Nirsevimab Prophylaxis for Reduction of Respiratory Syncytial Virus Complications in Hospitalised Infants: The Multi-Centre Study During the 2023–2024 Season in Andalusia, Spain (NIRSEGRAND)
Abstract
1. Introduction
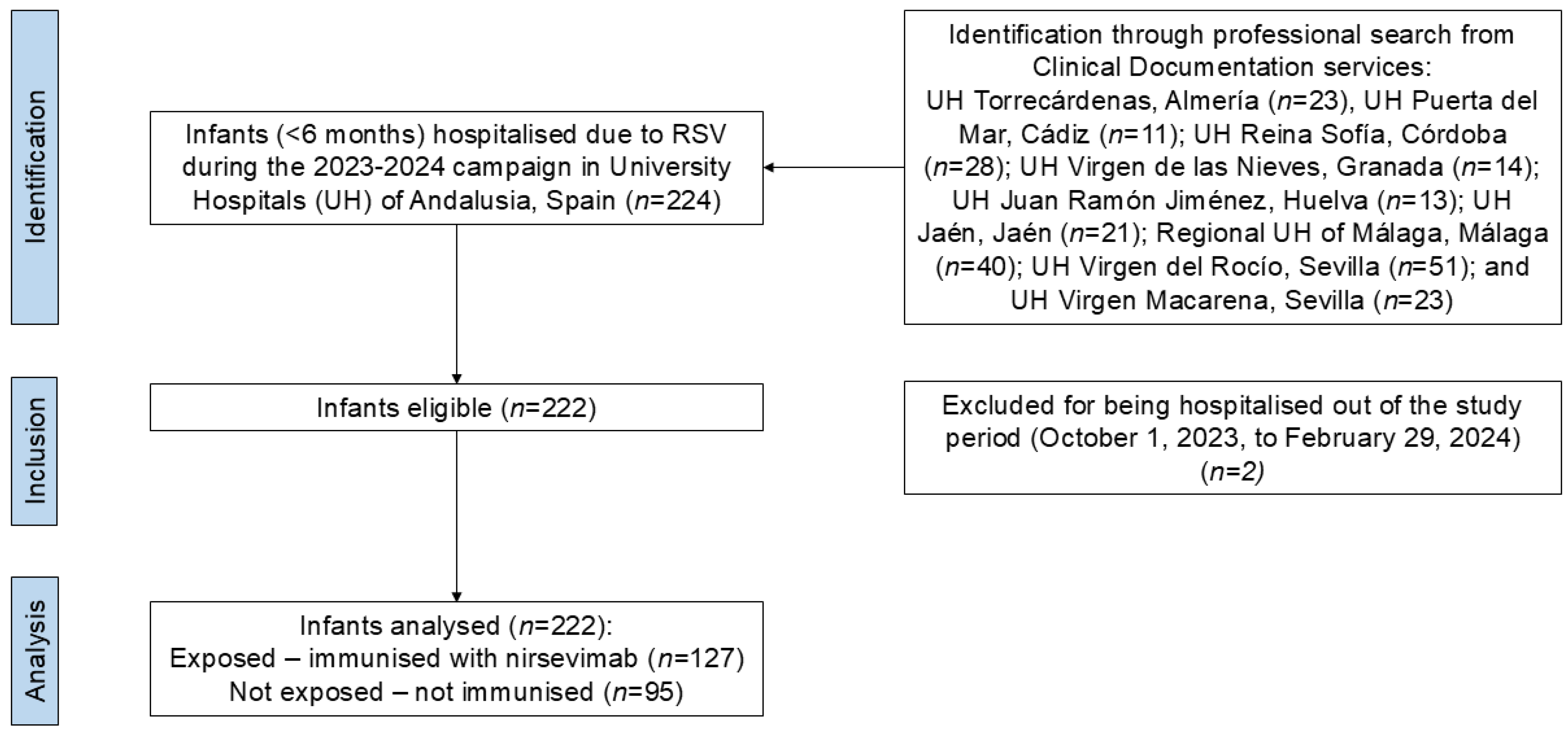
2. Materials and Methods
2.1. Study Setting and Design
2.2. Sources of Information and Variables
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Characteristics of the Sample
3.2. Bivariate Analysis
3.3. Multivariable Analysis of Dichotomous Outcomes
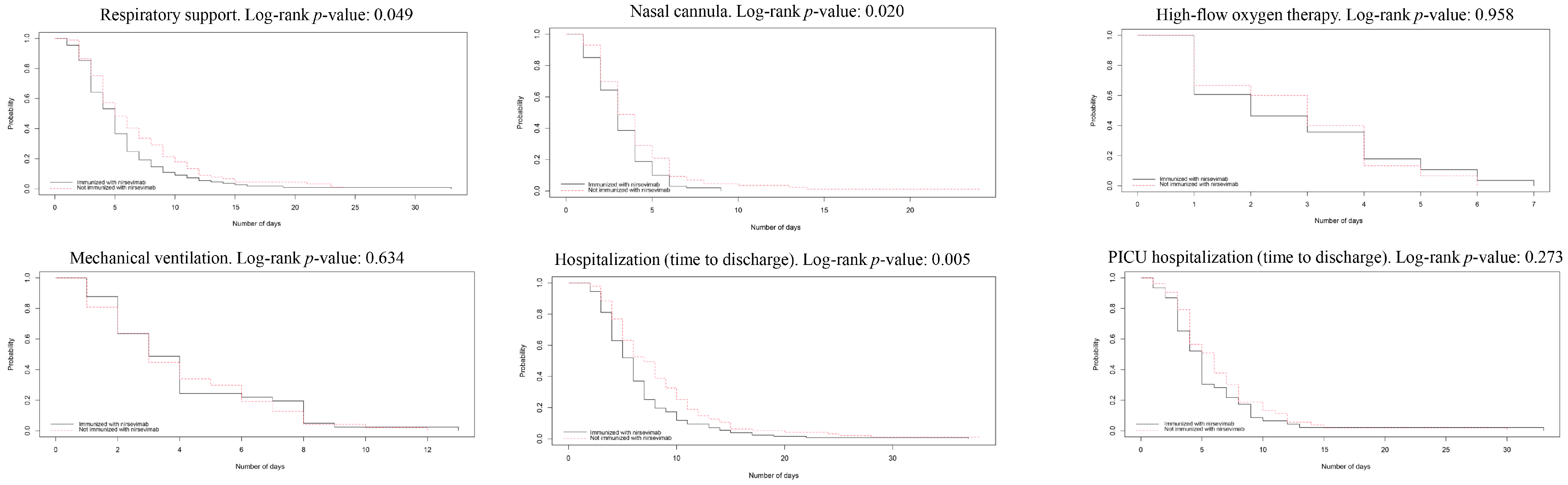
3.4. Survival Analysis for Temporal Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aPF | Adjusted preventive fraction |
| aOR | Adjusted odds ratio |
| cRR | Crude risk ratio |
| CI | Cumulative incidence |
| 95%CI | 95% confidence interval |
| IR | Incidence rate |
| IRR | Incidence rate ratio |
| HR | Hazard ratio |
| OR | Odds ratio |
| PF | Preventive fraction |
| PICU | Paediatric intensive care unit |
| RR | Risk ratio |
| RSV | Respiratory syncytial virus |
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, G.A. Respiratory syncytial virus mortality among young children. Lancet Glob. Health 2017, 5, e951–e952. [Google Scholar] [CrossRef] [PubMed]
- Treston, B.; Geoghegan, S. Exploring parental perspectives: Maternal RSV vaccination versus infant RSV monoclonal antibody. Hum. Vaccin. Immunother. 2024, 20, 2341505. [Google Scholar] [CrossRef]
- Britton, A.; Roper, L.E.; Kotton, C.N.; Hutton, D.W.; Fleming-Dutra, K.E.; Godfrey, M.; Ortega-Sanchez, I.R.; Broder, K.R.; Talbot, H.K.; Long, S.S.; et al. Use of Respiratory Syncytial Virus Vaccines in Adults Aged ≥60 Years: Updated Recommendations of the Advisory Committee on Immunization Practices—United States, 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 696–702. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios. Ficha Técnica Beyfortus 100 mg Solución Inyectable en Jeringa Precargada [Technical Datasheet Beyfortus 100 mg Solution for Injection in Prefilled Syringe]. Available online: https://cima.aemps.es/cima/dochtml/ft/1221689004/FT_1221689004.html (accessed on 3 January 2025).
- Simões, E.A.F.; Madhi, S.A.; Muller, W.J.; Atanasova, V.; Bosheva, M.; Cabañas, F.; Baca Cots, M.; Domachowske, J.B.; Garcia-Garcia, M.L.; Grantina, I.; et al. Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: A pooled analysis of randomised controlled trials. Lancet Child. Adolesc. Health 2023, 7, 180–189. [Google Scholar] [PubMed]
- Griffin, M.P.; Yuan, Y.; Takas, T.; Domachowske, J.B.; Madhi, S.A.; Manzoni, P.; Simões, E.A.F.; Esser, M.T.; Khan, A.A.; Dubovsky, F.; et al. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Eng. J. Med. 2020, 383, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Sanidad, Gobierno de España. Recomendaciones de Utilización de Nirsevimab Para la Prevención del Virus Respiratorio Sincitial (VRS) Para la Temporada 2023–2024 [Recommendations for the Use of Nirsevimab for the Prevention of respiratory Syncytial Virus (RSV) for the 2023–2024 Season]. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/Nirsevimab_2023.pdf (accessed on 3 January 2025).
- Ministerio de Sanidad, Gobierno de España. Actualización de Recomendaciones de Utilización de Nirsevimab [Update of Recommendations for the Use of Nirsevimab]. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/NirsevimabActualizacion.pdf (accessed on 3 January 2025).
- Turalde-Mapili, M.W.R.; Mapili, J.A.L.; Turalde, C.W.R.; Pagcatipunan, M.R. The efficacy and safety of nirsevimab for the prevention of RSV infection among infants: A systematic review and meta-analysis. Front. Pediat. 2023, 11, 1132740. [Google Scholar] [CrossRef]
- Sun, M.; Lai, H.; Na, F.; Li, S.; Qiu, X.; Tian, J.; Zhang, Z.; Ge, L. Monoclonal Antibody for the Prevention of Respiratory Syncytial Virus in Infants and Children: A Systematic Review and Network Meta-analysis. JAMA Netw. Open 2023, 6, e230023. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Cascio, A.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Giuri, P.G.; Gori, D.; Manzoni, P. Impact of Nirsevimab Immunization on Pediatric Hospitalization Rates: A Systematic Review and Meta-Analysis (2024). Vaccines 2024, 12, 640. [Google Scholar] [CrossRef] [PubMed]
- Drysdale, S.B.; Cathie, K.; Flamein, F.; Knuf, M.; Collins, A.M.; Hill, H.C.; Kaiser, F.; Cohen, R.; Pinquier, D.; Felter, C.T.; et al. Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants. N. Eng. J. Med. 2023, 389, 2425–2435. [Google Scholar] [CrossRef] [PubMed]
- Kuitunen, I.; Backman, K.; Gärdström, E.; Renko, M. Monoclonal antibody therapies in respiratory syncytial virus prophylaxis-An umbrella review. Pediat. Pulmonol. 2024, 59, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Ares-Gómez, S.; Mallah, N.; Santiago-Pérez, M.I.; Pardo-Seco, J.; Pérez-Martínez, O.; Otero-Barrós, M.T.; Suárez-Gaiche, N.; Kramer, R.; Jin, J.; Platero-Alonso, L.; et al. Effectiveness and impact of universal prophylaxis with nirsevimab in infants against hospitalisation for respiratory syncytial virus in Galicia, Spain: Initial results of a population-based longitudinal study. Lancet Infect. Dis. 2024, 24, 817–828. [Google Scholar] [CrossRef] [PubMed]
- López-Lacort, M.; Muñoz-Quiles, C.; Mira-Iglesias, A.; López-Labrador, F.X.; Mengual-Chuliá, B.; Fernández-García, C.; Carballido-Fernández, M.; Pineda-Caplliure, A.; Mollar-Maseres, J.; Shalabi Benavent, M.; et al. Early estimates of nirsevimab immunoprophylaxis effectiveness against hospital admission for respiratory syncytial virus lower respiratory tract infections in infants, Spain, October 2023 to January 2024. Euro Surveill. 2024, 29, 2400046. [Google Scholar] [CrossRef] [PubMed]
- Barbas Del Buey, J.F.; Íñigo Martínez, J.; Gutiérrez Rodríguez, M.Á.; Alonso García, M.; Sánchez-Gómez, A.; Lasheras Carbajo, M.D.; Jiménez Bueno, S.; Esteban Vasallo, M.D.; López Zambrano, M.A.; Calvo Rey, C.; et al. The effectiveness of nirsevimab in reducing the burden of disease due to respiratory syncytial virus (RSV) infection over time in the Madrid region (Spain): A prospective population-based cohort study. Front. Public Health 2024, 12, 1441786. [Google Scholar] [CrossRef] [PubMed]
- Coma, E.; Martinez-Marcos, M.; Hermosilla, E.; Mendioroz, J.; Reñé, A.; Fina, F.; Perramon-Malavez, A.; Prats, C.; Cereza, G.; Ciruela, P.; et al. Effectiveness of nirsevimab immunoprophylaxis against respiratory syncytial virus-related outcomes in hospital and primary care settings: A retrospective cohort study in infants in Catalonia (Spain). Arch. Dis. Child. 2024, 109, 736–741. [Google Scholar] [CrossRef]
- Mazagatos, C.; Mendioroz, J.; Rumayor, M.B.; Gallardo García, V.; Álvarez Río, V.; Cebollada Gracia, A.D.; Batalla Rebollo, N.; Barranco Boada, M.I.; Pérez-Martínez, O.; Lameiras Azevedo, A.S.; et al. Estimated Impact of Nirsevimab on the Incidence of Respiratory Syncytial Virus Infections Requiring Hospital Admission in Children < 1 Year, Weeks 40, 2023, to 8, 2024, Spain. Influenza Other Respir. Viruses 2024, 18, e13294. [Google Scholar]
- Spanish Ministry of Health. Evaluación de la Vacunación Frente a VRS en la Población Adulta [Evaluation of RSV Vaccination in Adult Population]. Available online: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/VRS_adultos.pdf (accessed on 26 January 2025).
- Jeziorski, E.; Ouziel, A.; Cotillon, M.; Bridonneau, C.; Bizot, E.; Basse, C.; Portefaix, A.; Dubos, F.; Béchet, S.M.; Domitien, L.; et al. Impact of nirsevimab on respiratory syncytial virus bronchiolitis in hospitalized infants: A real-world study. Pediatr. Infect. Dis. J. 2024, 10, 1097. [Google Scholar] [CrossRef]
- Sender, V.; Hentrich, K.; Henriques-Normark, B. Virus-induced changes of the respiratory tract environment promote secondary infections with Streptococcus pneumoniae. Front. Cell Infect. Microbiol. 2021, 11, 643326. [Google Scholar] [CrossRef]
- Chatzopoulou, F.; Gioula, G.; Kioumis, I.; Chatzidimitriou, D.; Exindari, M. Identification of complement-related host genetic risk factors associated with influenza A(H1N1)pdm09 outcome: Challenges ahead. Med. Microbiol. Immunol. 2019, 208, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Moline, H.L.; Tannis, A.; Toepfer, A.P.; Williams, J.V.; Boom, J.A.; Englund, J.A.; Halasa, N.B.; Staat, M.A.; Weinberg, G.A.; Selvarangan, R.; et al. Early Estimate of Nirsevimab Effectiveness for Prevention of Respiratory Syncytial Virus–Associated Hospitalization Among Infants Entering Their First Respiratory Syncytial Virus Season—New Vaccine Surveillance Network, October 2023–February 2024. MMWR Morb. Mortal. Wkly. Rep. 2024, 73, 209–214. [Google Scholar] [CrossRef]
| Characteristics at Admission | Total | Passive Immunisation with Nirsevimab | |
|---|---|---|---|
| Yes (n = 127) | No (n = 95) | ||
| Sex, n (%) | |||
| Men | 133 (59.9) | 82 (64.6) | 51 (53.7) |
| Women | 89 (40.1) | 45 (35.4) | 44 (46.3) |
| University Hospital (UH), n (%) | |||
| UH Virgen del Rocío, Sevilla | 50 (22.5) | 22 (17.3) | 28 (29.5) |
| Regional UH Malaga | 39 (17.6) | 21 (16.5) | 18 (19) |
| UH Reina Sofía, Cordoba | 28 (12.6) | 19 (15.0) | 9 (9.5) |
| UH Torrecárdenas Almería | 23 (10.4) | 10 (7.9) | 13 (13.7) |
| UH Virgen Macarena, Sevilla | 23 (10.4) | 14 (11.0) | 9 (9.5) |
| UH Jaén | 21 (9.5) | 16 (12.6) | 5 (5.3) |
| UH Virgen de las Nieves, Granada | 14 (6.0) | 8 (6.3) | 6 (6.3) |
| UH Juan Ramón Jiménez, Huelva | 13 (5.9) | 8 (6.3) | 5 (5.3) |
| UH Puerta del Mar, Cádiz | 11 (5.0) | 9 (7.1) | 2 (2.1) |
| Age at admission in days, median (IQR) | 59 (71) | 66 (55.0) | 45 (99.5) |
| Use of FilmArray, n (%) | 44 (19.8) | 28 (22.1) | 16 (16.8) |
| Any baseline disease, n (%) | 13 (5.9) | 10 (7.9) | 3 (3.2) |
| Congenital heart disease, n (%) | 7 (3.2) | 6 (4.7) | 1 (1.1) |
| Outcomes During Hospitalisation (Follow-Up) | Total | Passive Immunisation with Nirsevimab | p-Value | |
|---|---|---|---|---|
| Yes (n = 127) | No (n = 95) | |||
| Respiratory support, n (%) | 198 (89.2) | 109 (85.8) | 89 (93.7) | 0.062 1 |
| Conventional nasal cannula, n (%) | 187 (84.2) | 101 (79.5) | 86 (90.5) | 0.026 1 |
| High-flow oxygen therapy, n (%) | 43 (19.4) | 28 (22.1) | 15 (15.8) | 0.245 1 |
| Mechanical ventilation (invasive o non-invasive), n (%) | 89 (40.1) | 41 (32.3) | 48 (50.5) | 0.006 1 |
| Invasive mechanical ventilation, n (%) | 7 (3.2) | 5 (3.9) | 2 (2.1) | 0.701 3 |
| Nasogastric tube, n (%) | 102 (46.0) | 51 (40.2) | 51 (53.7) | 0.045 1 |
| Intravenous access, n (%) | 121 (54.5) | 67 (52.8) | 54 (56.8) | 0.545 1 |
| Antibiotic use, n (%) | 40 (18.0) | 24 (18.9) | 16 (16.8) | 0.693 1 |
| Chest imaging diagnostics, n (%) | 97 (43.7) | 49 (38.6) | 48 (50.5) | 0.076 1 |
| Co-infection, n (%) | 46 (20.7) | 35 (27.6) | 11 (11.6) | 0.004 1 |
| PICU admission, n (%) | 99 (44.6) | 46 (36.2) | 53 (55.8) | 0.004 1 |
| Mechanical ventilation in PICU, n (%) | 38 (17.1) | 17 (13.4) | 21 (22.1) | 0.088 1 |
| Length of PICU hospitalisation, median (IQR) | 5 (5) | 5 (4) | 6 (4) | 0.167 2 |
| Total length of hospitalisation, median (IQR) | 6 (5) | 6 (3.5) | 7 (5.5) | 0.003 2 |
| Outcomes During Follow-Up (Hospitalisation) | CIe (Nirsevimab) | CIo (no Nirsevimab) | RR (IC95%) | Effectiveness, PF (IC95%) |
|---|---|---|---|---|
| Respiratory support | 85.8% | 93.7% | 0.92 (0.84–1.00) | 8.4% (0.0–16.1) |
| Conventional nasal cannula | 79.5% | 90.5% | 0.88 (0.79–0.98) | 12.1% (2.0–21.3) |
| High-flow oxygen therapy | 22.0% | 15.8% | 1.40 (0.79–2.46) | - |
| Mechanical ventilation (invasive or non-invasive) | 32.3% | 50.5% | 0.63 (0.37–1.10) | - |
| Invasive mechanical ventilation | 3.9% | 2.1% | 1.87 (0.35–9.85) | - |
| Nasogastric tube | 40.2% | 53.7% | 0.75 (0.56–0.99) | 25.2% (0.0–43.6) |
| Intravenous access | 52.8% | 56.8% | 0.93 (0.73–1.18) | - |
| Antibiotic use | 18.9% | 16.8% | 1.12 (0.63–1.99) | - |
| Chest imaging tests | 38.6% | 50.5% | 0.76 (0.57–1.03) | - |
| Co-infection | 27.6% | 11.6% | 2.38 (1.28–4.44) | - |
| PICU admission | 36.2% | 55.8% | 0.65 (0.48–0.87) | 35.1% (13.1–51.5) |
| Invasive o non-invasive mechanical ventilation in PICU | 13.4% | 22.1% | 0.61 (0.34–1.08) | - |
| Outcomes | IR (Total Sample) | IRe (Nirsevimab Administration) | IRo (no Nirsevimab Administration) | IRR (IC95%) | PF (IC95%) | |||
|---|---|---|---|---|---|---|---|---|
| Cases/ Patients-Day | IR per 100 Patients-Day | Cases/ Patients-Day | IR per 100 Patients-Day | Cases/ Patients-Day | IR per 100 Patients-Day | |||
| Respiratory support | 198/347 | 57.06 | 109/209 | 52.15 | 89/138 | 64.49 | 0.81 (0.61–1.07) | - |
| Conventional nasal cannula | 187/826 | 22.64 | 101/478 | 21.13 | 86/348 | 24.71 | 0.86 (0.64–1.14) | - |
| High-flow oxygen therapy | 43/1563 | 2.75 | 28/780 | 3.59 | 15/783 | 1.92 | 1.87 (1.00–3.51) | - |
| Mechanical ventilation (invasive or non-invasive) | 88/1386 | 6.35 | 41/926 | 4.43 | 47/460 | 10.22 | 0.43 (0.29–0.66) | 56.7% (34.1–71.5%) |
| Invasive mechanical ventilation | 7/1731 | 0.40 | 5/882 | 0.57 | 2/849 | 0.24 | 2.41 (0.39–25.27) | - |
| Nasogastric tube | 102/948 | 10.76 | 51/521 | 9.79 | 51/427 | 11.94 | 0.82 (0.56–1.21) | - |
| Antibiotic use | 40/1552 | 2.58 | 24/765 | 3.14 | 16/787 | 2.03 | 1.54 (0.82–2.90) | |
| PICU admission | 99/857 | 11.55 | 46/547 | 8.41 | 53/310 | 17.10 | 0.49 (0.33–0.73) | 50.8% (27.0–66.9%) |
| Outcome | cOR (IC95%) | aOR (IC95%) | Effectiveness. aPF |
|---|---|---|---|
| Respiratory support | 0.41 (0.16–1.07) | 0.38 (0.14–1.03) | - |
| Conventional nasal cannula | 0.41 (0.18–0.91) | 0.36 (0.15–0.87) | 64% (13–85%) |
| High-flow oxygen therapy | 1.51 (0.75–3.02) | 1.32 (0.58–3.00) | - |
| Mechanical ventilation (invasive or non-invasive) | 0.47 (0.27–0.81) | 0.52 (0.27–0.99) | 48% (1–73%) |
| Invasive mechanical ventilation | 1.91 (0.36–10.04) | 3.52 (0.55–22.56) | - |
| Nasogastric tube | 0.58 (0.34–0.99) | 0.62 (0.34–1.13) | - |
| Intravenous access | 0.85 (0.88–1.98) | 1.07 (0.57–2.00) | - |
| Antibiotic use | 1.15 (0.57–2.31) | 1.26 (0.60–2.66) | - |
| Chest imaging tests | 0.62 (0.36–1.05) | 0.73 (0.39–1.35) | - |
| Co-infection | 2.91 (1.39–6.08) | 3.42 (1.52–7.68) | - |
| PICU admission | 0.45 (0.26–0.77) | 0.46 (0.25–0.86) | 54% (14–75%) |
| Mechanical ventilation in PICU | 0.54 (0.27–1.10) | 0.74 (0.27–2.05) | - |
| Outcome | Patients | Exposed (Nirsevimab), Days | Not Exposed (No Nirsevimab), Days | Log-Rank Test p-Value | cHR (IC95%) for Being Administered with Nirsevimab | aHR (IC95%) for Being Administered with Nirsevimab | ||
|---|---|---|---|---|---|---|---|---|
| n | Median (IQR) | Mean (sd) | Median (IQR) | Mean (sd) | ||||
| Total length of hospitalisation | 222 | 6 (4–8) | 6.62 (4.58) | 7 (5–11) | 8.47 (5.79) | 0.005 | 0.68 (0.52–0.89) | 0.70 (0.53–0.92) |
| Length of respiratory support | 198 | 5 (3–6) | 5.56 (4.26) | 5 (4–9) | 6.81 (4.87) | 0.048 | 0.76 (0.57–1.01) | 0.75 (0.56–1.01) |
| Length of nasal cannula usage | 187 | 3 (2–4) | 3.24 (1.68) | 3 (2–5) | 4.12 (3.19) | 0.020 | 0.71 (0.53–0.95) | 0.69 (0.51–0.93) |
| Length of high-flow oxygen therapy | 43 | 2 (1–4) | 2.75 (1.86) | 3 (1–4) | 2.87 (1.64) | 0.958 | 1.04 (0.55–1.97) | 1.07 (0.51–2.28) |
| Length of mechanical ventilation 1 | 89 | 4 (2–7) | 5.03 (4.72) | 3 (2–6) | 4.35 (3.17) | 0.471 | 1.14 (0.75–1.73) | 1.12 (0.71–1.77) |
| Length of PICU hospitalisation | 99 | 5 (3–7) | 5.65 (4.99) | 6 (4–8) | 6.47 (4.64) | 0.273 | 0.81 (0.54–1.20) | 0.76 (0.49–1.18) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Pérez, D.; Korobova, A.; Croche-Santander, F.d.B.; Cordón-Martínez, A.; Díaz-Morales, O.; Martínez-Campos, L.; Pérez-González, E.; Martínez-Padilla, M.d.C.; Santos-Pérez, J.L.; Brioso-Galiana, J.; et al. Nirsevimab Prophylaxis for Reduction of Respiratory Syncytial Virus Complications in Hospitalised Infants: The Multi-Centre Study During the 2023–2024 Season in Andalusia, Spain (NIRSEGRAND). Vaccines 2025, 13, 175. https://doi.org/10.3390/vaccines13020175
Moreno-Pérez D, Korobova A, Croche-Santander FdB, Cordón-Martínez A, Díaz-Morales O, Martínez-Campos L, Pérez-González E, Martínez-Padilla MdC, Santos-Pérez JL, Brioso-Galiana J, et al. Nirsevimab Prophylaxis for Reduction of Respiratory Syncytial Virus Complications in Hospitalised Infants: The Multi-Centre Study During the 2023–2024 Season in Andalusia, Spain (NIRSEGRAND). Vaccines. 2025; 13(2):175. https://doi.org/10.3390/vaccines13020175
Chicago/Turabian StyleMoreno-Pérez, David, Aleksandra Korobova, Francisco de Borja Croche-Santander, Ana Cordón-Martínez, Olga Díaz-Morales, Leticia Martínez-Campos, Elena Pérez-González, María del Carmen Martínez-Padilla, Juan Luis Santos-Pérez, Jaime Brioso-Galiana, and et al. 2025. "Nirsevimab Prophylaxis for Reduction of Respiratory Syncytial Virus Complications in Hospitalised Infants: The Multi-Centre Study During the 2023–2024 Season in Andalusia, Spain (NIRSEGRAND)" Vaccines 13, no. 2: 175. https://doi.org/10.3390/vaccines13020175
APA StyleMoreno-Pérez, D., Korobova, A., Croche-Santander, F. d. B., Cordón-Martínez, A., Díaz-Morales, O., Martínez-Campos, L., Pérez-González, E., Martínez-Padilla, M. d. C., Santos-Pérez, J. L., Brioso-Galiana, J., Sánchez-Códez, M. I., Del Diego-Salas, J., Rivera-Izquierdo, M., & Lorusso, N. (2025). Nirsevimab Prophylaxis for Reduction of Respiratory Syncytial Virus Complications in Hospitalised Infants: The Multi-Centre Study During the 2023–2024 Season in Andalusia, Spain (NIRSEGRAND). Vaccines, 13(2), 175. https://doi.org/10.3390/vaccines13020175





