Collective Immunity to the Measles, Mumps, and Rubella Viruses in the Kyrgyz Population
Abstract
1. Introduction
2. Materials and Methods
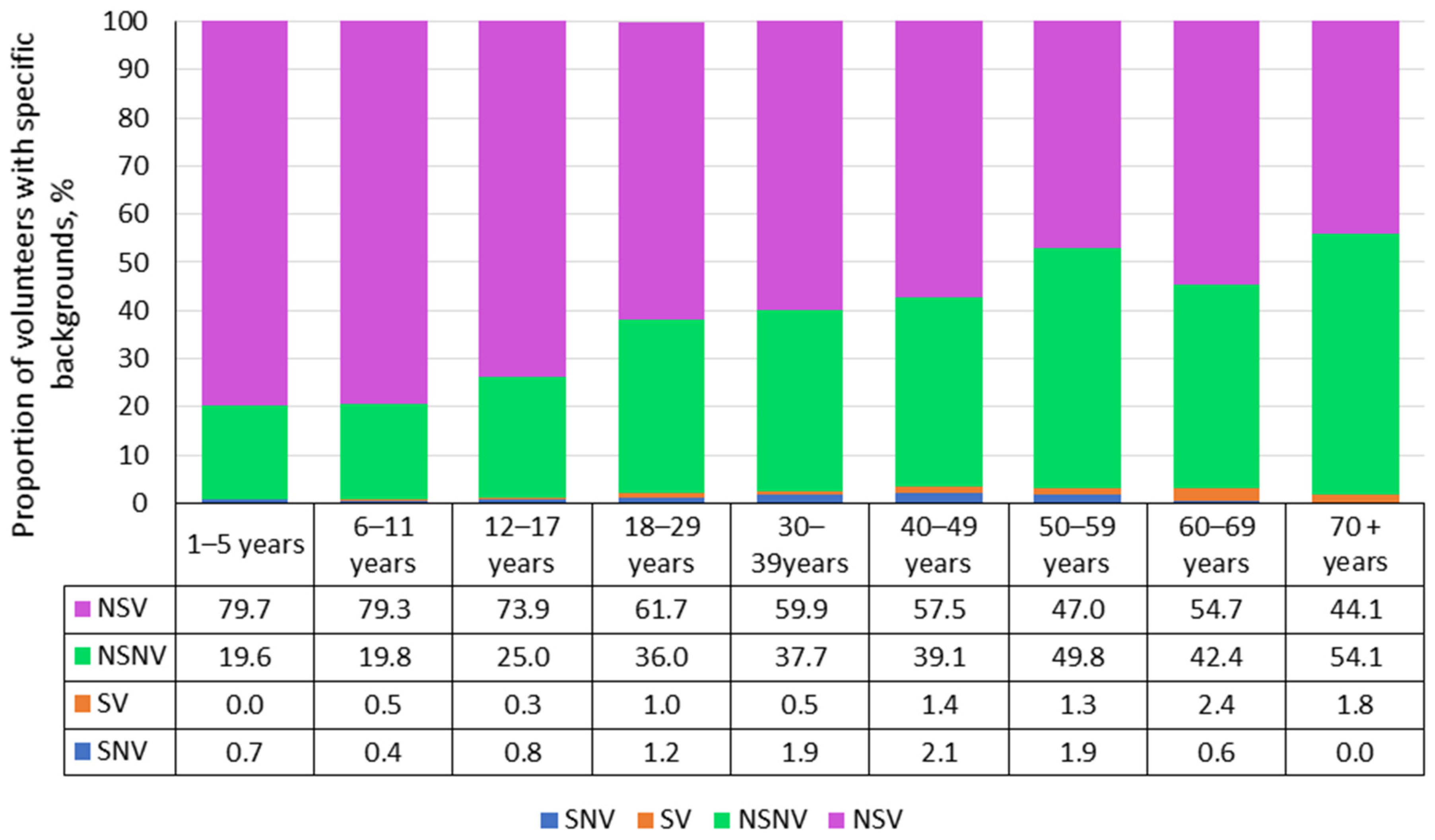
2.1. Characteristics of the Surveyed Volunteer Cohort
2.2. Research Methods
3. Results
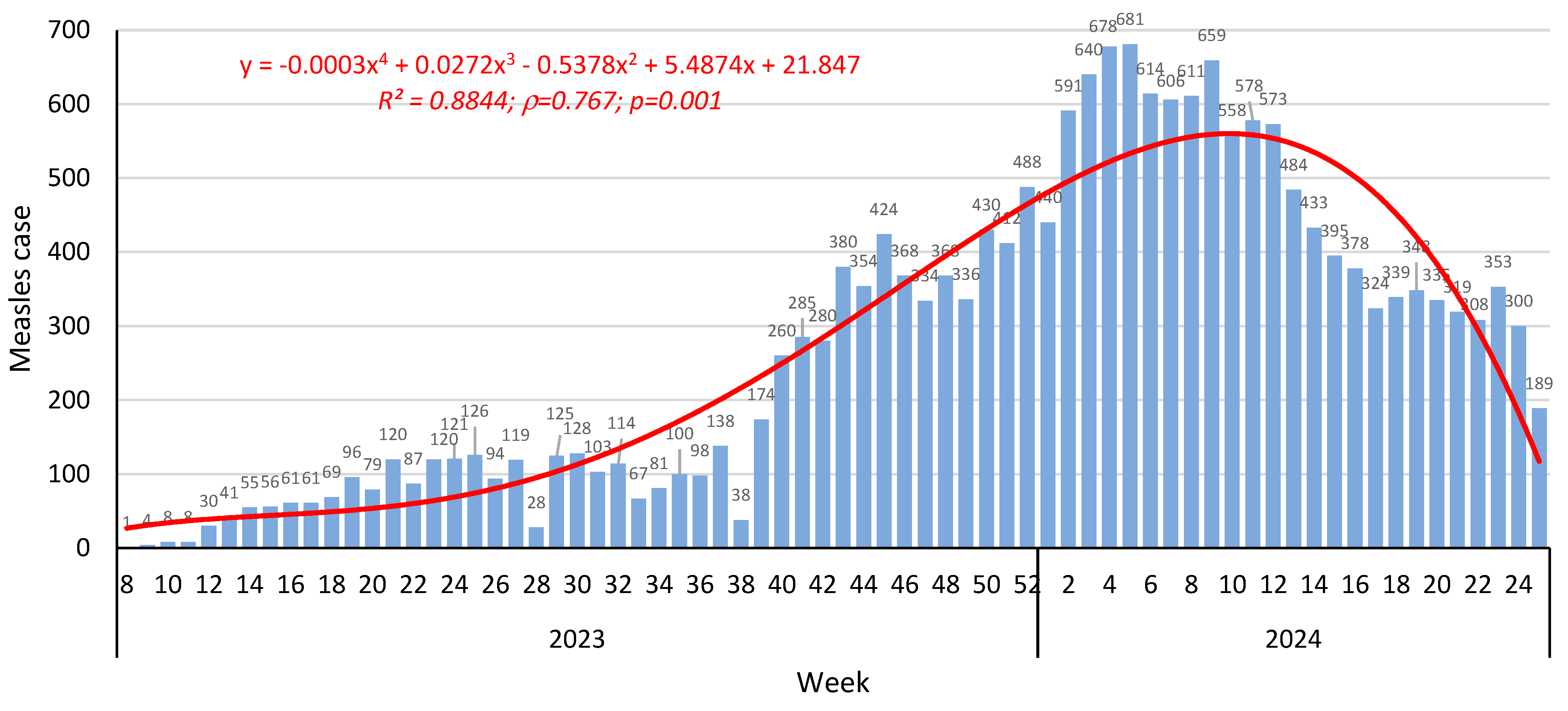
3.1. Measles
3.1.1. Epidemiological Situation
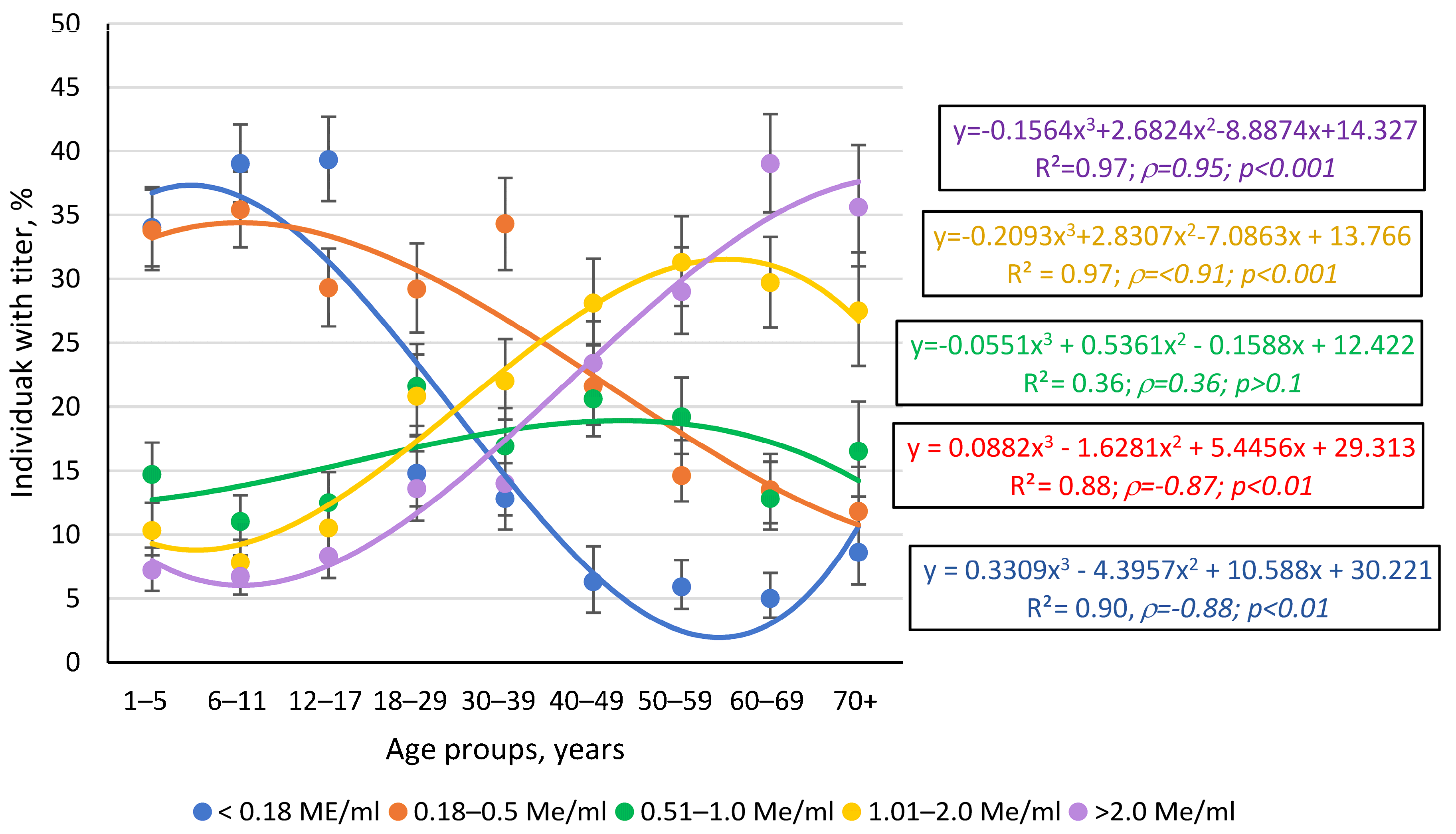
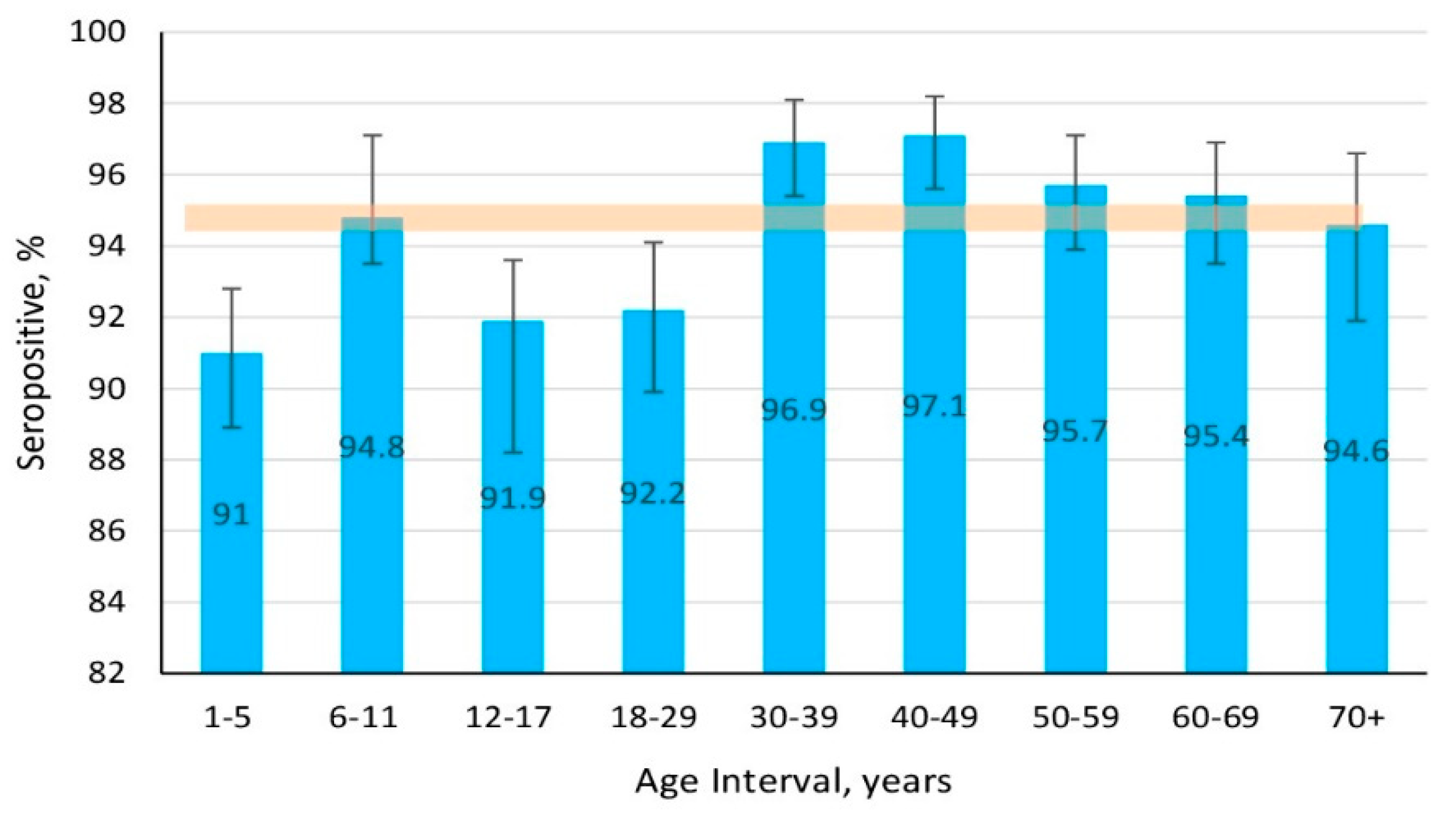
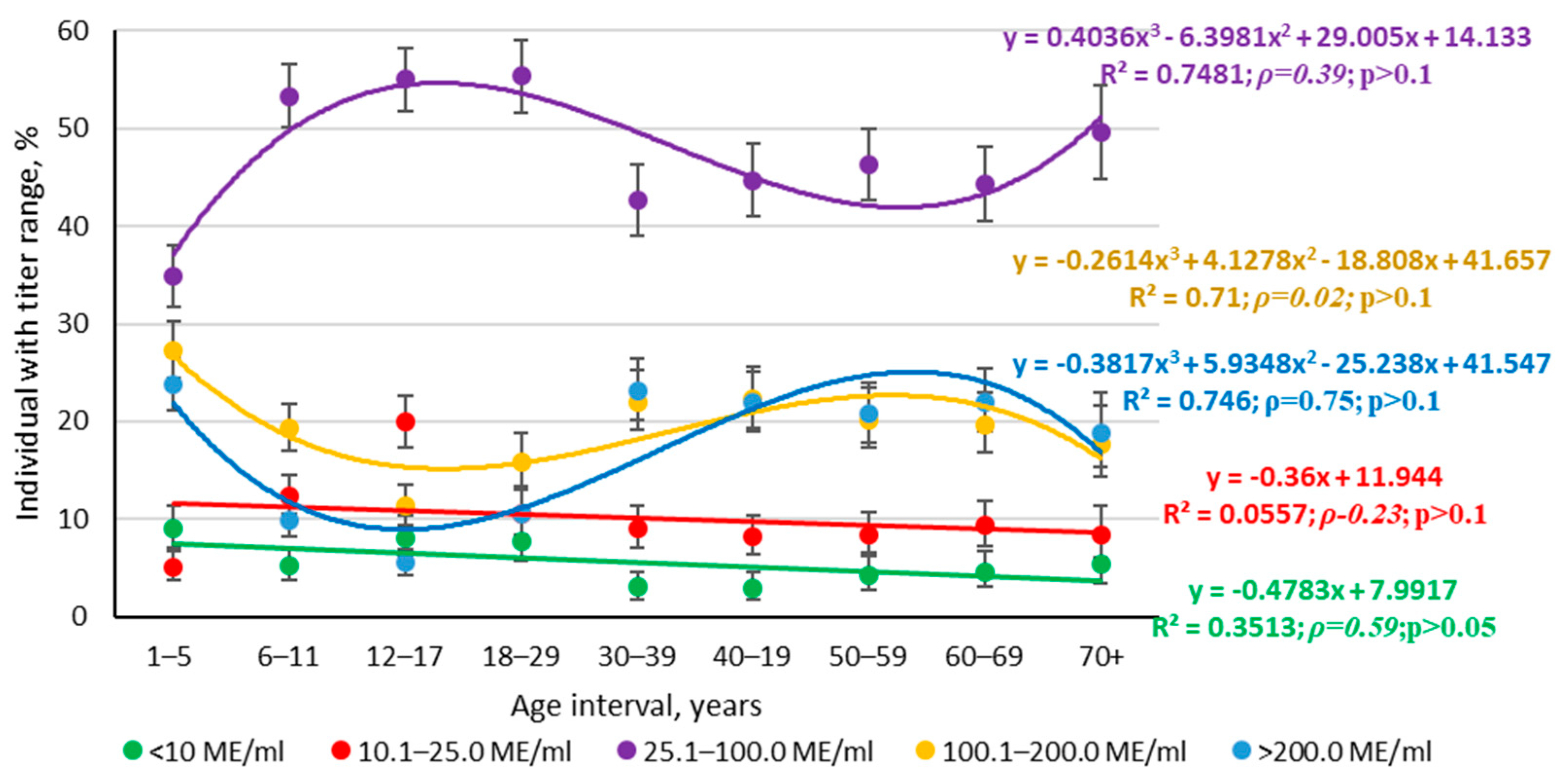
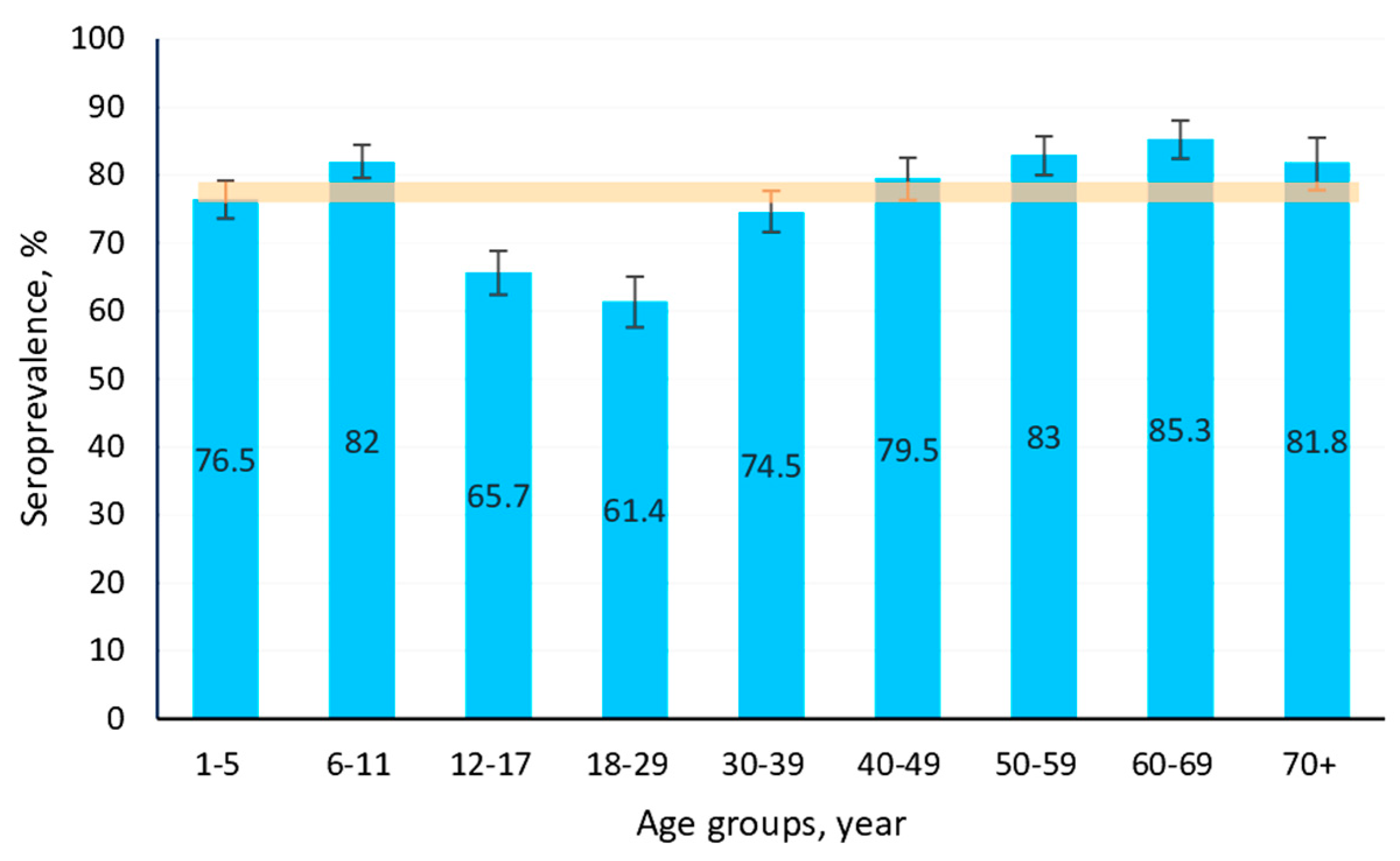
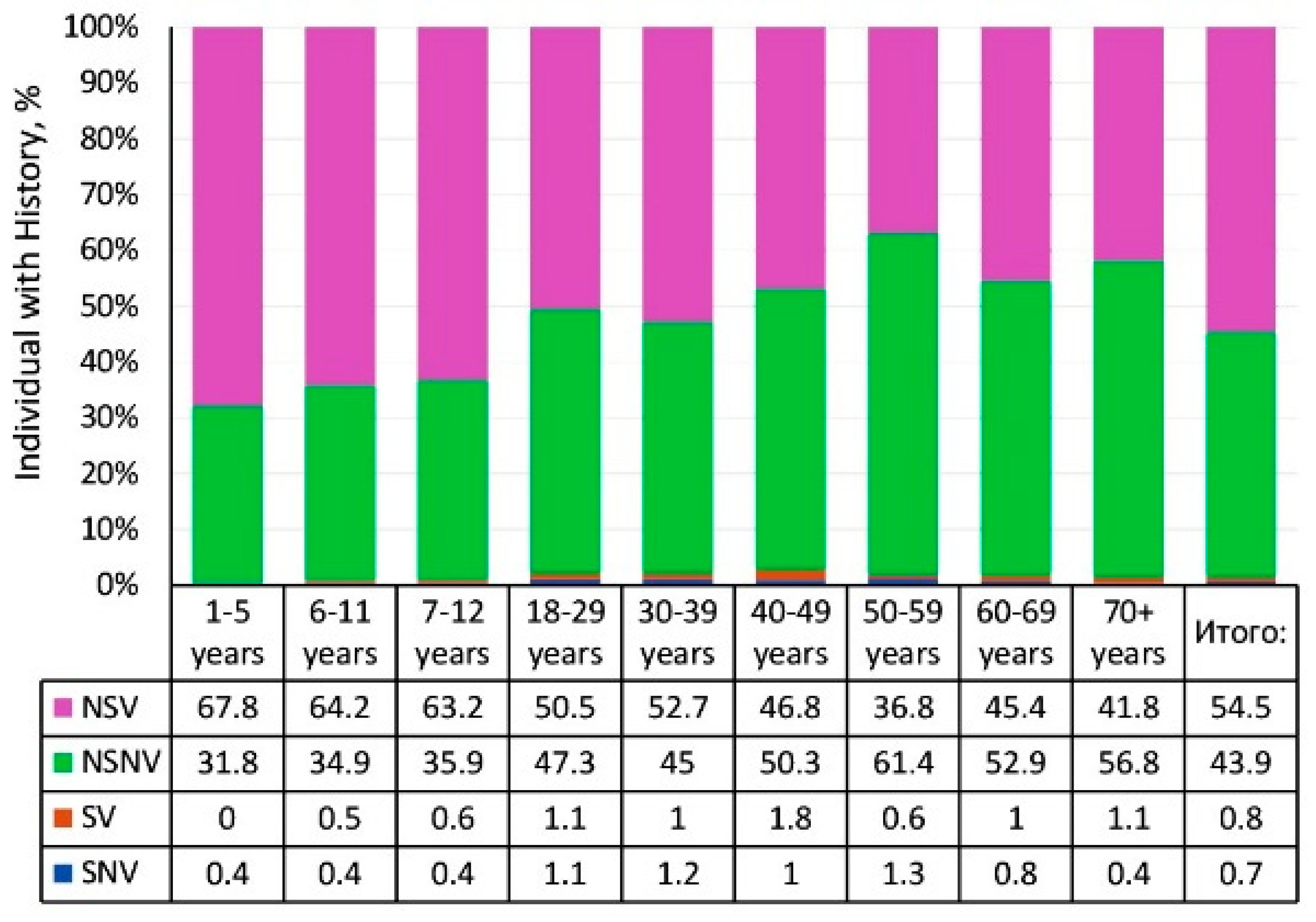
3.1.2. Structure of Measles Collective Immunity
3.2. Rubella
3.2.1. Epidemiological Situation
3.2.2. Structure of Rubella Collective Immunity
3.3. Mumps
3.3.1. Epidemiological Situation
3.3.2. Structure of Mumps Collective Immunity
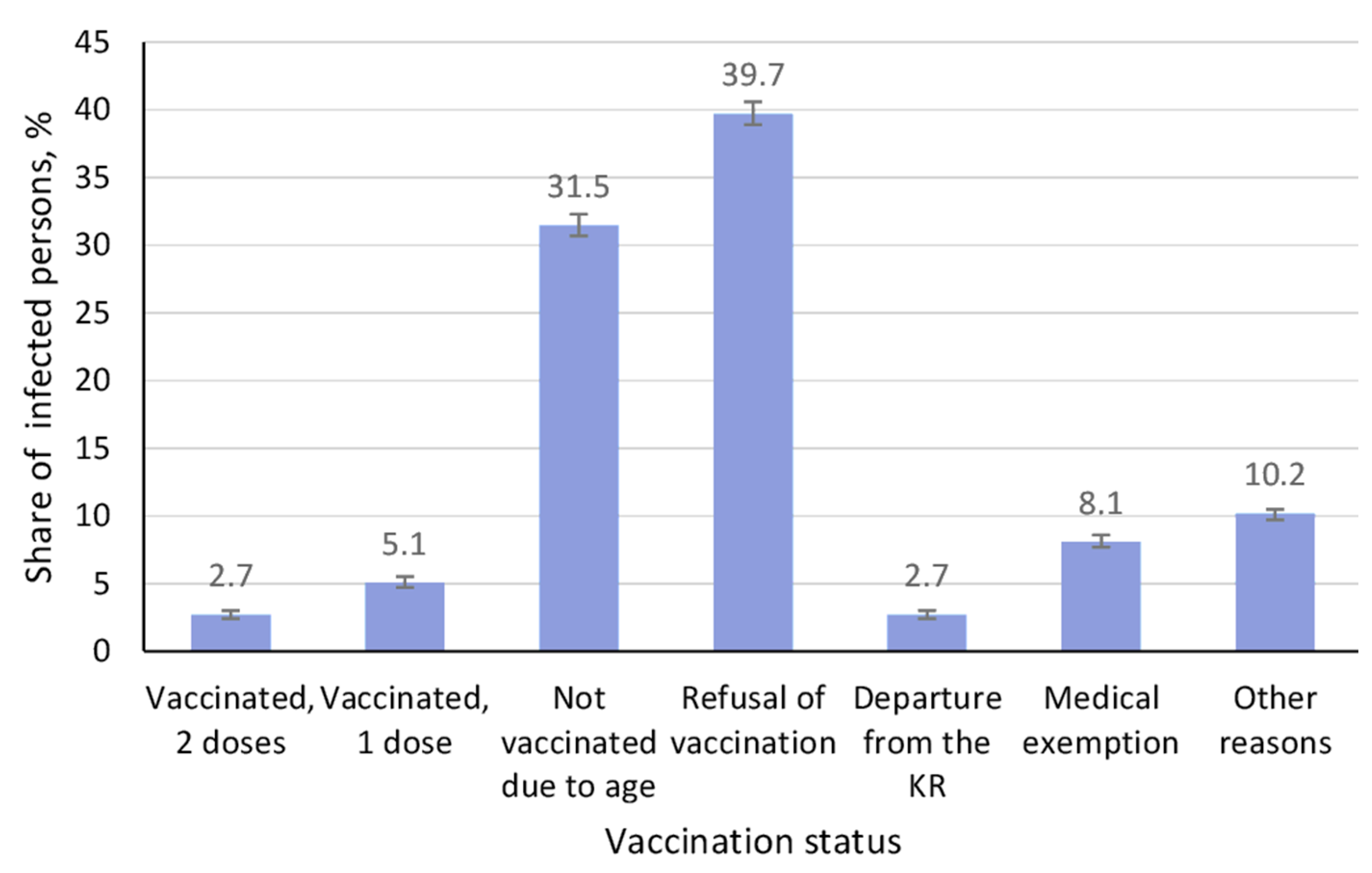
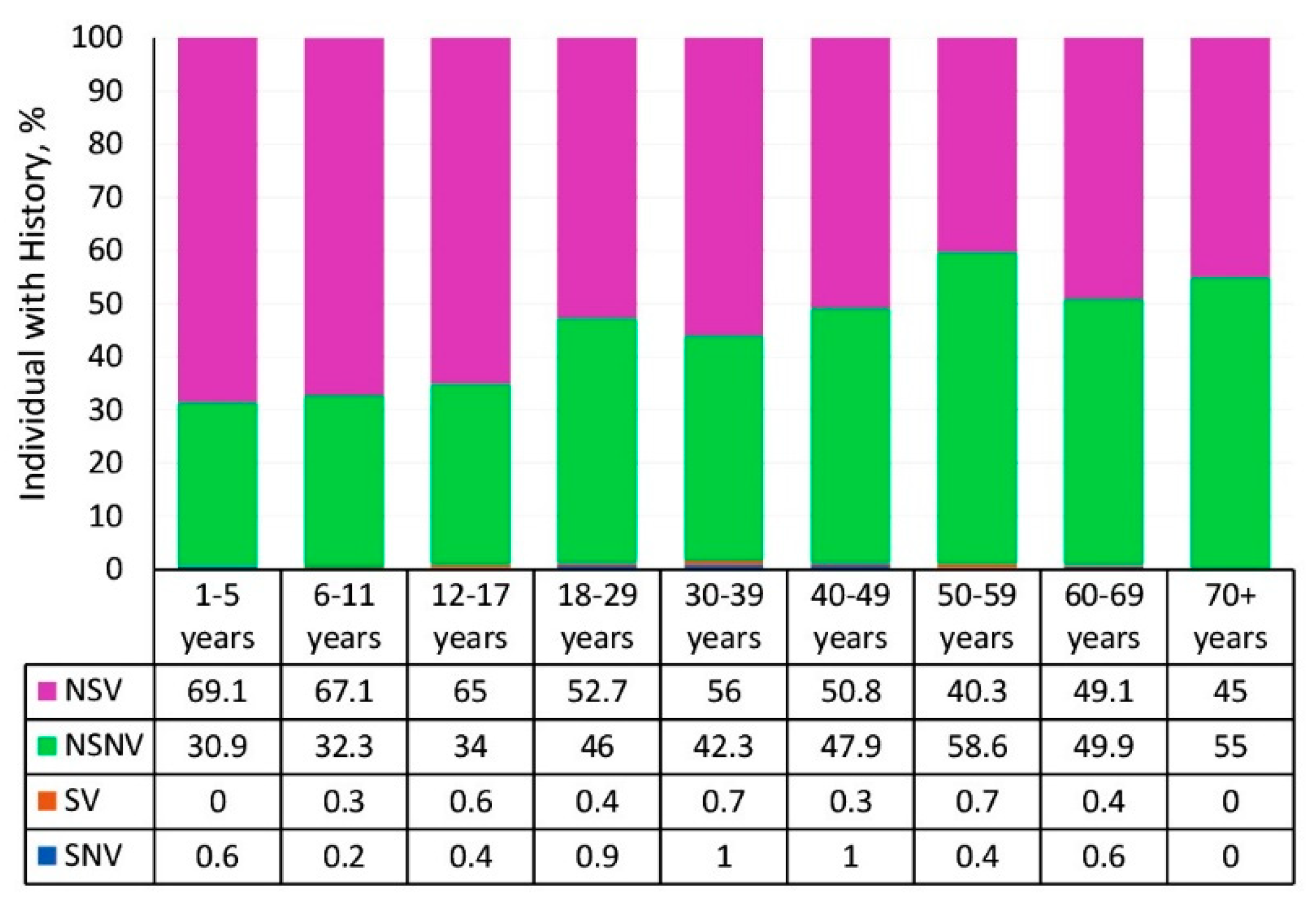
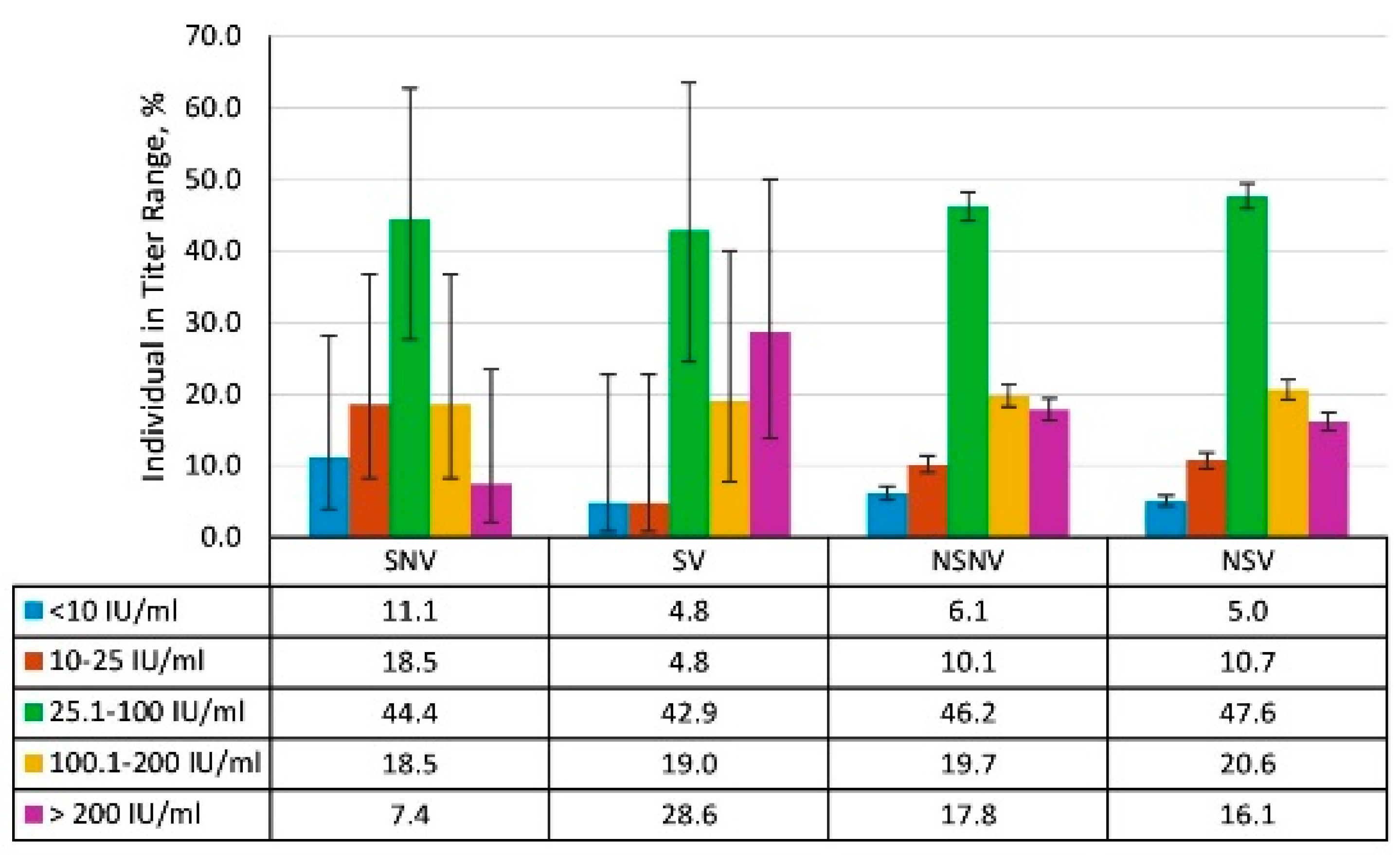
3.4. Vaccination Status
4. Discussion
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Popova, A.Y.; Smirnov, V.S.; Andreeva, E.E.; Babura, E.A.; Balakhonov, S.V.; Bashketova, N.S.; Bugorkova, S.A.; Bulanov, M.V.; Valeullina, N.N.; Vetrov, V.V.; et al. SARS-CoV-2 Seroprevalence Structure of the Russian Population during the COVID-19 Pandemic. Viruses 2021, 13, 1648. [Google Scholar] [CrossRef]
- Popova, A.Y.; Kasymov, O.T.; Smolenski, V.Y.; Smirnov, V.S.; Egorova, S.A.; Nurmatov, Z.S.; Milichkina, A.M.; Gulmira, S.; Suranbaeva, G.S.; Kuchuk, T.E.; et al. SARS-CoV-2 herd immunity of the Kyrgyz population in 2021. Med. Microbiol. Immunol. 2022, 211, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.Y.; Egorova, S.A.; Smirnov, V.S.; Ezhlova, E.B.; Milichkina, A.M.; Melnikova, A.A.; Bashketova, N.S.; Istorik, O.A.; Buts, L.V.; Ramsay, E.S.; et al. Immunity to measles, rubella and mumps herd immunity to vaccine preventable infections in SaintPetersburg and the Leningrad region: Serological status of measles, mumps, and rubella. Russ. J. Infect. Immun. 2024, 14, 1187–1208. [Google Scholar] [CrossRef]
- Popova, A.Y.; Totolian, A.A. Methodology for assessing herd immunity to the SARS-CoV-2 virus in the context of the COVID-19 pandemic. Russ. J. Infect. Immun. 2021, 11, 609–616. (In Russian) [Google Scholar] [CrossRef]
- Gadroen, K.; Dodd, C.N.; Muscle, G.M.C.; de Ridder, M.A.J.; Weibel, D.; Mina, M.J.; Grenfell, B.T.; Sturkenboom, M.C.J.M.; van de Vijver, D.A.M.C.; de Swart, R.L. Impact and longevity of measles-associated immune suppression: A matched cohort study using data from the THIN general practice database in the UK. BMJ Open 2018, 8, e021465. [Google Scholar] [CrossRef]
- George, K.; Govindaraj, G. Infections in Inborn Errors of Immunity with Combined Immune Deficiency: A Review. Pathogens 2023, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Zarka, S.; Ankol, O.E.; Rozhavski, V.; Davidovitch, N.; Aboudy, Y.; Balicer, R.D. Seroprevalence of measles, mumps and rubella among young adults, after 20 years of universal 2-dose MMR vaccination in Israel. Hum. Vaccines Immunother. 2015, 11, 1400–1405. [Google Scholar] [CrossRef]
- Fadda, M.; Depping, M.K.; Schulz, P.J. Addressing issues of vaccination literacy and psychological empowerment in the measles-mumps-rubella (MMR) vaccination decision-making: A qualitative study. BMC Public Health 2015, 15, 836. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data. Available online: http://ourworldindata.org (accessed on 3 May 2023).
- Mumps. Annual Epidemiological Report for 2022. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/mumps-annual-epidemiological-report-for-2022.pdf (accessed on 22 August 2024).
- Friedrich, N.; Poethko-Müller, C.; Kuhnert, R.; Matysiak-Klose, D.; Koch, J.; Wichmann, O.; Santibanez, S.; Mankertz, A. Seroprevalence of Measles-, Mumps-, and Rubella-specific antibodies in the German adult population—Cross-sectional analysis of the German Health Interview and Examination Survey for Adults (DEGS1). Lancet Reg. Health Eur. 2021, 7, 100128. [Google Scholar] [CrossRef] [PubMed]
- Tabacchi, G.; Costantino, C.; Napoli, G.; Marchese, V.; Cracchiolo, M.; Casuccio, A.; Vitale, F.; Esculapio Working Group. Determinants of European parents’ decision on the vaccination of their children against measles, mumps and rubella: A systematic review and meta-analysis. Hum. Vaccines Immunother. 2016, 12, 1909–1923. [Google Scholar] [CrossRef]
- Baumgaertner, B.; Carlisle, J.E.; Justwan, F. The influence of political ideology and trust on willingness to vaccinate. PLoS ONE 2018, 13, e0191728. [Google Scholar] [CrossRef] [PubMed]
- 14 Wagner, C.E.; Prentice, J.A.; Saad-Roy, C.M.; Yang, L.; Grenfell, B.T.; Levin, S.A.; Laxminarayan, R. Economic and Behavioral Influencers of Vaccination and Antimicrobial Use. Front. Public Health 2020, 8, 614113. [Google Scholar] [CrossRef] [PubMed]
- 16 Wang, Z.; Yan, R.; He, H.; Li, Q.; Chen, G.; Yang, S.; Chen, E. Difficulties in Eliminating Measles and Controlling Rubella and Mumps: A Cross-Sectional Study of a First Measles and Rubella Vaccination and a Second Measles, Mumps, and Rubella Vaccination. PLoS ONE 2014, 9, e89361. [Google Scholar] [CrossRef]
- Tischer, A.; Andrews, N.; Kafatos, G.; Nardone, A.; Berbers, G.; Davidkin, I.; Aboudy, Y.; Backhouse, J.; Barbara, C.; Bartha, K.; et al. Standardization of measles, mumps and rubella assays to enable comparisons of seroprevalence data across 21 European countries and Australia. Epidemiol. Infect. 2007, 135, 787–798. [Google Scholar] [CrossRef]
- Di Pietrantonj, C.; Rivetti, A.; Marchione, P.; Debalini, M.G.; Demarchelier, V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst. Rev. 2020, 2020, CD004407. [Google Scholar] [CrossRef]
- Kuter, B.J.; Marshall, G.S.; Fergie, J.; Schmidt, E.; Pawaskar, M. Prevention of measles, mumps and rubella: 40 years of global experience with M-M-RII. Hum. Vaccines Immunother. 2021, 17, 5372–5383. [Google Scholar] [CrossRef]
- Medical Statistics. Available online: https://medstatistic.ru/ (accessed on 1 June 2023).
- Local Moivre-Laplace Theorem. Available online: https://www.matematicus.ru/teoriya-veroyatnosti/lokalnaya-teorema-laplasa (accessed on 10 August 2024).
- Wald, A.; Wolfowitz, J. Confidence limits for continuous distribution functions. Ann. Math. Stat. 1939, 10, 105–118. [Google Scholar] [CrossRef]
- Agresti, A.; Coull, B.A. Approximate is better than “exact” for interval estimation of binomial proportions. Am. Stat. 1998, 52, 119–126. [Google Scholar] [CrossRef]
- White, S.J.; Boldt, K.L.; Holditch, S.J.; Poland, G.A.; Jacobson, R.M. Measles, Mumps, and Rubella. Clin. Obstet. Gynecol. 2012, 55, 550–559. [Google Scholar] [CrossRef]
- Béraud, G.; Abrams, S.; Beutels, P.; Dervaux, B.; Hens, N. Resurgence risk for measles, mumps and rubella in France in 2018 and 2020. Eurosurveillance 2018, 23, 1700796. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.M.; Bolotin, S.; Lim, G.; Heffernan, J.; Deeks, S.L.; Li, Y.; Crowcroft, N.S. The basic reproduction number (R0) of measles: A systematic review. Lancet Infect. Dis. 2017, 17, e420–e428. [Google Scholar] [CrossRef] [PubMed]
- Glasser, J.W.; Feng, Z.; Omer, S.B.; Smith, P.J.; Rodewald, L.E. An assessment of the impact of heterogeneity in vaccine uptake due to religious and philosophical exemptions on the potential for outbreaks. Lancet Infect. Dis. 2016, 16, 599–605. [Google Scholar] [CrossRef]
- Randolph, H.E.; Barreiro, L.B. Herd Immunity: Understanding COVID-19. Immunity 2020, 52, 737–741. [Google Scholar] [CrossRef]
- Mossong, J.; Muller, C.P. Estimation of the basic reproduction number of measles during an outbreak in a partially vaccinated population. Epidemiol. Infect. 2000, 124, 273–278. [Google Scholar] [CrossRef]
- Goodson, J.L.; Chu, S.Y.; Rota, P.A.; Moss, W.J.; Featherstone, D.A.; Vijayaraghavan, M.; Thompson, K.M.; Martin, R.; Reef, S.; Strebel, P.M. Research priorities for global measles and rubella control and eradication. Vaccine 2012, 30, 4709–4716. [Google Scholar] [CrossRef] [PubMed]
- Hickman, C.; Hyde, T.; Sowers, S.; Mercader, S.; McGrew, M.; Williams, N.; Beeler, J.A.; Audet, S.; Kiehl, B.; Nandy, R.; et al. Laboratory characterization of measles virus infection in previously vaccinated and unvaccinated individuals. J. Infect. Dis. 2011, 204 (Suppl. 1), S549–S558. [Google Scholar] [CrossRef]
- Mutsaerts, E.A.M.L.; Nunes, M.C.; van Rijswijk, M.N.; Klipstein-Grobusch, K.; Grobbee, D.E.; Madhi, S.A. Safety and Immunogenicity of Measles Vaccination in HIV-Infected and HIV-Exposed Uninfected Children: A Systematic Review and Meta-Analysis. EClinicalMedicine 2018, 1, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Coughlin, M.M.; Beck, A.S.; Bankamp, B.; Rota, P.A. Perspective on Global Measles Epidemiology and Control and the Role of Novel Vaccination Strategies. Viruses 2017, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Summary of WHO Position Paper on Measles. Available online: https://cdn.who.int/media/docs/default-source/immunization/position_paper_documents/measles/who-pp-measles-vaccine-summary-2017.pdf?sfvrsn=e546119a_2 (accessed on 12 May 2023).
- Meacham, W. Postinfection immunity to measles was known to common folk well before its discovery by science. Am. J. Med. Sci. 2014, 347, 502–503. [Google Scholar] [CrossRef]
- Mohamed El Shehab, A.J. Provision of Taking Vaccines and the Role of the Government on making it Mandatory: COVID-19 Vaccine as a Model. (Arabian). Online J. Res. Islam. Stud. 2022, 9, 103–126. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Ahmed, M.; Hussain, S. The Anti-vaccination Movement: A Regression in Modern Medicine. Cureus 2018, 10, e2919. [Google Scholar] [CrossRef] [PubMed]
- Mawson, A.R.; Croft, A.M. Rubella Virus Infection, the Congenital Rubella Syndrome, and the Link to Autism. Int. J. Environ. Res. Public Health 2019, 16, 3543. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.; Strebel, P.; Orenstein, W.; Icenogle, J.; Poland, G.A. Rubella. Lancet 2015, 385, 2297–2307. [Google Scholar] [CrossRef]
- Papadopoulos, T.; Vynnycky, E. Estimates of the basic reproduction number for rubella using seroprevalence data and indicator-based approaches. PLoS Comput. Biol. 2022, 18, e1008858. [Google Scholar] [CrossRef] [PubMed]
- Rozhnova, G.; Metcalf, C.J.E.; Grenfell, B.T. Characterizing the dynamics of rubella relative to measles: The role of stochasticity. J. R. Soc. Interface 2013, 10, 20130643. [Google Scholar] [CrossRef] [PubMed]
- Plans-Rubió, P. Evaluation of the establishment of herd immunity in the population by means of serological surveys and vaccination coverage. Hum. Vaccines Immunother. 2012, 8, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Motaze, N.V.; Edoka, I.; Wiysonge, C.S.; Metcalf, C.J.E.; Winter, A.K. Rubella Vaccine Introduction in the South African Public Vaccination Schedule: Mathematical Modelling for Decision Making. Vaccines 2020, 8, 383. [Google Scholar] [CrossRef]
- Plans, P. New preventive strategy to eliminate measles, mumps and rubella from Europe based on the serological assessment of herd immunity levels in the population. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 961–966. [Google Scholar] [CrossRef]
- Shah, N.; Ghosh, A.; Kumar, K.; Dutta, T.; Mahajan, M. A review of safety and immunogenicity of a novel measles, mumps, rubella (MMR) vaccine. Hum. Vaccines Immunother. 2024, 20, 2302685. [Google Scholar] [CrossRef]
- Wu, H.; Wang, F.; Tang, D.; Han, D. Mumps Orchitis: Clinical Aspects and Mechanisms. Front. Immunol. 2021, 12, 582946. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.W.; Tae, B.S.; Chang, H.K.; Song, M.S.; Cheon, J.; Park, J.Y.; Bae, J.H. Epidemiology of mumps, mumps complications, and mumps orchitis in Korea using the National Health Insurance Service database. Investig. Clin. Urol. 2023, 64, 412–417. [Google Scholar] [CrossRef]
- Trabert, B.; Graubard, B.I.; Erickson, R.L.; McGlynn, K.A. Childhood infections, orchitis and testicular germ cell tumours: A report from the STEED study and a meta-analysis of existing data. Br. J. Cancer 2012, 106, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-B.; Chang, H.-L.; Chen, K.-T. Current Status of Mumps Virus Infection: Epidemiology, Pathogenesis, and Vaccine. Int. J. Environ. Res. Public Health 2020, 17, 1686. [Google Scholar] [CrossRef] [PubMed]
- Rubin, S.; Kennedy, R.; Poland, G. Emerging mumps infection. Pediatr. Infect. Dis. J. 2016, 35, 799–801. [Google Scholar] [CrossRef]
- Edmunds, W.J.; Gay, N.J.; Kretzschmar, M.; Pebody, R.G.; Wachmann, H. The pre-vaccination epidemiology of measles, mumps and rubella in Europe: Implications for modelling studies. Epidemiol. Infect. 2000, 125, 635–650. [Google Scholar] [CrossRef]
- Beleni, A.-I.; Borgmann, S. Mumps in the Vaccination Age: Global Epidemiology and the Situation in Germany. Int. J. Environ. Res. Public Health 2018, 15, 1618. [Google Scholar] [CrossRef]
- Almansour, I. Mumps Vaccines: Current Challenges and Future Prospects. Front. Microbiol. 2020, 11, 1999. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Best, J.; MacMahon, E. Mumps and the UK epidemic 2005. BMJ, 2005; 330, 1132. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, T.; Zhu, Y.; Hu, Q.; Wang, Y.; Zhao, Z.; Rui, J.; Lin, S.; Liu, X.; Xu, J.; et al. Estimating the transmissibility of mumps: A modelling study in Wuhan City, China. Front. Med. 2021, 8, 683720. [Google Scholar] [CrossRef]
- Yang, T.; Wang, Y.; Zhao, Q.; Guo, X.; Yu, S.; Zhao, Z.; Deng, B.; Huang, J.; Liu, W.; Su, Y.; et al. Age-specific transmission dynamic of mumps: A long-term large-scale modeling study in Jilin Province, China. Front. Public Health 2022, 10, 968702. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.; Beutels, P.; Hens, N. Assessing Mumps Outbreak Risk in Highly Vaccinated Populations Using Spatial Seroprevalence Data. Am. J. Epidemiol. 2014, 179, 1006–1017. [Google Scholar] [CrossRef]
- Winter, A.K.; Moss, W.J. Rubella. Lancet 2022, 399, 1336–1346. [Google Scholar] [CrossRef]



| Age Interval, Years | Individuals | Share of Cohort | Gender | ||
|---|---|---|---|---|---|
| % | 95% CI | Males n (%) | Females n (%) | ||
| 1–5 | 909 | 13.7 | 12.9–14.6 | 416 (45.8) | 493 (54.2) |
| 6–11 | 1025 | 15.5 | 14.6–16.4 | 460 (44.9) | 565 (55.1) |
| 12–17 | 877 | 13.3 | 12.5–14.1 | 354 (40.4) | 523 (59.6) |
| 18–29 | 668 | 10.1 | 9.4–10.9 | 136 (20.4) | 532 (79.6) |
| 30–39 | 686 | 10.4 | 9.7–11.1 | 121 (17.6) | 565 (82.4) |
| 40–49 | 698 | 10.5 | 9.8–11.3 | 107 (15.3) | 591 (84.7) |
| 50–59 | 692 | 10.5 | 9.8–11.2 | 103 (14.9) | 590 (85.3) |
| 60–69 | 654 | 9.9 | 9.2–10.6 | 107 (16.4) | 547 (83.6) |
| 70+ | 407 | 6.2 | 5.5–6.7 | 104 (25.6) | 303 (74.4) |
| Total | 6617 | 100.0 | - | 1908 (28.8) | 4709 (71.2) |
| City/Region | Population, Thousand | Volunteers | Share, % | 95% CI | Gender | |
|---|---|---|---|---|---|---|
| Males n (%) | Females n (%) | |||||
| Bishkek city | 1165.5 | 1132 | 17.1 | 16.2–18.0 | 343 (30.3) | 789 (69.7) |
| Osh city | 366.7 | 268 | 4.1 | 3.6–4.6 | 51 (19.0) | 217 (81.0) |
| Osh region | 1490.1 | 1410 | 21.3 | 20.4–22.3 | 392 (27.8) | 1018 (72.2) |
| Batken region | 583.4 | 563 | 8.5 | 7.9–9.2 | 170 30.2) | 393 (69.8) |
| Jalal-Abad region | 1335.8 | 1218 | 18.4 | 17.5–19.4 | 386 31.7) | 832 (68.3) |
| Talas region | 277.1 | 268 | 4.1 | 3.6–4.6 | 82 (30.6) | 186 (69.4) |
| Issyk-Kul region | 544.4 | 538 | 8.1 | 7.5–8.8 | 171(31.8) | 367 (68.2) |
| Naryn region | 312.1 | 339 | 5.1 | 4.6–5.7 | 83 (24.5) | 256 (75.5) |
| Chüy region | 1086.8 | 881 | 13.3 | 12.5–14.2 | 230 (26.1) | 651 (73.9) |
| Total | 7169.9 | 6617 | 100 | - | 1908 (28.8) | 4709 (71.2) |
| Activity | Individuals | Share, % | 95% CI |
|---|---|---|---|
| Preschooler | 648 | 9.8 | 9.1–10.5 |
| Schoolchild | 1632 | 24.7 | 23.6–25.7 |
| Student | 164 | 2.5 | 2.1–2.9 |
| Medicine | 1276 | 19.3 | 18.4–20.3 |
| Science + the Arts | 47 | 0.7 | 0.5–0.9 |
| Business | 86 | 1.3 | 1.1–1.6 |
| Education | 198 | 3.0 | 2.6–3.4 |
| Production + Transport | 51 | 0.8 | 0.6–1.0 |
| State-military Service | 184 | 2.8 | 2.4–3.2 |
| Office Work | 81 | 1.2 | 1.0–1.5 |
| Info. Technology (IT) | 66 | 1.0 | 1.8–1.3 |
| Agriculture | 173 | 2.6 | 2.2–3.0 |
| Other | 668 | 10.1 | 9.3–10.8 |
| Unemployed | 731 | 11.1 | 10.3–11.8 |
| Pensioner | 612 | 9.2 | 8.6–10.0 |
| Total | 6617 | 100 | - |
| City/Region | Cases | Per 100 K Population | ||
|---|---|---|---|---|
| Overall | Among Children 1–14 Years | Overall | Among Children 1–14 Years | |
| Bishkek city | 5 | 5 | 0.4 | 2.2 |
| Osh city | 1 | 1 | 0.3 | 1.0 |
| Batken region | 0 | 0 | 0 | 0 |
| Jalal-Abad region | 2 | 2 | 0.2 | 0.4 |
| Issyk-Kul region | 0 | 0 | 0 | 0 |
| Naryn region | 0 | 0 | 0 | 0 |
| Osh region | 0 | 0 | 0 | 0 |
| Talas region | 0 | 0 | 0 | 0 |
| Chüy region | 3 | 3 | 0.3 | 0.9 |
| Total | 11 | 11 | 0.2 | 0.5 |
| City/Region | Cases | Per 100 K Population | ||
|---|---|---|---|---|
| Overall | Among Children 1–14 Years | Overall | Among Children 1–14 Years | |
| Bishkek city | 30 | 17 | 2.6 | 7.4 |
| Osh city | 7 | 2 | 1.9 | 1.9 |
| Batken region | 27 | 19 | 4.7 | 8.8 |
| Jalal-Abad region | 18 | 13 | 1.4 | 2.7 |
| Issyk-Kul region | 4 | 3 | 0.7 | 1.7 |
| Naryn region | 1 | 1 | 0.3 | 1 |
| Osh region | 8 | 4 | 0.5 | 0.7 |
| Talas region | 7 | 7 | 2.5 | 7.1 |
| Chüy region | 59 | 51 | 5.5 | 15.2 |
| Total | 162 | 117 | 2.3 | 5.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, A.Y.; Smirnov, V.S.; Egorova, S.A.; Nurmatov, Z.S.; Milichkina, A.M.; Drozd, I.V.; Dadanova, G.S.; Zhumagulova, G.D.; Danilova, E.M.; Kasymbekov, Z.O.; et al. Collective Immunity to the Measles, Mumps, and Rubella Viruses in the Kyrgyz Population. Vaccines 2025, 13, 249. https://doi.org/10.3390/vaccines13030249
Popova AY, Smirnov VS, Egorova SA, Nurmatov ZS, Milichkina AM, Drozd IV, Dadanova GS, Zhumagulova GD, Danilova EM, Kasymbekov ZO, et al. Collective Immunity to the Measles, Mumps, and Rubella Viruses in the Kyrgyz Population. Vaccines. 2025; 13(3):249. https://doi.org/10.3390/vaccines13030249
Chicago/Turabian StylePopova, Anna Yurievna, Viacheslav Sergeevich Smirnov, Svetlana Alexandrovna Egorova, Zuridin Sharipovich Nurmatov, Angelika Marsovna Milichkina, Irina Victorovna Drozd, Gulzada Saparbekovna Dadanova, Gulnara Dzhumadylovna Zhumagulova, Ekaterina Mikhailovna Danilova, Zharkynbek Orozbekovich Kasymbekov, and et al. 2025. "Collective Immunity to the Measles, Mumps, and Rubella Viruses in the Kyrgyz Population" Vaccines 13, no. 3: 249. https://doi.org/10.3390/vaccines13030249
APA StylePopova, A. Y., Smirnov, V. S., Egorova, S. A., Nurmatov, Z. S., Milichkina, A. M., Drozd, I. V., Dadanova, G. S., Zhumagulova, G. D., Danilova, E. M., Kasymbekov, Z. O., Drobyshevskaya, V. G., Sattarova, G. Z., Zhimbaeva, O. B., Ramsay, E. S., Nuridinova, Z. N., Ivanov, V. A., Urmanbetova, A. K., & Totolian, A. A. (2025). Collective Immunity to the Measles, Mumps, and Rubella Viruses in the Kyrgyz Population. Vaccines, 13(3), 249. https://doi.org/10.3390/vaccines13030249







