimated problem. Epidemiol. Infect. 2020, 148, e116. [Google Scholar] [CrossRef]
- Tominaga, T.; Hachiya, M.; Tatsuzaki, H.; Akashi, M. The Accident at the Fukushima Daiichi Nuclear Power Plant in 2011. Health Phys. 2014, 106, 630–637. [Google Scholar] [CrossRef]
- Kayama, M.; Akiyama, T.; Ohashi, A.; Horikoshi, N.; Kido, Y.; Murakata, T.; Kawakami, N. Experiences of Municipal Public Health Nurses Following Japan’s Earthquake, Tsunami, and Nuclear Disaster. Public Health Nurs. 2014, 31, 517–525. [Google Scholar] [CrossRef]
- Ishiwatari, M. Institutional Coordination of Disaster Management: Engaging National and Local Governments in Japan. Nat. Hazards Rev. 2021, 22, 04020059. [Google Scholar] [CrossRef]
- Yumiya, Y.; Goto, A.; Murakami, M.; Ohira, T.; Rudd, R.E. Communication between Health Professionals and Community Residents in Fukushima: A Focus on the Feedback Loop. Health Commun. 2019, 35, 1274–1282. [Google Scholar] [CrossRef]
- Kobashi, Y.; Kawamura, T.; Shimazu, Y.; Zhao, T.; Sugiyama, A.; Nakayama, A.; Kaneko, Y.; Nishikawa, Y.; Omata, F.; Takita, M.; et al. Humoral immunity after second dose of BNT162b2 vaccine in Japanese communities: An observational cross-sectional study, Fukushima Vaccination Community Survey. Sci. Rep. 2022, 12, 18929. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, Y.; Nishikawa, Y.; Kawamura, T.; Kodama, T.; Shimazu, Y.; Obara, D.; Zhao, T.; Tsubokura, M. Seroprevalence of SARS-CoV-2 antibodies among hospital staff in rural Central Fukushima, Japan: A historical cohort study. Int. Immunopharmacol. 2021, 98, 107884. [Google Scholar] [CrossRef] [PubMed]
- Pagheh, A.S.; Ziaee, A.; Abrari Romenjan, K.; Rezaei, F.; Bahman, B.; Alamzadeh, E.; Elhamirad, S.; Ziaee, M. SARS-COV-2 IgG specific antibodies persistence in recovered COVID-19 individuals and its association with severity and time of illness. New Microbes New Infect. 2023, 52, 101096. [Google Scholar] [CrossRef]
- Stead, D.; Adeniyi, O.V.; Singata-Madliki, M.; Abrahams, S.; Batting, J.; Jelliman, E.; Parrish, A. Cumulative incidence of SARS-CoV-2 and associated risk factors among healthcare workers: A cross-sectional study in the Eastern Cape, South Africa. BMJ Open 2022, 12, e058761. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, M.; Wang, M.; Zhao, Y.; Zheng, Z.; Li, X.; Zhang, Y.; Wang, T.; Zeng, S.; Hu, W.; et al. Asymptomatic infection by SARS-CoV-2 in healthcare workers: A study in a large teaching hospital in Wuhan, China. Int. J. Infect. Dis. 2020, 99, 219–225. [Google Scholar] [CrossRef]
- Thiruvengadam, R.; Chattopadhyay, S.; Mehdi, F.; Desiraju, B.K.; Chaudhuri, S.; Singh, S.; Bhartia, V.; Kshetrapal, P.; Mouli Natchu, U.C.; Wadhwa, N.; et al. Longitudinal serology of SARS-CoV-2-Infected individuals in India: A prospective cohort study. Am. J. Trop. Med. Hyg. 2021, 105, 66–72. [Google Scholar] [CrossRef]
- Kharroubi, G.; Cherif, I.; Ghawar, W.; Dhaouadi, N.; Yazidi, R.; Chaabane, S.; Snoussi, M.A.; Salem, S.; Ben Hammouda, W.; Ben Hammouda, S.; et al. Incidence and risk factors of SARS-CoV-2 infection among workers in a public health laboratory in Tunisia. Arch. Virol. 2023, 168, 69. [Google Scholar] [CrossRef]
- Sanada, T.; Honda, T.; Yasui, F.; Yamaji, K.; Munakata, T.; Yamamoto, N.; Kurano, M.; Matsumoto, Y.; Kohno, R.; Toyama, S.; et al. Serologic survey of IgG against SARS-CoV-2 among hospital visitors without a history of SARS-CoV-2 infection in Tokyo, 2020–2021. J. Epidemiol. 2022, 32, 105–111. [Google Scholar] [CrossRef]
- Marklund, E.; Leach, S.; Nyström, K.; Lundgren, A.; Liljeqvist, J.Å.; Nilsson, S.; Yilmaz, A.; Andersson, L.M.; Bemark, M.; Gisslén, M. Longitudinal follow up of immune responses to SARS-CoV-2 in health care workers in Sweden with several different commercial IgG-assays, measurement of neutralizing antibodies and CD4+ T-cell Responses. Front. Immunol. 2021, 12, 750448. [Google Scholar] [CrossRef]
- Turbett, S.E.; Anahtar, M.; Dighe, A.S.; Garcia Beltran, W.; Miller, T.; Scott, H.; Durbin, S.M.; Bharadwaj, M.; Thomas, J.; Gogakos, T.S.; et al. Evaluation of three commercial SARS-CoV-2 serologic assays and their performance in two-test algorithms. J. Clin. Microbiol. 2020, 59, e01892-20. [Google Scholar] [CrossRef]
- Algaissi, A.; Alfaleh, M.A.; Hala, S.; Abujamel, T.S.; Alamri, S.S.; Almahboub, S.A.; Alluhaybi, K.A.; Hobani, H.I.; Alsulaiman, R.M.; AlHarbi, R.H.; et al. SARS-CoV-2 S1 and N-based serological assays reveal rapid seroconversion and induction of specific antibody response in COVID-19 patients. Sci. Rep. 2020, 10, 16561. [Google Scholar] [CrossRef] [PubMed]
- Lisboa Bastos, M.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for Covid-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef] [PubMed]
- Igawa, G.; Ai, T.; Yamamoto, T.; Ito, K.; Nojiri, S.; Saito, K.; Wakita, M.; Fukuda, H.; Hori, S.; Misawa, S.; et al. Antibody response and seroprevalence in healthcare workers after the BNT162b2 vaccination in a University Hospital at Tokyo. Sci. Rep. 2022, 12, 8707. [Google Scholar] [CrossRef]
- Speer, C.; Göth, D.; Benning, L.; Buylaert, M.; Schaier, M.; Grenz, J.; Nusshag, C.; Kälble, F.; Kreysing, M.; Reichel, P.; et al. Early humoral responses of hemodialysis patients after COVID-19 vaccination with BNT162b2. Clin. J. Am. Soc. Nephrol. 2021, 16, 1073–1082. [Google Scholar] [CrossRef]
- Anand, S.; Montez-Rath, M.E.; Han, J.; Garcia, P.; Cadden, L.; Hunsader, P.; Kerschmann, R.; Beyer, P.; Dittrich, M.; Block, G.A.; et al. Antibody response to COVID-19 vaccination in patients receiving dialysis. J. Am. Soc. Nephrol. 2021, 32, 2435–2438. [Google Scholar] [CrossRef]
| Variable | n (%) |
|---|---|
| Age | 52 (39–67) |
| Under 41 | 572 (27.2) |
| 41–65 | 977 (46.4) |
| Over 65 | 555 (26.3) |
| Sex: female | 1220 (58.0) |
| Type of third dose vaccination (n = 2070) | |
| mRNA-1273 | 1401 (67.7) |
| BNT162b | 669 (32.3) |
| Type of fourth dose vaccination (n = 1406) | |
| mRNA-1273 | 337 (24.0) |
| BNT162b | 1008 (71.7) |
| Others | 61 (4.3) |
| Smoking habit (n = 2069) | 392 (19.0) |
| Alcohol consumption (n = 2058) | 904 (43.9) |
| Daily medicine | |
| Steroid (n = 2072) | 40 (1.9) |
| Immunosuppression (n = 2067) | 21 (1.0) |
| Biologics (n = 2065) | 11 (0.5) |
| Comorbidity | |
| Hypertension (n = 2098) | 555 (26.5) |
| Diabetes (n = 2098) | 155 (7.4) |
| Dyslipidemia (n = 2098) | 248 (11.8) |
| BMI (n = 1923) | |
| Under 18.5 | 120 (6.2) |
| 18.5–under 25 | 1221 (63.5) |
| 25–30 | 443 (23.0) |
| Over 30 | 139 (7.2) |
| Not Infected | Infected | Infected and Hospitalized | |
|---|---|---|---|
| IgG(N) | |||
| Never | 1838 | 24 | 1 |
| 1 time | 40 | 142 | 3 |
| 2 times | 14 | 26 | 4 |
| 3 times | 1 | 0 | 0 |
| 4 times | 0 | 0 | 0 |
| 5 times | 9 | 2 | 0 |
| T-spot(N) | |||
| Never | 789 | 45 | 3 |
| 1 time | 50 | 60 | 0 |
| 2 times | 22 | 9 | 1 |
| 3 times | 0 | 1 | 0 |
| Geometric Mean (95% CI) | |
|---|---|
| IgG(S) at T5 | |
| Not infected (with PCR test) | |
| IgG(N)-negative (n = 1838) | 1541.7 (1469.8–1617.0) |
| IgG(N)-positive between T1 and T5 (n = 64) | 3965.8 (3071.0–5121.4) |
| Infected (with PCR test) | |
| IgG(N)-negative (n = 25) | 2700.6 (1800.6–4050.5) |
| IgG(N)-positive between T1 and T5 (n = 177) | 5400.8 (4963.4–5876.8) |
| T-spot(S) at T5 | |
| Not infected (with PCR test) | |
| IgG(N)-negative (n = 1525) | 12.6 (11.9–13.3) |
| IgG(N)-positive between T1 and T5 (n = 52) | 15.2 (11.2–20.6) |
| Infected (with PCR test) | |
| IgG(N)-negative (n = 17) | 17.9 (11.8–27.1) |
| IgG(N)-positive between T1 and T5 (n = 161) | 21.2 (18.6–24.3) |
| Geometric Mean (95% CI) | |
|---|---|
| IgG(S) at 5T | |
| Not infected (with PCR test) | |
| T-spot(N)-negative (n = 789) | 1556.7 (1435.1–1688.6) |
| T-spot(N)-positive between T1 and T5 (n = 72) | 2268.2 (1770.3–2906.1) |
| Infected (with PCR test) | |
| T-spot(N)-negative (n = 48) | 4872.7 (3824.6–6208.1) |
| T-spot(N)-positive between T1 and T5 (n = 70) | 5277.0 (4668.8–5964.3) |
| T-spot(S) at 5T | |
| Not infected (with PCR test) | |
| T-spot(N)-negative (n = 755) | 11.4 (10.5–12.3) |
| T-spot(N)-positive between T1 and T5 (n = 71) | 15.0 (11.6–19.1) |
| Infected (with PCR test) | |
| T-spot(N)-negative (n = 48) | 15.1 (11.8–19.4) |
| T-spot(N)-positive between T1 and T5 (n = 70) | 26.4 (21.7–32.0) |
| IgG(N)-Negative (n = 25) | IgG(N)-Positive (n = 177) | p-Value | |
|---|---|---|---|
| Fever | 19 (79.2) | 131 (74.0) | 0.59 |
| Dysgeusia | 2 (8.3) | 33 (18.6) | 0.21 |
| Cough | 13 (54.2) | 121 (68.4) | 0.166 |
| Sore throat | 10 (41.7) | 112 (63.3) | 0.042 |
| Headache | 9 (37.5) | 74 (41.8) | 0.69 |
| Joint and muscle pain | 6 (25.0) | 51 (28.8) | 0.70 |
| Diarrhea | 2 (8.3) | 27 (15.3) | 0.37 |
| Rash | 0 (0.0) | 4 (2.3) | 0.46 |
| Fatigue | 10 (41.7) | 107 (60.5) | 0.080 |
| Eye symptoms | 0 (0.0) | 2 (1.1) | 0.60 |
| Difficulty breathing | 2 (8.3) | 33 (18.6) | 0.21 |
| Chest pain | 1 (4.2) | 8 (4.5) | 0.94 |
| Difficulty talking | 1 (4.2) | 4 (2.3) | 0.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchiyama, T.; Kobashi, Y.; Kawamura, T.; Nishikawa, Y.; Nakayama, A.; Oguro, F.; Kaneko, Y.; Yamamoto, C.; Ito, N.; Zhao, T.; et al. Discrepancy in SARS-CoV-2 Infection Status Among PCR, Serological, and Cellular Immunity Assays of Nucleocapsids: A Historical Cohort Study. Vaccines 2025, 13, 259. https://doi.org/10.3390/vaccines13030259
Uchiyama T, Kobashi Y, Kawamura T, Nishikawa Y, Nakayama A, Oguro F, Kaneko Y, Yamamoto C, Ito N, Zhao T, et al. Discrepancy in SARS-CoV-2 Infection Status Among PCR, Serological, and Cellular Immunity Assays of Nucleocapsids: A Historical Cohort Study. Vaccines. 2025; 13(3):259. https://doi.org/10.3390/vaccines13030259
Chicago/Turabian StyleUchiyama, Taiga, Yurie Kobashi, Takeshi Kawamura, Yoshitaka Nishikawa, Aya Nakayama, Fumiya Oguro, Yudai Kaneko, Chika Yamamoto, Naomi Ito, Tianchen Zhao, and et al. 2025. "Discrepancy in SARS-CoV-2 Infection Status Among PCR, Serological, and Cellular Immunity Assays of Nucleocapsids: A Historical Cohort Study" Vaccines 13, no. 3: 259. https://doi.org/10.3390/vaccines13030259
APA StyleUchiyama, T., Kobashi, Y., Kawamura, T., Nishikawa, Y., Nakayama, A., Oguro, F., Kaneko, Y., Yamamoto, C., Ito, N., Zhao, T., Saito, H., Abe, T., Kodama, T., & Tsubokura, M. (2025). Discrepancy in SARS-CoV-2 Infection Status Among PCR, Serological, and Cellular Immunity Assays of Nucleocapsids: A Historical Cohort Study. Vaccines, 13(3), 259. https://doi.org/10.3390/vaccines13030259






