A Novel Vaccine for Bovine Diarrhea Complex Utilizing Recombinant Enterotoxigenic Escherichia coli and Salmonella Expressing Surface-Displayed Chimeric Antigens from Enterohemorrhagic Escherichia coli O157:H7
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of EHEC Recombinant Proteins in ETEC and Salmonella Dublin
2.2. Inactivation of Recombinant ETEC and Salmonella Dublin
2.3. Cellular Localization of the Chimera Protein Detection
2.4. Western Blot Assay
2.5. Strains and Production of Coronavirus and Rotavirus
2.6. Fluorescent Focus Reduction Assay
2.7. Animal Models and Immunization
2.8. IgG Specific Antibody ELISA
2.9. Specific Antibody BCoV and BRoVA ELISA
2.10. Antigen- Specific IL-17, IFN-γ and IL-5 Production by Spleen Cells
2.11. Statistical Analysis
3. Results
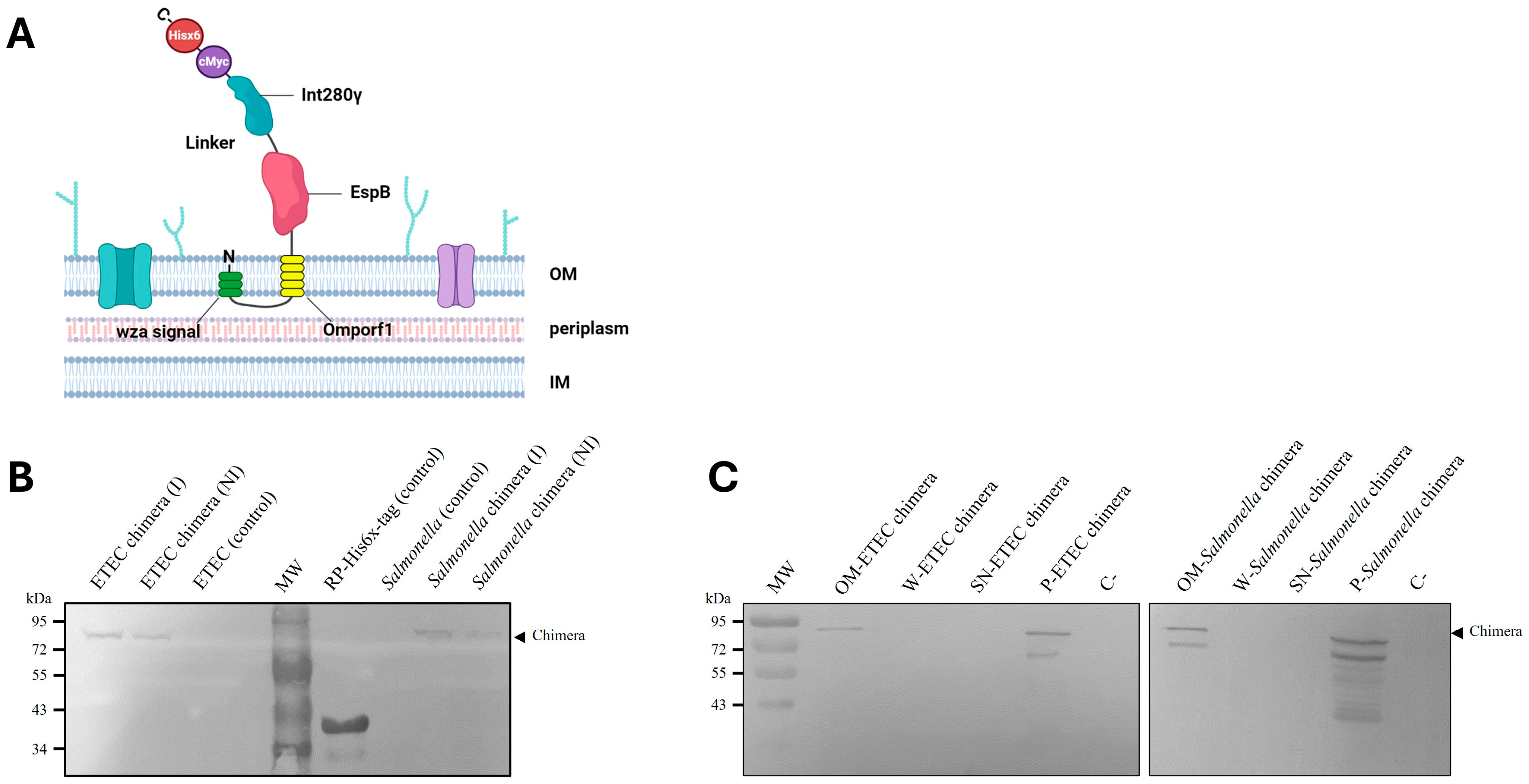
3.1. Design, Cloning and Introduction into Carrier Bacteria of Chimera Protein
3.2. Membrane-Anchoring of Chimeric Protein to ETEC and Salmonella Dublin
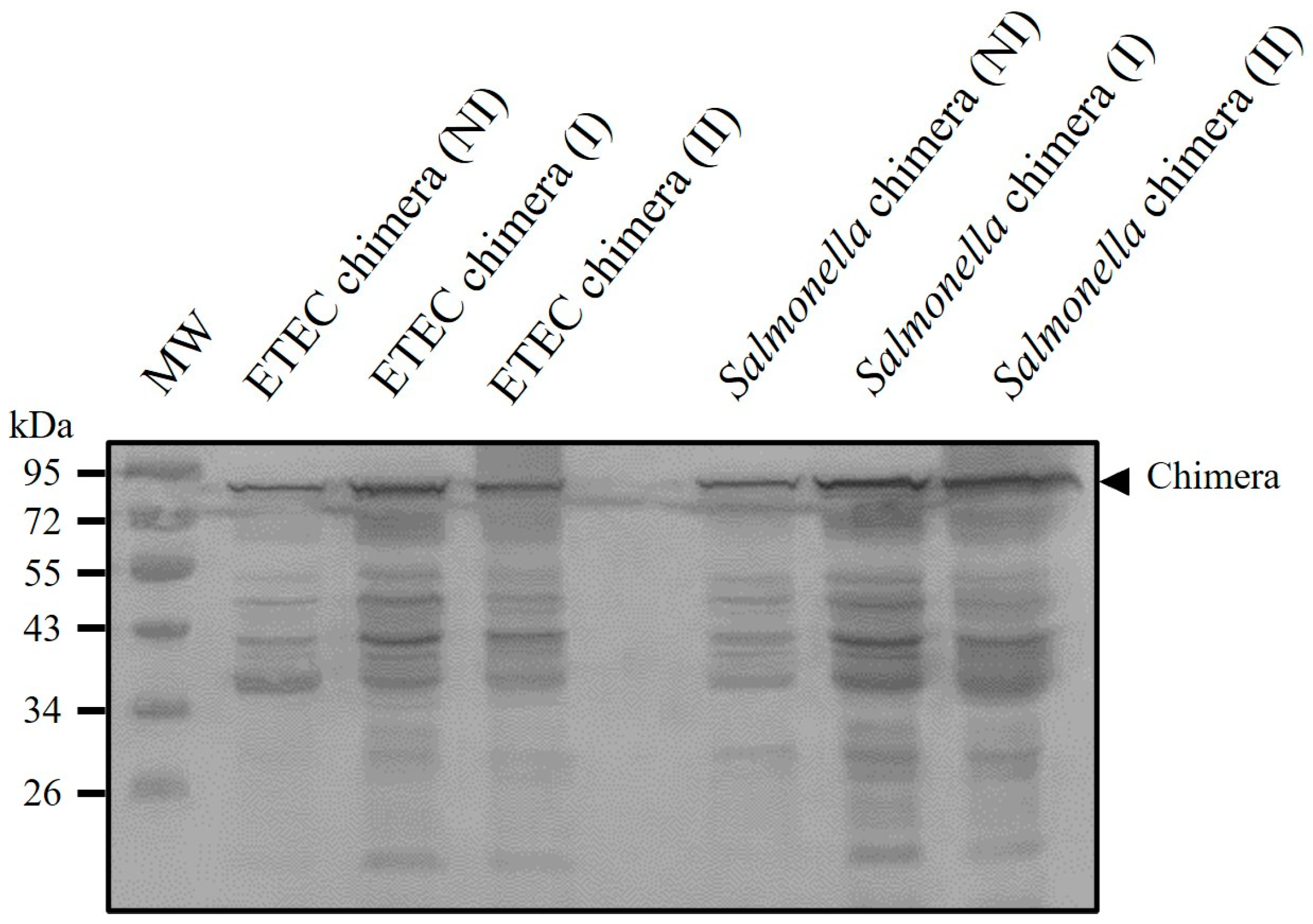
3.3. Inactivation of Recombinant ETEC and Salmonella Dublin and Antigenic Preservation in Vaccine Formulation
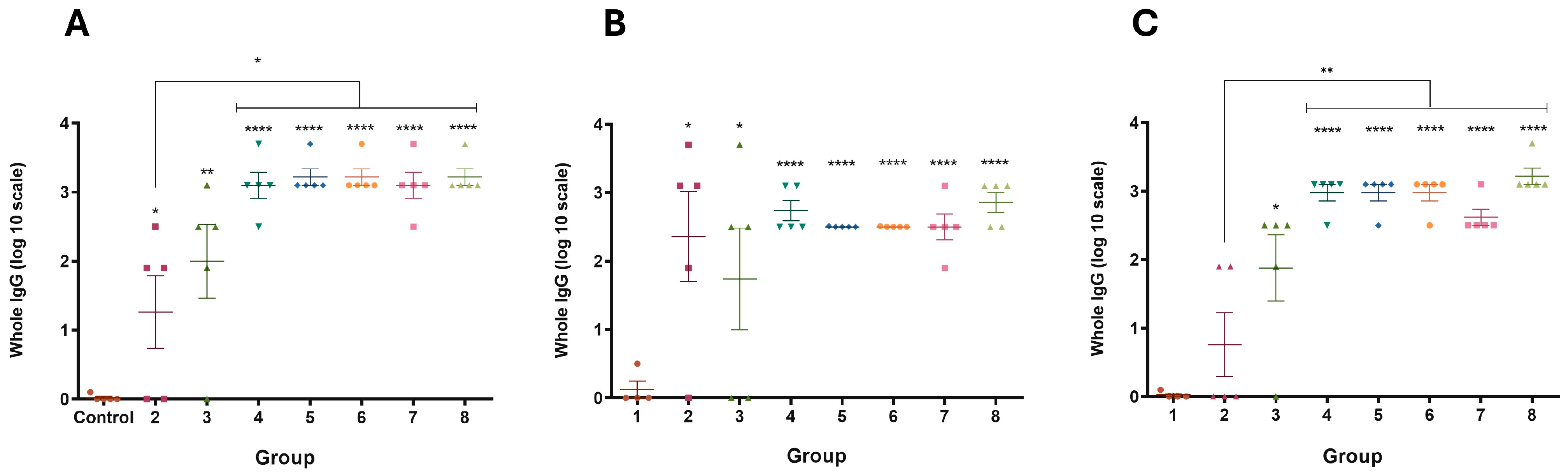
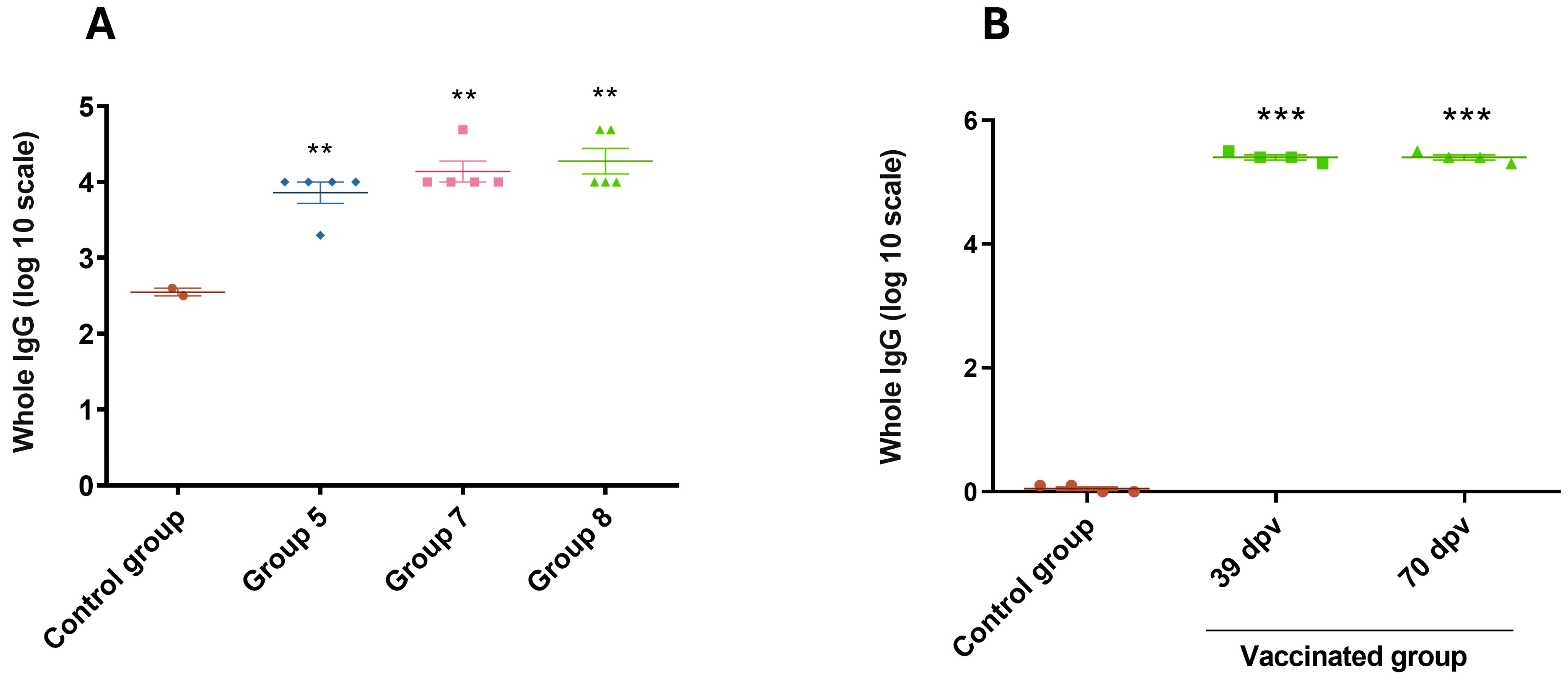
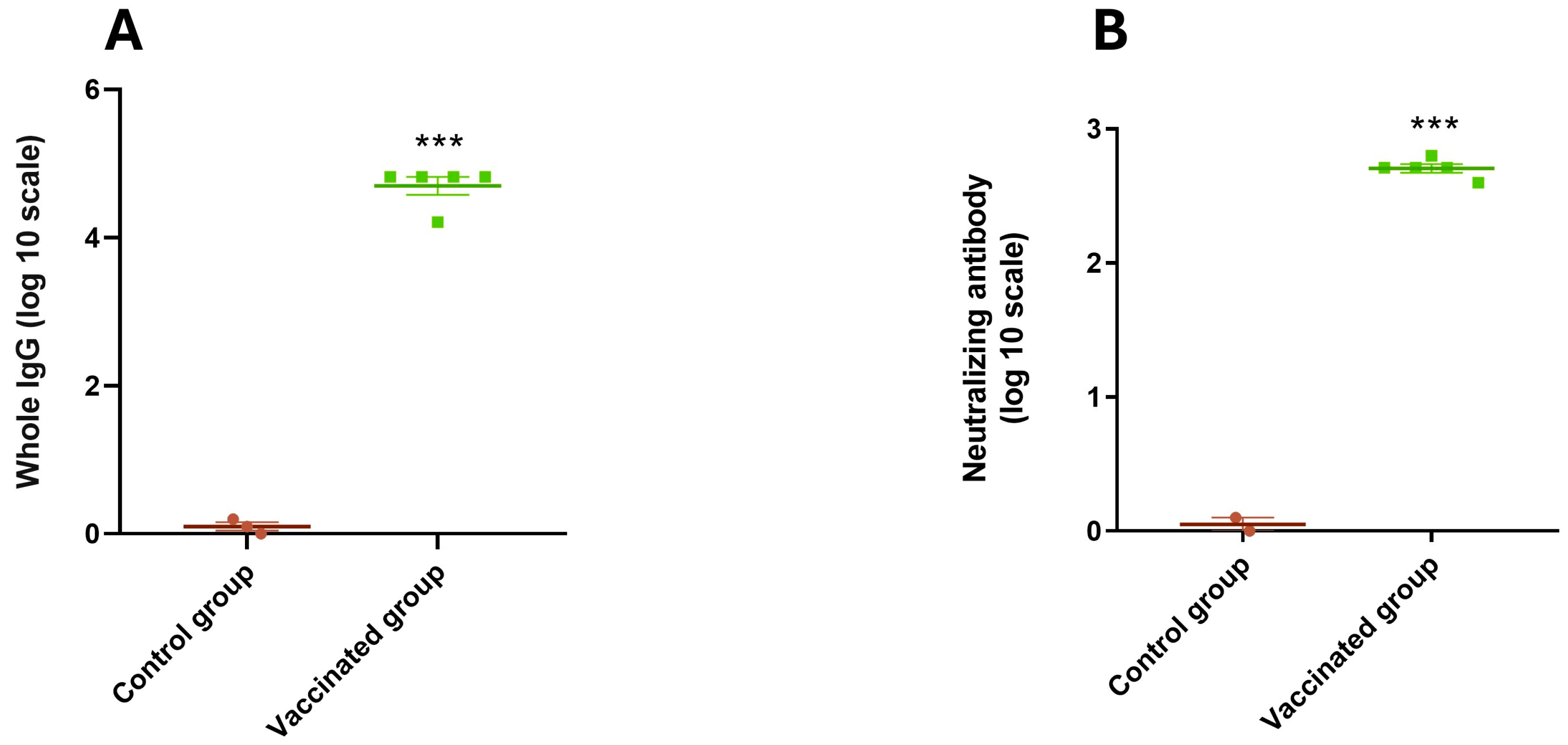
3.4. Immune Response of Mice and Guinea Pigs
3.5. Evaluation of Immune Response Against Chimera-Carrying Bacteria
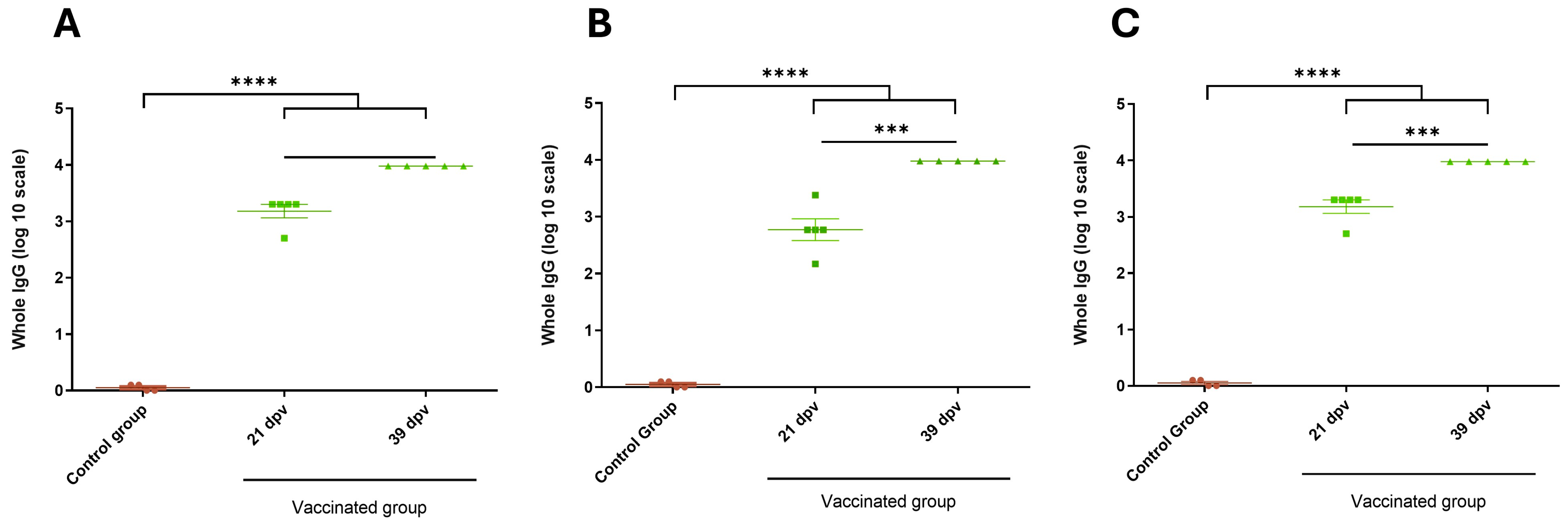
3.6. Antibody Titers Against BRoVA UK and BCoV Mebus
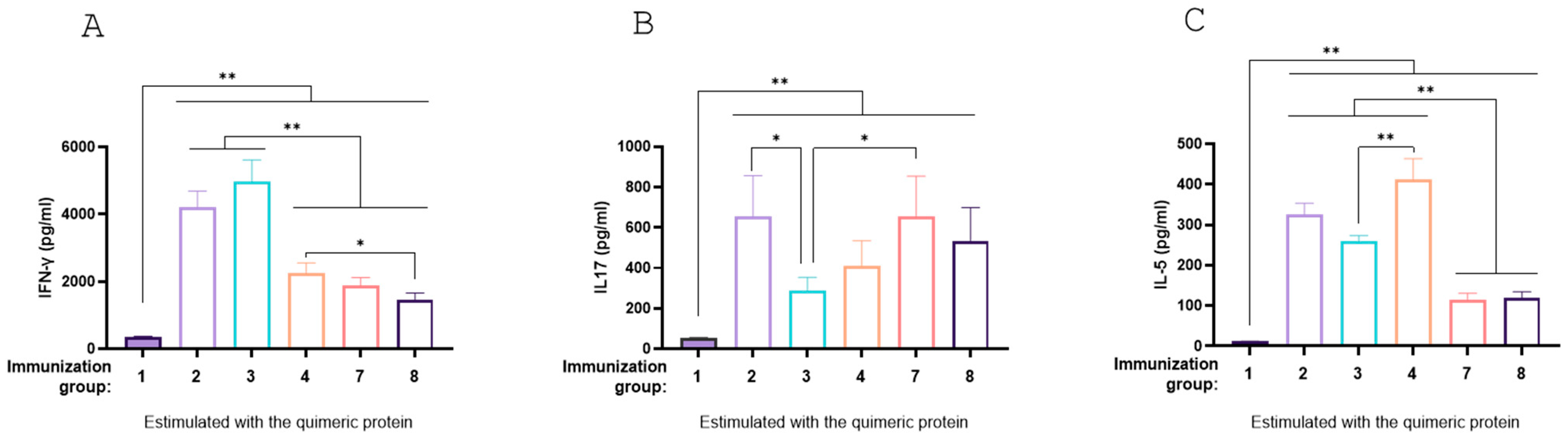
3.7. Antigen-Specific Immune Response Profiles Across Vaccination Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palermo, M.S.; Exeni, R.A.; Fernández, G.C. Hemolytic Uremic Syndrome: Pathogenesis and Update of Interventions. Expert Rev. Anti-Infect. Ther. 2009, 7, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Nataro, J.P.; Kaper, J.B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 1998, 11, 142–201. [Google Scholar] [CrossRef]
- Rivas, M.; Chinen, I.; Miliwebsky, E.; Masana, M. Risk Factors for Shiga Toxin-Producing Escherichia Coli-Associated Human Diseases. Microbiol. Spectr. 2014, 2, 359–380. [Google Scholar] [CrossRef]
- Naylor, S.W.; Low, J.C.; Besser, T.E.; Mahajan, A.; Gunn, G.J.; Pearce, M.C.; Mckendrick, I.J.; Smith, D.G.E.; Gally, D.L. Lymphoid Follicle-Dense Mucosa at the Terminal Rectum Is the Principal Site of Colonization of Enterohemorrhagic Escherichia coli O157:H7 in the Bovine Host. Infect. Immun. 2003, 71, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Midgley, J.; Desmarchelier, P. Pre-Slaughter Handling of Cattle and Shiga Toxin-Producing Escherichia coli (STEC). Lett. Appl. Microbiol. 2001, 32, 307–311. [Google Scholar] [CrossRef]
- Matthews, L.; Reeve, R.; Gally, D.L.; Low, J.C.; Woolhouse, M.E.J.; McAteer, S.P.; Locking, M.E.; Chase-Topping, M.E.; Haydon, D.T.; Allison, L.J.; et al. Predicting the Public Health Benefit of Vaccinating Cattle against Escherichia Coli O157. Proc. Natl. Acad. Sci. USA 2013, 110, 16265–16270. [Google Scholar] [CrossRef]
- Bretschneider, G.; Berberov, E.M.; Moxley, R.A. Isotype-Specific Antibody Responses against Escherichia coli O157:H7 Locus of Enterocyte Effacement Proteins in Adult Beef Cattle Following Experimental Infection. Vet. Immunol. Immunopathol. 2007, 118, 229–238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rabinovitz, B.C.; Larzábal, M.; Vilte, D.A.; Cataldi, A.; Mercado, E.C. The Intranasal Vaccination of Pregnant Dams with Intimin and EspB Confers Protection in Neonatal Mice from Escherichia Coli (EHEC) O157:H7 Infection. Vaccine 2016, 34, 2793–2797. [Google Scholar] [CrossRef]
- Cataldi, A.; Yevsa, T.; Vilte, D.A.; Schulze, K.; Castro-Parodi, M.; Larzábal, M.; Ibarra, C.; Mercado, E.C.; Guzmán, C. A Efficient Immune Responses against Intimin and EspB of Enterohaemorragic Escherichia Coli after Intranasal Vaccination Using the TLR2/6 Agonist MALP-2 as Adjuvant. Vaccine 2008, 26, 5662–5667. [Google Scholar] [CrossRef]
- Babiuk, S.; Asper, D.; Rogan, D.; Mutwiri, G.; Potter, A. Subcutaneous and Intranasal Immunization with Type III Secreted Proteins Can Prevent Colonization and Shedding of Escherichia coli O157:H7 in Mice. Microb. Pathog. 2008, 45, 7–11. [Google Scholar] [CrossRef]
- Mayr, U.B.; Haller, C.; Haidinger, W.; Atrasheuskaya, A.; Bukin, E.; Lubitz, W.; Ignatyev, G. Bacterial Ghosts as an Oral Vaccine: A Single Dose of Escherichia coli O157:H7 Bacterial Ghosts Protects Mice against Lethal Challenge. Infect. Immun. 2005, 73, 4810–4817. [Google Scholar] [CrossRef] [PubMed]
- Iannino, F.; Herrmann, C.K.; Roset, M.S.; Briones, G. Development of a Dual Vaccine for Prevention of Brucella Abortus Infection and Escherichia Coli O157:H7 Intestinal Colonization. Vaccine 2015, 33, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Fingermann, M.; Avila, L.; De Marco, M.B.; Vázquez, L.; Di Biase, D.N.; Müller, A.V.; Lescano, M.; Dokmetjian, J.C.; Fernández Castillo, S.; Pérez Quiñoy, J.L. OMV-Based Vaccine Formulations against Shiga Toxin Producing Escherichia Coli Strains Are Both Protective in Mice and Immunogenic in Calves. Hum. Vaccin. Immunother. 2018, 14, 2208–2213. [Google Scholar] [CrossRef]
- Montero, D.A.; Del Canto, F.; Salazar, J.C.; Céspedes, S.; Cádiz, L.; Arenas-Salinas, M.; Reyes, J.; Oñate, Á.; Vidal, R.M. Immunization of Mice with Chimeric Antigens Displaying Selected Epitopes Confers Protection against Intestinal Colonization and Renal Damage Caused by Shiga Toxin-Producing Escherichia Coli. NPJ Vaccines 2020, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Garcia-Betancourt, R.; Vidal, R.M.; Velasco, J.; Palacios, P.A.; Schneider, D.; Vega, C.; Gómez, L.; Montecinos, H.; Soto-Shara, R.; et al. A Chimeric Protein-Based Vaccine Elicits a Strong IgG Antibody Response and Confers Partial Protection against Shiga Toxin-Producing Escherichia coli in Mice. Front. Immunol. 2023, 14, 1186368. [Google Scholar] [CrossRef] [PubMed]
- Konadu, E.; Donohue-Rolfe, A.; Calderwood, S.B.; Pozsgay, V.; Shiloach, J.; Robbins, J.B.; Szu, S.C. Syntheses and Immunologic Properties of Escherichia coli O157 O-Specific Polysaccharide and Shiga Toxin 1 B Subunit Conjugates in Mice. Infect. Immun. 1999, 67, 6191–6193. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, M.; Roch, F.F.; Conrady, B. Prevalence of Worldwide Neonatal Calf Diarrhoea Caused by Bovine Rotavirus in Combination with Bovine Coronavirus, Escherichia coli K99 and Cryptosporidium Spp.: A Meta-Analysis. Animals 2021, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Q.; Wang, Q.; Zhang, Y. Novel Bacterial Surface Display Systems Based on Outer Membrane Anchoring Elements from the Marine Bacterium Vibrio Anguillarum. Appl. Environ. Microbiol. 2008, 74, 4359. [Google Scholar] [CrossRef]
- Vilte, D.A.; Larzábal, M.; Garbaccio, S.; Gammella, M.; Rabinovitz, B.C.; Elizondo, A.M.; Cantet, R.J.C.; Delgado, F.; Meikle, V.; Cataldi, A.; et al. Reduced Faecal Shedding of Escherichia coli O157:H7 in Cattle Following Systemic Vaccination with γ-Intimin C280 and EspB Proteins. Vaccine 2011, 29, 3962–3968. [Google Scholar] [CrossRef]
- Tô, T.L.; Ward, L.A.; Yuan, L.; Saif, L.J. Serum and Intestinal Isotype Antibody Responses and Correlates of Protective Immunity to Human Rotavirus in a Gnotobiotic Pig Model of Disease. J. Gen. Virol. 1998, 79 Pt 11, 2661–2672. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty per Cent Endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Vega, C.; Bok, M.; Chacana, P.; Saif, L.; Fernandez, F.; Parreño, V. Egg Yolk IgY: Protection against Rotavirus Induced Diarrhea and Modulatory Effect on the Systemic and Mucosal Antibody Responses in Newborn Calves. Vet. Immunol. Immunopathol. 2011, 142, 156–169. [Google Scholar] [CrossRef]
- Smith, D.R. Vaccination of Cattle against Escherichia coli O157:H7. Microbiol. Spectr. 2014, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Potter, A.A.; Klashinsky, S.; Li, Y.; Frey, E.; Townsend, H.; Rogan, D.; Erickson, G.; Hinkley, S.; Klopfenstein, T.; Moxley, R.A.; et al. Decreased Shedding of Escherichia coli O157:H7 by Cattle Following Vaccination with Type III Secreted Proteins. Vaccine 2004, 22, 362–369. [Google Scholar] [CrossRef]
- McNeilly, T.N.; Mitchell, M.C.; Rosser, T.; McAteer, S.; Low, J.C.; Smith, D.G.E.; Huntley, J.F.; Mahajan, A.; Gally, D.L. Immunization of Cattle with a Combination of Purified Intimin-531, EspA and Tir Significantly Reduces Shedding of Escherichia coli O157:H7 Following Oral Challenge. Vaccine 2010, 28, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.L.; Larzábal, M.; Song, H.; Cheng, T.; Wang, Y.; Smith, L.Y.; Cataldi, A.A.; Ow, D.W. Enterohemorrhagic Escherichia coli O157:H7 Antigens Produced in Transgenic Lettuce Effective as an Oral Vaccine in Mice. Theor. Appl. Genet. 2023, 136, 214. [Google Scholar] [CrossRef]
- Bexsero|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/bexsero (accessed on 12 January 2025).
- Trumenba|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/trumenba (accessed on 12 January 2025).
- Lymerix (Lipoprotein Outer Surface A Vaccine): Side Effects, Uses, Dosage, Interactions, Warnings. Available online: https://www.rxlist.com/lymerix-drug.htm (accessed on 12 January 2025).
- Chauhan, S.; Khasa, Y.P. Challenges and Opportunities in the Process Development of Chimeric Vaccines. Vaccines 2023, 11, 1828. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.Y.C.; Van, T.T.H.; Smooker, P.M. Live-Attenuated Bacterial Vectors: Tools for Vaccine and Therapeutic Agent Delivery. Vaccines 2015, 3, 940–972. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs. Front. Immunol. 2020, 11, 529088. [Google Scholar] [CrossRef]
- Demento, S.L.; Siefert, A.L.; Bandyopadhyay, A.; Sharp, F.A.; Fahmy, T.M. Pathogen-Associated Molecular Patterns on Biomaterials: A Paradigm for Engineering New Vaccines. Trends Biotechnol. 2011, 29, 294. [Google Scholar] [CrossRef] [PubMed]
- Vos, Q.; Lees, A.; Wu, Z.Q.; Snapper, C.M.; Mond, J.J. B-Cell Activation by T-Cell-Independent Type 2 Antigens as an Integral Part of the Humoral Immune Response to Pathogenic Microorganisms. Immunol. Rev. 2000, 176, 154–170. [Google Scholar] [CrossRef]
- Wamhoff, E.C.; Ronsard, L.; Feldman, J.; Knappe, G.A.; Hauser, B.M.; Romanov, A.; Case, J.B.; Sanapala, S.; Lam, E.C.; Denis, K.J.S.; et al. Enhancing Antibody Responses by Multivalent Antigen Display on Thymus-Independent DNA Origami Scaffolds. Nat. Commun. 2024, 15, 795. [Google Scholar] [CrossRef] [PubMed]
- Nicchi, S.; Giuliani, M.; Giusti, F.; Pancotto, L.; Maione, D.; Delany, I.; Galeotti, C.L.; Brettoni, C. Decorating the Surface of Escherichia coli with Bacterial Lipoproteins: A Comparative Analysis of Different Display Systems. Microb. Cell Fact. 2021, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.A.; Decaris, N.; Parreño, V.; Brandão, P.E.; Ayres, H.; Gomes, V. Efficacy of Prepartum Vaccination against Neonatal Calf Diarrhea in Nelore Dams as a Prevention Measure. BMC Vet. Res. 2022, 18, 323. [Google Scholar] [CrossRef]



| (A) | ||
| Groups of Mice | Treatments | Details |
| 1 | Control | 150 µL of PBS |
| 2 | EspB and Int280γ | 1 µg of EspB and 1 µg of Int280γ dissolved in 150 µL of PBS |
| 3 | Chimera protein (low dose) | 2 µg of Chimera protein dissolved in 150 µL of PBS |
| 4 | Chimera protein (high dose) | 10 µg of Chimera protein dissolved in 150 µL of PBS |
| 5 | Inactivated ETEC B41Arg expressing Chimera protein | 1.108 CFU of inactivated ETEC B41Arg expressing Chimera protein resuspended in PBS |
| 6 | Inactivated Salmonella Dublin expressing Chimera protein | 1.108 CFU of inactivated Salmonella Dublin expressing Chimera protein resuspended in PBS |
| 7 | Inactivated ETEC B41Arg and Salmonella Dublin expressing Chimera protein | 1.108 CFU of inactivated ETEC B41Arg and Salmonella Dublin, both expressing Chimera protein resuspended in PBS |
| 8 | Inactivated ETEC B41Arg and Salmonella Dublin expressing Chimera proteins + BRoVA UK and BCoVB Mebus | 1.108 CFU of inactivated ETEC B41Arg and Salmonella Dublin expressing Chimera proteins resuspended with 1.107 FFU BRoVA UK and BCoVB Mebus |
| (B) | ||
| Groups of guinea pigs | Treatments | Details |
| Control | Control | 1 mL of PBS |
| Vaccinated | Inactivated ETEC B41Arg and Salmonella Dublin expressing Chimera proteins + BRoVA UK and BCoVB Mebus | 1.108 CFU of inactivated ETEC B41Arg and Salmonella Dublin expressing Chimera proteins resuspended with 1.107 FFU BRoVA UK and BCoVB Mebus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez, H.; Vilte, D.A.; Hozbor, D.; Zurita, E.; Bottero, D.; Casabonne, M.C.; Cataldi, Á.A.; Wigdorovitz, A.; Larzábal, M. A Novel Vaccine for Bovine Diarrhea Complex Utilizing Recombinant Enterotoxigenic Escherichia coli and Salmonella Expressing Surface-Displayed Chimeric Antigens from Enterohemorrhagic Escherichia coli O157:H7. Vaccines 2025, 13, 124. https://doi.org/10.3390/vaccines13020124
Ramírez H, Vilte DA, Hozbor D, Zurita E, Bottero D, Casabonne MC, Cataldi ÁA, Wigdorovitz A, Larzábal M. A Novel Vaccine for Bovine Diarrhea Complex Utilizing Recombinant Enterotoxigenic Escherichia coli and Salmonella Expressing Surface-Displayed Chimeric Antigens from Enterohemorrhagic Escherichia coli O157:H7. Vaccines. 2025; 13(2):124. https://doi.org/10.3390/vaccines13020124
Chicago/Turabian StyleRamírez, Hernán, Daniel A. Vilte, Daniela Hozbor, Eugenia Zurita, Daniela Bottero, María C. Casabonne, Ángel A. Cataldi, Andrés Wigdorovitz, and Mariano Larzábal. 2025. "A Novel Vaccine for Bovine Diarrhea Complex Utilizing Recombinant Enterotoxigenic Escherichia coli and Salmonella Expressing Surface-Displayed Chimeric Antigens from Enterohemorrhagic Escherichia coli O157:H7" Vaccines 13, no. 2: 124. https://doi.org/10.3390/vaccines13020124
APA StyleRamírez, H., Vilte, D. A., Hozbor, D., Zurita, E., Bottero, D., Casabonne, M. C., Cataldi, Á. A., Wigdorovitz, A., & Larzábal, M. (2025). A Novel Vaccine for Bovine Diarrhea Complex Utilizing Recombinant Enterotoxigenic Escherichia coli and Salmonella Expressing Surface-Displayed Chimeric Antigens from Enterohemorrhagic Escherichia coli O157:H7. Vaccines, 13(2), 124. https://doi.org/10.3390/vaccines13020124






