The Humoral Immune Response Against COVID-19 Through Vaccination in Hemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. Assessment of Humoral Immunities
2.3. Statistical Analysis
3. Results
3.1. Antibody Levels at the Time of the First Blood Sample Collection
3.2. Antibody Levels at the Time of the Second Blood Sample Collection
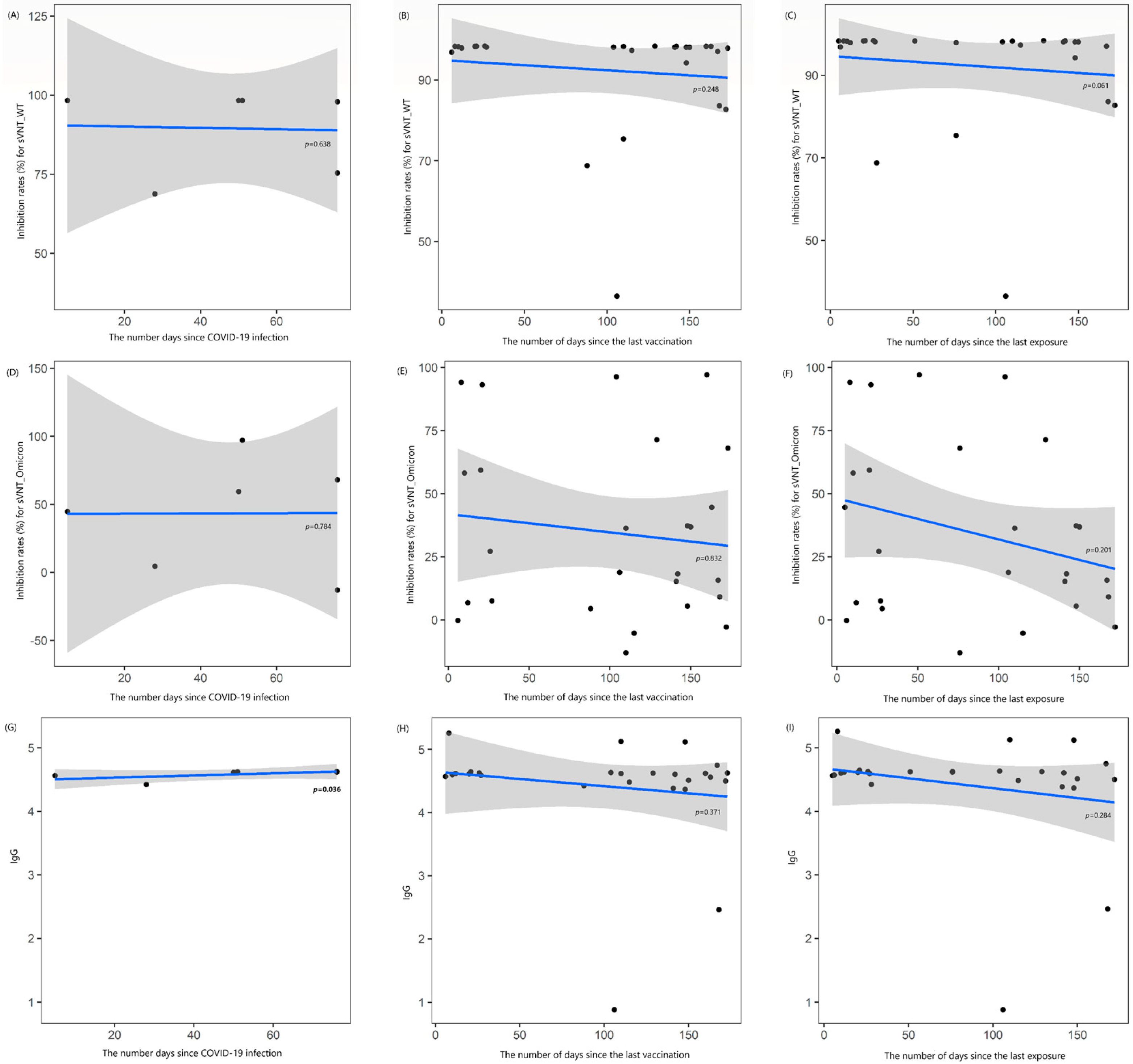
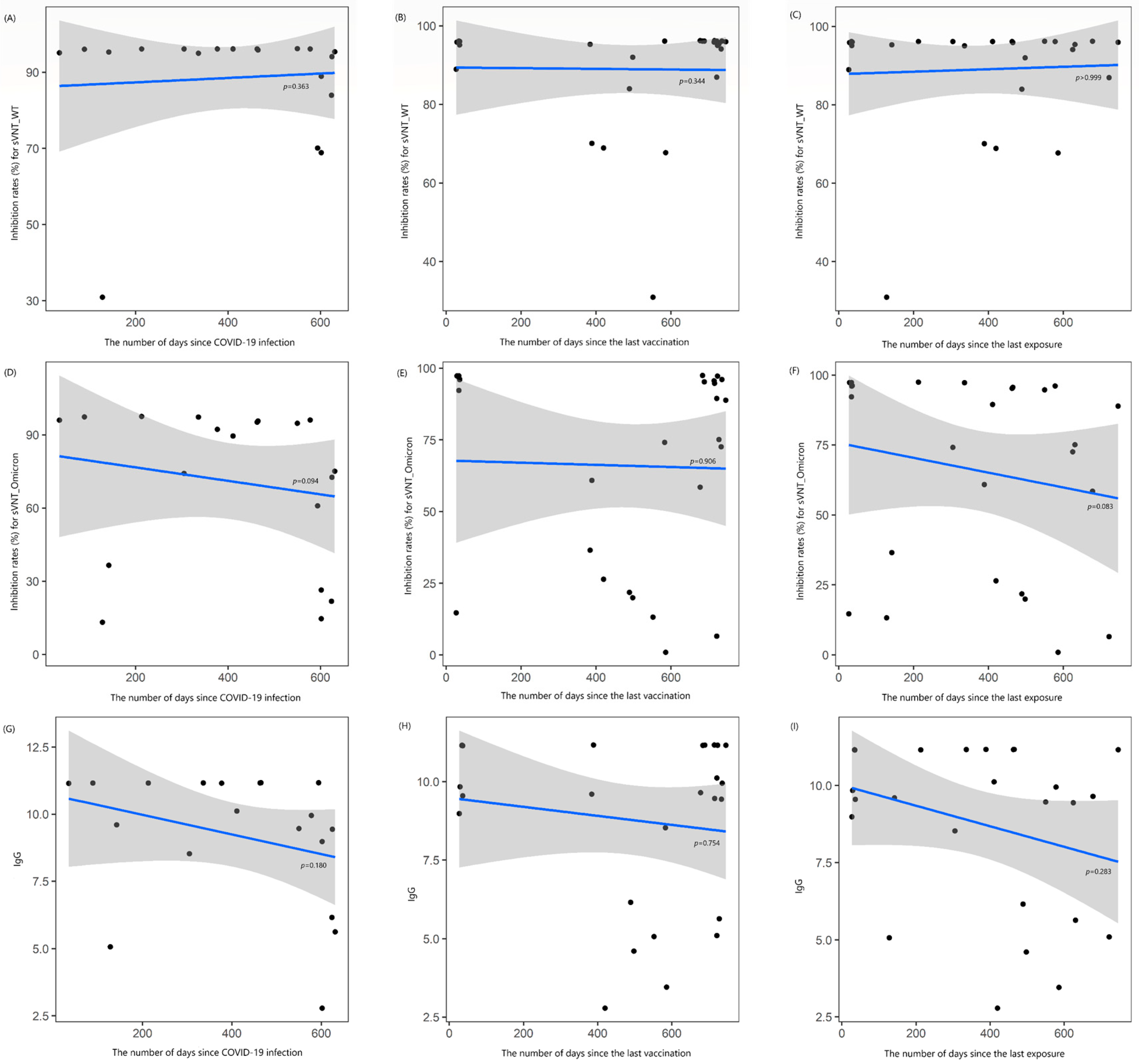
3.3. Changes in Antibody Values over Time Intervals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney diseases |
| HD | Hemodialysis |
| NAb | Neutralizing antibody |
| sVNT | Surrogate virus neutralization test |
| IgG | Spike-specific immunoglobulin G |
| ELISA | Enzyme-linked immunosorbent assay |
| OD | Optical density |
| PRNT | Plaque reduction neutralization test |
| ARD | Autoimmune rheumatic diseases |
References
- Klein, S.L.; Roberts, C. Sex Hormones and Immunity to Infection, 2nd ed.; Springer: Berlin, Germany, 2014. [Google Scholar]
- Nahid, P.; Jarlsberg, L.G.; Kato-Maeda, M.; Segal, M.R.; Osmond, D.H.; Gagneux, S.; Dobos, K.; Gold, M.; Hopewell, P.C.; Lewinsohn, D.M. Interplay of strain and race/ethnicity in the innate immune response to M. tuberculosis. PLoS ONE 2018, 13, e0195392. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.-H.; Chung, J.-W.; Hwang, M.-H.; Kim, M.-C. Systemic Adverse Events and Use of Antipyretics Predict the Neutralizing Antibody Positivity Early after the First Dose of ChAdOx1 Coronavirus Disease Vaccine. J. Clin. Med. 2021, 10, 2844. [Google Scholar] [CrossRef]
- Choi, S.-H.; Park, J.Y.; Park, J.H.; Kim, M.-C.; Kweon, O.J.; Chung, J.-W. Investigation of the Neutralizing Antibody Response of Healthcare Workers at a Korean University Hospital Six Months After the Introduction of the COVID-19 Vaccine. Ann. Lab. Med. 2022, 42, 612–615. [Google Scholar] [CrossRef]
- Choi, S.-H.; Park, J.Y.; Kweon, O.J.; Park, J.H.; Kim, M.-C.; Lim, Y.; Chung, J.-W. Immune Responses After Vaccination with Primary 2-Dose ChAdOx1 Plus a Booster of BNT162b2 or Vaccination with Primary 2-Dose BNT162b2 Plus a Booster of BNT162b2 and the Occurrence of Omicron Breakthrough Infection. J. Korean Med. Sci. 2023, 38, e155. [Google Scholar] [CrossRef]
- Vaccinating Dialysis Patients and Healthcare Personnel. Available online: https://archive.cdc.gov/#/details?url=https://www.cdc.gov/vaccines/covid-19/planning/vaccinate-dialysis-patients-hcp.html (accessed on 20 January 2025).
- Ovcar, E.; Patyna, S.; Kohmer, N.; Heckel-Kratz, E.; Ciesek, S.; Rabenau, H.F.; Hauser, I.A.; de Groot, K. Riding the Omicron BA.5 Wave: Improved Humoral Response after Vaccination with Bivalent Omicron BA.4-5-Adapted mRNA SARS-CoV-2 Vaccine in Chronic Hemodialysis Patients. Vaccines 2023, 11, 1428. [Google Scholar] [CrossRef]
- Krueger, K.M.; Ison, M.G.; Ghossein, C. Practical Guide to Vaccination in All Stages of CKD, Including Patients Treated by Dialysis or Kidney Transplantation. Am. J. Kidney Dis. 2020, 75, 417–425. [Google Scholar] [CrossRef]
- Toda, M.; Yoshifuji, A.; Nakayama, T.; Mise-Omata, S.; Oyama, E.; Uwamino, Y.; Namkoong, H.; Komatsu, M.; Yoshimura, A.; Hasegawa, N.; et al. Cellular and Humoral Immune Responses after Breakthrough Infection in Patients Undergoing Hemodialysis. Vaccines 2023, 11, 1214. [Google Scholar] [CrossRef]
- Yoshifuji, A.; Toda, M.; Oyama, E.; Nakayama, T.; Mise-Omata, S.; Kikuchi, K.; Yoshizawa, M.; Kato, N.; Wakai, H.; Koibuchi, K.; et al. Cellular and humoral immune responses to COVID-19 booster vaccination in Japanese dialysis patients. Clin. Exp. Nephrol. 2024, 28, 674–682. [Google Scholar] [CrossRef]
- Taylor, S.C.; Hurst, B.; Charlton, C.L.; Bailey, A.; Kanji, J.N.; McCarthy, M.K.; Morrison, T.E.; Huey, L.; Annen, K.; DomBourian, M.G.; et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J. Clin. Microbiol. 2021, 59, e02438-20. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ao, L.; Ke, M.; Chen, Z.; Chen, M.; Peng, M.; Ling, N.; Hu, P.; Cai, D.; et al. Association between immunosuppressants and poor antibody responses to SARS-CoV-2 vaccines in patients with autoimmune liver diseases. Front. Immunol. 2022, 13, 988004. [Google Scholar]
- Kim, W.J.; Choi, S.H.; Park, J.Y.; Song, J.S.; Chung, J.W.; Choi, S.T. SARS-CoV-2 Omicron escapes mRNA vaccine booster-induced antibody neutralisation in patients with autoimmune rheumatic diseases: An observational cohort study. Ann. Rheum. Dis. 2022, 81, 1585–1593. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; No, J.S.; Kim, J.A.; Park, A.K.; Lee, H.; Kim, J.M.; Lee, N.J.; Kim, C.K.; Lee, C.Y.; Woo, S.; et al. Genomic epidemiology of SARS-CoV-2 variants in South Korea between January 2020 and February 2023. Virology 2023, 587, 109869. [Google Scholar] [CrossRef] [PubMed]
- No, J.S.; Noh, J.Y.; Lee, C.Y.; Kim, I.H.; Kim, J.A.; Ahn, Y.J.; Lee, H.; Kim, J.M.; Lee, N.J.; Lee, D.W.; et al. Dynamics of SARS-CoV-2 variants during the XBB wave in the Republic of Korea. Virus Res. 2024, 350, 199471. [Google Scholar] [CrossRef] [PubMed]
- Goggins, E.; Sharma, B.; Ma, J.Z.; Gautam, J.; Bowman, B. Humoral immunity trends in a hemodialysis cohort following SARS-CoV-2 mRNA booster: A cohort study. Health Sci. Rep. 2024, 7, e1858. [Google Scholar] [CrossRef]
- Bathish, Y.; Tuvia, N.; Eshel, E.; Lange, T.T.; Eberhardt, C.S.; Edelstein, M.; Abu-Jabal, K. B and T cell responses to the 3rd and 4th dose of the BNT162b2 vaccine in dialysis patients. Hum. Vaccines Immunother. 2024, 20, 2292376. [Google Scholar] [CrossRef]
- Bednarski, E.; Del Rio Estrada, P.M.; DaSilva, J.; Boukadida, C.; Zhang, F.; Luna-Villalobos, Y.A.; Rodríguez-Rangel, X.; Pitén-Isidro, E.; Luna-García, E.; Díaz Rivera, D.; et al. Antibody and memory B-cell Immunity in a Heterogeneously SARS-CoV-2-Infected and -Vaccinated Population. mBio 2022, 13, e0084022. [Google Scholar] [CrossRef]
- Dinarello, C.A. Biologic basis for interleukin-1 in disease. Blood 1996, 87, 2095–2147. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Nakae, S.; Komiyama, Y.; Nambu, A.; Sudo, K.; Iwase, M.; Homma, I.; Sekikawa, K.; Asano, M.; Iwakura, Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002, 17, 375–387. [Google Scholar] [CrossRef]
- Rizk, J.G.; Kalantar-Zadeh, K.; Mehra, M.R.; Lavie, C.J.; Rizk, Y.; Forthal, D.N. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs 2020, 80, 1267–1292. [Google Scholar] [CrossRef]
- Margo, S.F. Medication Safety Concerns Surrounding Immunomodulators. US Pharm. 2016, 41, HS2–HS8. Available online: https://www.uspharmacist.com/article/medication-safety-concerns-surrounding-immunomodulators (accessed on 20 January 2025).
- Joo, Y.; Kim, D.K.; Jeon, Y.G.; Kim, A.-R.; Do, H.N.; Yoon, S.-Y.; Kim, J.S.; Jung, S.W.; Hwang, H.S.; Moon, J.-Y.; et al. Comparison of Humoral Response between Third and Fourth Doses of COVID-19 Vaccine in Hemodialysis Patients. Vaccines 2023, 11, 1584. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Minn, D.; Hong, S.; Jeong, S.; Kim, S.; Lee, C.H.; Kim, B. Immunogenicity of COVID-19 Vaccination in Patients with End-Stage Renal Disease Undergoing Maintenance Hemodialysis: The Efficacy of a Mix-and-Match Strategy. J. Korean Med. Sci. 2022, 37, e180. [Google Scholar] [CrossRef] [PubMed]
| 1st Period (2022) | 2nd Period (2023) | |
|---|---|---|
| N (%) | ||
| Sex | ||
| Female | 14 (53.8) | |
| Male | 12 (46.2) | |
| Mean age (±SD 1, years) | 72.4 ± 10.8 | |
| On immunosuppressive therapy | 3 (11.5) | |
| Vaccination status | ||
| Only primary series | 2 (7.7) | - |
| Booster, once | 17 (65.4) | 13 (50.0) |
| Booster, twice | 7 (26.9) | 7 (26.9) |
| Booster, thrice | - | 2 (7.7) |
| Booster, four times | - | 4 (15.4) |
| Booster with Omicron variant | - | 7 (26.9) |
| BNT162b2 | - | 6 (85.7) |
| BA.1 | - | 1 |
| BA.4/5 | - | 3 |
| XBB.1.5 | - | 5 |
| mRNA-1273 | - | 1 (14.3) |
| BA.1 | - | 1 |
| XBB.1.5 | - | 1 |
| Confirmed COVID-19 history | ||
| Yes | 8 (30.8) | 19 (73.1) |
| None | 18 (69.2) | 7 (26.9) |
| sVNT_1 | sVNT_2 | Omicron_1 | Omicron_2 | IgG_1 | IgG_2 | |
|---|---|---|---|---|---|---|
| % | ||||||
| Sex | ||||||
| Female | 94.9 | 91.6 | 28.0 | 71.5 | 4.6 | 9.0 |
| Male | 89.4 | 85.9 | 42.2 | 59.5 | 4.2 | 8.5 |
| p-value | 0.423 | 0.587 | 0.231 | 0.681 | 0.877 | 0.625 |
| On immunosuppressive therapy | ||||||
| Yes | 77.5 | 64.2 | 33.8 | 28.9 | 3.4 | 6.0 |
| None | 94.3 | 92.2 | 40.9 | 70.8 | 4.6 | 9.1 |
| p-value | 0.572 | 0.024 | 0.705 | 0.045 | 0.602 | 0.054 |
| Bivalent vaccination status | ||||||
| Booster | - | 94.8 | - | 75.7 | - | 10.2 |
| No booster | - | 86.8 | - | 62.4 | - | 8.3 |
| p-value | - | 0.727 | - | 0.162 | - | 0.470 |
| History of COVID-19 infection | ||||||
| 1st sampling period | ||||||
| Yes | 91.6 | - | 34.2 | - | 4.6 | - |
| None | 92.7 | - | 34.8 | - | 4.3 | - |
| p-value | >0.999 | - | 0.892 | - | 0.637 | - |
| 2nd sampling period | ||||||
| Yes | - | 88.6 | - | 70.9 | - | 9.2 |
| None | - | 90.1 | - | 52.6 | - | 7.6 |
| p-value | - | 0.793 | - | 0.340 | - | 0.214 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Choi, S.-H.; Lim, Y.K.; Shin, J.; Kwon, S.; Kim, H.; Chung, J.-W. The Humoral Immune Response Against COVID-19 Through Vaccination in Hemodialysis Patients. Vaccines 2025, 13, 170. https://doi.org/10.3390/vaccines13020170
Park JY, Choi S-H, Lim YK, Shin J, Kwon S, Kim H, Chung J-W. The Humoral Immune Response Against COVID-19 Through Vaccination in Hemodialysis Patients. Vaccines. 2025; 13(2):170. https://doi.org/10.3390/vaccines13020170
Chicago/Turabian StylePark, Ji Young, Seong-Ho Choi, Yong Kwan Lim, Jungho Shin, Soie Kwon, Haein Kim, and Jin-Won Chung. 2025. "The Humoral Immune Response Against COVID-19 Through Vaccination in Hemodialysis Patients" Vaccines 13, no. 2: 170. https://doi.org/10.3390/vaccines13020170
APA StylePark, J. Y., Choi, S.-H., Lim, Y. K., Shin, J., Kwon, S., Kim, H., & Chung, J.-W. (2025). The Humoral Immune Response Against COVID-19 Through Vaccination in Hemodialysis Patients. Vaccines, 13(2), 170. https://doi.org/10.3390/vaccines13020170







