Coverage and Drivers of Vaccinations in Patients with Autoimmune Rheumatic Diseases: An Italian Multicentric Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
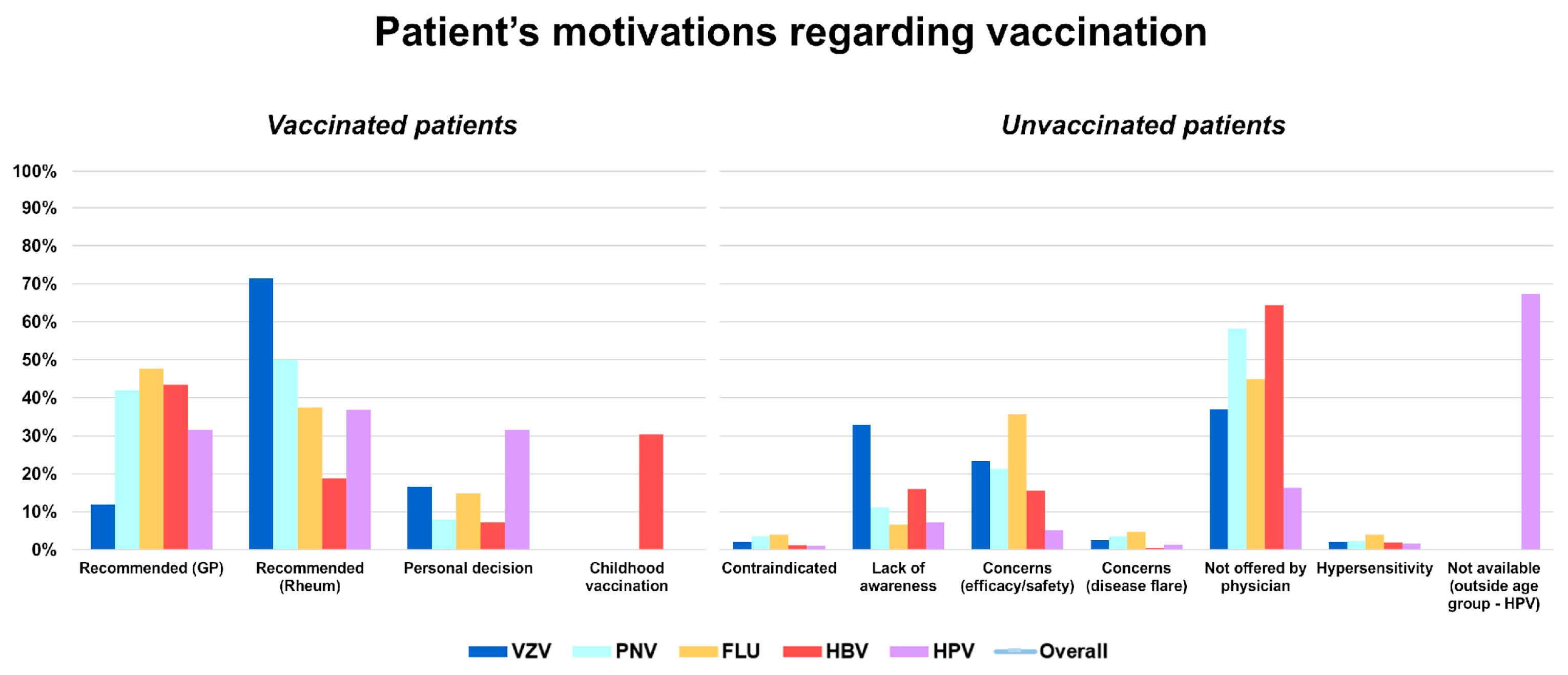
3. Results
Motivation Analysis
4. Discussion
4.1. National and International Context
4.2. Drivers of Vaccine Uptake and Barriers
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARDs | Autoimmune rheumatic diseases |
| SLE | Systemic lupus erythematosus |
| RA | Rheumatoid arthritis |
| VZV | varicella-zoster virus |
| HBV | hepatitis B virus |
| HPV | human papillomavirus |
| ACR | American College of Rheumatology |
| SIR | Italian Society of Rheumatology |
| BMI | body mass index |
| csDMARDs | conventional synthetic Disease Modifying Anti Rheumatic Drugs |
| bDMARDs | biological DMARDs |
| tsDMARDs | targeted synthetic DMARDs |
| GP | general practitioner |
| IQR | interquartile range |
| OR | odds ratios |
| CI | confidence intervals |
| AEs | adverse events |
References
- Weaver, A.; Troum, O.; Hooper, M.; Koenig, A.S.; Chaudhari, S.; Feng, J.; Wenkert, D. Rheumatoid arthritis disease activity and disability affect the risk of serious infections events in RADIUS1. J. Rheumatol. 2013, 40, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.R.; Clarke, A.E. Systemic lupus erythematosus and risk of infection. Expert Rev. Clin. Immunol. 2020, 16, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Park, S.H. Infectious complications in SLE after immunosuppressive therapies. Curr. Opin. Rheumatol. 2003, 15, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Danza, A.; Ruiz-Irastorza, G. Infection risk in systemic lupus erythematososus patients: Susceptibility factors and preventive strategies. Lupus 2013, 22, 1286–1294. [Google Scholar] [CrossRef]
- Immunization Coverage and Vaccine-Preventable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed on 21 April 2025).
- Bass, A.R.; Chakravarty, E.; Akl, E.A.; Bingham, C.O.; Calabrese, L.; Cappelli, L.C.; Johnson, S.R.; Imundo, L.F.; Winthrop, K.L.; Arasaratnam, R.J.; et al. 2022 American College of Rheumatology Guideline for Vaccinations in Patients With Rheumatic and Musculoskeletal Diseases. Arthritis Care Res. 2023, 75, 449–464. [Google Scholar] [CrossRef]
- Furer, V.; Rondaan, C.; Heijstek, M.W.; Agmon-Levin, N.; Van Assen, S.; Bijl, M.; Breedveld, F.C.; D’Amelio, R.; Dougados, M.; Kapetanovic, M.C.; et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann. Rheum. Dis. 2020, 79, 39–52. [Google Scholar] [CrossRef]
- Colmegna, I.; Useche, M.L.; Rodriguez, K.; McCormack, D.; Alfonso, G.; Patel, A.; Ramanakumar, A.V.; Rahme, E.; Bernatsky, S.; Hudson, M.; et al. Immunogenicity and safety of high-dose versus standard-dose inactivated influenza vaccine in rheumatoid arthritis patients: A randomised, double-blind, active-comparator trial. Lancet Rheumatol. 2020, 2, e14–e23. [Google Scholar] [CrossRef]
- Lenfant, T.; Jin, Y.; Kirchner, E.; A Hajj-Ali, R.; Calabrese, L.H.; Calabrese, C. Safety of recombinant zoster vaccine: A retrospective study of 622 rheumatology patients. Rheumatology 2021, 60, 5149–5157. [Google Scholar] [CrossRef]
- Dhar, J.P.; Essenmacher, L.; Dhar, R.; Magee, A.; Ager, J.; Sokol, R.J. The safety and immunogenicity of Quadrivalent HPV (qHPV) vaccine in systemic lupus erythematosus. Vaccine 2017, 35, 2642–2646. [Google Scholar] [CrossRef]
- Liao, Z.; Tang, H.; Xu, X.; Liang, Y.; Xiong, Y.; Ni, J. Immunogenicity and Safety of Influenza Vaccination in Systemic Lupus Erythematosus Patients Compared with Healthy Controls: A Meta-Analysis. PLoS ONE 2016, 11, e0147856. [Google Scholar]
- Alunno, A.; Carubbi, F.; Tan, A.L.; Sen, P.; Cavagna, L.; Joshi, M.; Day, J.; Saha, S.; Gutiérrez, C.E.T.; Caballero-Uribe, C.V.; et al. COVID-19 severity, breakthrough infections and vaccine safety in young individuals with autoimmune diseases: Insights from the COVAD study. Rheumatol. Int. 2024, 44, 1725–1731. [Google Scholar] [CrossRef]
- Sandler, D.S.; Ruderman, E.M.; Brown, T.; Lee, J.Y.; Mixon, A.; Liss, D.T.; Baker, D.W. Understanding vaccination rates and attitudes among patients with rheumatoid arthritis. Am. J. Manag. Care 2016, 22, 161–167. [Google Scholar] [PubMed]
- Nguyen, M.; Lindegaard, H.; Hendricks, O.; Friis-Møller, N. Factors associated with influenza and pneumococcal vaccine uptake among rheumatoid arthritis patients in Denmark invited to participate in a pneumococcal vaccine trial (Immunovax_RA). Scand. J. Rheumatol. 2017, 46, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Neusser, S.; Neumann, A.; Nieden, P.Z.; Speckemeier, C.; Schlierenkamp, S.; Walendzik, A.; Karbach, U.; Andreica, I.; Vaupel, K.; Baraliakos, X.; et al. Facilitators and barriers of vaccine uptake in patients with autoimune inflammatory rheumatic disease: A scoping review. RMD Open 2022, 8, e002562. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18, Erratum in Ann. Rheum. Dis. 2023, 82, e76. https://doi.org/10.1136/ard-2022-223356corr1. [Google Scholar] [CrossRef] [PubMed]
- Fanouriakis, A.; Kostopoulou, M.; Alunno, A.; Aringer, M.; Bajema, I.; Boletis, J.N.; Cervera, R.; Doria, A.; Gordon, C.; Govoni, M.; et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann. Rheum. Dis. 2019, 78, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Calendario Vaccinale Per la Vita. Available online: http://www.sitinazionale.org (accessed on 27 November 2025).
- Ministero Della Salute. Available online: https://www.salute.gov.it/ (accessed on 7 October 2025).
- Istituto Superiore di Sanità. Available online: https://www.epicentro.iss.it/ (accessed on 7 October 2025).
- World Health Organization. Global Reported Cases of Vaccine-Preventable Diseases (VPDs). Available online: https://immunizationdata.who.int/ (accessed on 7 October 2025).
- Di Valerio, Z.; La Fauci, G.; Scognamiglio, F.; Salussolia, A.; Montalti, M.; Capodici, A.; Fantini, M.P.; Odone, A.; Costantino, C.; Soldà, G.; et al. Pneumococcal vaccine uptake among high-risk adults and children in Italy: Results from the OBVIOUS project survey. BMC Public Health 2024, 24, 736. [Google Scholar] [CrossRef]
- Costello, R.; Winthrop, K.L.; Pye, S.R.; Brown, B.; Dixon, W.G. Influenza and Pneumococcal Vaccination Uptake in Patients with Rheumatoid Arthritis Treated with Immunosuppressive Therapy in the UK: A Retrospective Cohort Study Using Data from the Clinical Practice Research Datalink. PLoS ONE 2016, 11, e0153848. [Google Scholar] [CrossRef]
- Furer, V.; Weil, C.; Chodik, G.; Slav, S.A.; Blonder, S.N.; Fisher-Shoval, Y.; Barak, M.; Elkayam, O. Real-World Coverage With Influenza, Pneumococcal, and Herpes Zoster Vaccines Among Patients With Rheumatic Diseases in a Nationwide Healthcare Plan. J. Rheumatol. 2024, 51, 505–516. [Google Scholar] [CrossRef]
- Hmamouchi, I.; Winthrop, K.; Launay, O.; Dougados, M. Low rate of influenza and pneumococcal vaccine coverage in rheumatoid arthritis: Data from the international COMORA cohort. Vaccine 2015, 33, 1446–1452. [Google Scholar] [CrossRef]
- McCarthy, E.M.; de Barra, E.; Bergin, C.; Cunnane, G.; Doran, M. Influenza and pneumococcal vaccination and varicella status in inflammatory arthritis patients. Ir. Med. J. 2011, 104, 208–211. [Google Scholar]
- Salussolia, A.; Capodici, A.; Scognamiglio, F.; La Fauci, G.; Soldà, G.; Montalti, M.; Di Valerio, Z.; Fantini, M.P.; Odone, A.; Costantino, C.; et al. Herpes zoster (HZ) vaccine coverage and confidence in Italy: A Nationwide cross-sectional study, the OBVIOUS project. BMC Infect. Dis. 2024, 24, 438. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, A.; Tamarri, F.; Angelini, R.; Bakken, E.; Concari, I.; Giannoccaro, E.; Domeniconi, G.; Morri, M.; Reali, C.; Righi, F.; et al. Herpes Zoster Vaccine Uptake and Active Campaign Impact, a Multicenter Retrospective Study in Italy. Vaccines 2024, 12, 51. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abada, S.; Li, J.; Tarasovsky, G.; Wilson, C.; Yazdany, J.; Whooley, M.A.; Schmajuk, G. Recombinant Zoster Vaccination Among US Veterans Receiving Immunosuppressive Medications. JAMA Netw. Open 2024, 7, e2439945. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; Tanaka, Y.; Lee, E.B.; Wollenhaupt, J.; Al Enizi, A.; Azevedo, V.F.; Curtis, J.R. Prevention and management of herpes zoster in patients with rheumatoid arthritis and psoriatic arthritis: A clinical review. Clin. Exp. Rheumatol. 2022, 40, 162–172. [Google Scholar] [CrossRef]
- Gatwood, J.; McGuiness, C.; Yasuda, M.; Chen, C.; Gupta, V.; Stempniewicz, N. Recombinant zoster vaccine uptake in US adults with rheumatic disease: A mixed methods analysis. Arthritis Rheumatol. 2024, 76 (Suppl. 9), 3514–3515. [Google Scholar]
- Krasselt, M.; Wagner, U.; Seifert, O. Influenza, Pneumococcal and Herpes Zoster Vaccination Rates in Patients with Autoimmune Inflammatory Rheumatic Diseases. Vaccines 2023, 11, 760. [Google Scholar] [CrossRef]
- Curtis, J.R.; Conrad, D.M.; Krueger, W.S.; Gara, A.P.; Winthrop, K.L. Real-world data on the use of the Shingrix vaccine among patients with inflammatory arthritis and risk of cardiovascular events following herpes zoster. Arthritis Res. Ther. 2025, 27, 108. [Google Scholar] [CrossRef]
- Available online: https://www.salute.gov.it/new/it/banche-dati/vaccinazione-contro-il-papilloma-virus-hpv-coperture-vaccinali/ (accessed on 21 April 2025).
- Goulenok, T.; Sacré, K. Human papillomavirus et lupus systémique: Une revue systématique [Human papillomavirus and systemic lupus erythematosus: A systematic review]. La Rev. Med. Interne 2025, 46, 164–173. [Google Scholar] [CrossRef]
- Dhar, J.P.; Essenmacher, L.; Dhar, R.; Ragina, N.; Sokol, R.J. Lack of Uptake of Prophylactic Human Papilloma Virus Vaccine Among Women With Systemic Lupus Erythematosus Seen at a Regional Medical Center. J. Clin. Rheumatol. 2019, 25, 348–350. [Google Scholar] [CrossRef]
- Feldman, C.H.; Hiraki, L.T.; Lii, H.; Seeger, J.D.; Kim, S.C. Human papillomavirus vaccine uptake among individuals with systemic inflammatory diseases. PLoS ONE 2015, 10, e0117620. [Google Scholar] [CrossRef] [PubMed]
- Lyrio, L.D.; Grassi, M.F.R.; Santana, I.U.; Olavarria, V.G.; Gomes, A.D.N.; CostaPinto, L.; Oliveira, R.P.C.; Aquino, R.D.C.R.; Santiago, M.B. Prevalence of cervical human papillomavirus infection in women with systemic lupus erythematosus. Rheumatol. Int. 2013, 33, 335–340. [Google Scholar] [PubMed]
- Klumb, E.; Pinto, A.; Jesus, G.; Araujo, M.; Jascone, L.; Gayer, C.; Ribeiro, F.; Albuquerque, E.; Macedo, J. Are women with lupus at higher risk of HPV infection? Lupus 2010, 19, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Stroffolini, T.; Lombardi, A.; Ciancio, A.; Fontana, R.; Colloredo, G.; Marignani, M.; Vinci, M.; Morisco, F.; Babudieri, S.; Ferrigno, L.; et al. Hepatitis B vaccine coverage and risk factors for lack of vaccination in subjects with HBsAg negative liver cirrhosis in Italy: Still, much work should be done. Dig. Liver Dis. 2021, 53, 1315–1319. [Google Scholar] [CrossRef]
- Qendro, T.; de la Torre, M.L.; Panopalis, P.; Hazel, E.; Ward, B.J.; Colmegna, I.; Hudson, M. Suboptimal Immunization Coverage among Canadian Rheumatology Patients in Routine Clinical Care. J. Rheumatol. 2020, 47, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Tsiligianni, I.; Bouloukaki, I.; Papazisis, G.; Paganas, A.; Chatzimanolis, E.; Kalatharas, M.; Platakis, I.; Tirodimos, I.; Dardavesis, T.; Tsimtsiou, Z. Vaccination coverage and predictors of influenza, pneumococcal, herpes zoster, tetanus, measles, and hepatitis B vaccine uptake among adults in Greece. Public Health 2023, 224, 195–202. [Google Scholar] [CrossRef]
- Kirik, A.; Şahin, N.; Baykul, M.; Bodur, H.; Güler, T.; Çevik, R.; Uğur, S.; Durmaz, Y.; Karahan, A.Y.; Devrimsel, G.; et al. Low vaccination rates and awareness status in patients with rheumatoid arthritis: A nationwide cross-sectional survey study. Rheumatol. Int. 2025, 45, 116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okoli, G.N.; Moon, A.G.; Soos, A.E.; Neilson, C.J.; Harper, D.M. Hepatitis B vaccination initiation and vaccination series completion: An in-depth systematic evidence review, with meta-analysis of associations with individual socioeconomic and health-related factors. Vaccine 2025, 55, 127051. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.; McBain, L.; Grainger, R. Rheumatologists fail to advise people with RA to get immunised, which matters if you are under 65: An audit in a New Zealand rheumatology service. N. Z. Med. J. 2016, 129, 72–78. [Google Scholar]
- Lawson, E.F.; Trupin, L.; Yelin, E.H.; Yazdany, J. Reasons for failure to receive pneumococcal and influenza vaccinations among immunosuppressed patients with systemic lupus erythematosus. Semin. Arthritis Rheum. 2015, 44, 666–671. [Google Scholar] [CrossRef]
- Hua, C.; Morel, J.; Ardouin, E.; Ricard, E.; Foret, J.; Mathieu, S.; Combe, B.; Lukas, C. Reasons for non-vaccination in French rheumatoid arthritis and spondyloarthritis patients. Rheumatology 2015, 54, 748–750. [Google Scholar] [CrossRef]
- Brocq, O.; Acquacalda, E.; Berthier, F.; Albert, C.; Bolla, G.; Millasseau, E.; Destombe, C.; Azulay, J.; Asquier, C.; Florent, A.; et al. Influenza and pneumococcal vaccine coverage in 584 patients taking biological therapy for chronic inflammatory joint: A retrospective study. Jt. Bone Spine 2016, 83, 155–159. [Google Scholar] [CrossRef]
- Miyake, H.; Sada, R.M.; Tsugihashi, Y.; Hatta, K. Single-centre, cross-sectional study on the factors and reasons for non-vaccination among patients with rheumatoid arthritis. Mod. Rheumatol. 2023, 34, 79–86. [Google Scholar] [CrossRef]
- Kojima, S.; Iwamoto, T.; Kobayashi, Y.; Kato, M.; Takizawa, F.; Ida, T.; Suzuki, J.; Toda, Y.; Miyachi, K.; Iwata, A.; et al. Immunogenicity and influence on disease activity of recombinant zoster vaccine in patients with rheumatoid arthritis treated with DMARDs. RMD Open 2024, 10, e003902. [Google Scholar] [CrossRef] [PubMed]


| Overall N = 325 | RA N = 226 | SLE N = 99 | p-Value | |
|---|---|---|---|---|
| Gender, (%) | 0.006 | |||
| Females | 275 (84.6%) | 183 (81.0%) | 92 (92.9%) | |
| Males | 50 (15.4%) | 43 (19.0%) | 7 (7.1%) | |
| Age, yrs, median [IQR] | 60.0 [50.0–70.0] | 64.0 [55.0–74.0] | 51.0 [41.0–59.0] | <0.001 |
| Group 18–49, (%) | 77 (23.7%) | 33 (14.6%) | 44 (44.4%) | |
| Group 50–64, (%) | 131 (40.3%) | 84 (37.2%) | 47 (47.5%) | |
| Group ≥ 65, (%) | 117 (36.0%) | 109 (48.2%) | 8 (8.1%) | |
| BMI, median [IQR] | 24.2 [22.4–27.2] | 24.3 [22.5–27.2] | 24.1 [21.7–27.4] | 0.376 |
| Smoking status, (%) | 0.985 | |||
| Never | 209 (64.3%) | 146 (64.6%) | 63 (63.6%) | |
| Previous | 60 (18.5%) | 41 (18.1%) | 19 (19.2%) | |
| Current | 56 (17.2%) | 39 (17.3%) | 17 (17.2%) | |
| Education level, (%) | <0.001 | |||
| Primary school | 120 (37.0%) | 99 (43.8%) | 21 (21.4%) | |
| High school diploma | 136 (42.0%) | 91 (40.3%) | 45 (45.9%) | |
| University degree | 68 (21.0%) | 36 (15.9%) | 32 (32.7%) | |
| Unknown | 1 | 0 | 1 | |
| Disease Activity a, (%) | n.a. | |||
| Remission/Low | 252 (79.5%) | 174 (78.4%) | 78 (82.1%) | |
| Moderate/High | 65 (20.5%) | 48 (21.6%) | 17 (17.9%) | |
| Unknown | 8 | 4 | 4 | |
| Comorbidities, (%) | 0.224 | |||
| Absent | 137 (42.2%) | 90 (39.8%) | 47 (47.5%) | |
| Present | 188 (57.8%) | 136 (60.2%) | 52 (52.5%) | |
| Immunosuppression level, (%) | <0.001 | |||
| Mild | 68 (21.1%) | 17 (7.6%) | 51 (52.0%) | |
| Moderate | 36 (11.2%) | 23 (10.3%) | 13 (13.3%) | |
| High | 218 (67.7%) | 184 (82.1%) | 34 (34.7%) | |
| Unknown | 3 | 2 | 1 |
| Vaccine by Gender | RA | SLE | ||||||
|---|---|---|---|---|---|---|---|---|
| Males N = 43 | Females N = 183 | p Value | Males N = 7 | Females N = 92 | p Value | |||
| VZV, (%) | 8 (18.6%) | 26 (14.2%) | 0.625 | 2 (28.6%) | 6 (6.5%) | 0.098 | ||
| HPV, (%) | - | 9 (4.9%) | 0.213 | - | 10 (10.9%) | >0.999 | ||
| PNV, (%) | 16 (37.2%) | 66 (36.1%) | >0.999 | 1 (14.3%) | 17 (18.5%) | >0.999 | ||
| FLU, (%) | 27 (62.8%) | 109 (59.6%) | 0.829 | 2 (28.6%) | 38 (41.3%) | 0.698 | ||
| HBV, (%) | 4 (9.3%) | 32 (17.5%) | 0.249 | 4 (57.1%) | 29 (31.5%) | 0.217 | ||
| Vaccine by age group | Age 18–49 N = 33 | Age 50–64 N = 84 | Age ≥ 65 N = 109 | p -value | Age 18–49 N = 44 | Age 50–64 N = 47 | Age ≥ 65 N = 8 | p -value |
| VZV, (%) | 6 (18.2%) | 10 (11.9%) | 18 (16.5%) | 0.581 | 2 (4.5%) | 5 (10.6%) | 1 (12.5%) | 0.426 |
| HPV, (%) | 8 (24.2%) | 1 (1.2%) | - | <0.001 | 9 (20.5%) | 1 (2.1%) | - | 0.011 |
| PNV, (%) | 5 (15.2%) | 21 (25.0%) | 56 (51.4%) | <0.001 | 6 (13.6%) | 8 (17.0%) | 4 (50.0%) | 0.071 |
| FLU, (%) | 10 (30.3%) | 41 (48.8%) | 85 (78.0%) | <0.001 | 10 (22.7%) | 24 (51.1%) | 6 (75.0%) | 0.001 |
| HBV, (%) | 17 (51.5%) | 8 (9.5%) | 11 (10.1%) | <0.001 | 26 (59.1%) | 6 (12.8%) | 1 (12.5%) | <0.001 |
| Vaccine by education level | Primary school N = 99 | High school N = 91 | University N = 36 | p -value | Primary school N = 21 | High school N = 45 | University N = 32 | p -value |
| VZV, (%) | 15 (15.2%) | 13 (14.3%) | 6 (16.7%) | 0.944 | 2 (9.5%) | 4 (8.9%) | 2 (6.2%) | >0.999 |
| HPV, (%) | 2 (2.0%) | 5 (5.5%) | 2 (5.6%) | 0.393 | 1 (4.8%) | 5 (11.1%) | 4 (12.5%) | 0.758 |
| PNV, (%) | 45 (45.5%) | 24 (26.4%) | 13 (36.1%) | 0.024 | 7 (33.3%) | 6 (13.3%) | 5 (15.6%) | 0.131 |
| FLU, (%) | 65 (65.7%) | 49 (53.8%) | 22 (61.1%) | 0.250 | 12 (57.1%) | 13 (28.9%) | 15 (46.9%) | 0.065 |
| HBV, (%) | 7 (7.1%) | 20 (22.0%) | 9 (25.0%) | 0.005 | 2 (9.5%) | 16 (35.6%) | 15 (46.9%) | 0.011 |
| Vaccine by immunosuppression level | Mild N = 17 | Moderate N = 23 | High N = 184 | p -value | Mild N = 51 | Moderate N = 13 | High N = 34 | p -value |
| VZV, (%) | 4 (23.5%) | 1 (4.3%) | 28 (15.2%) | 0.257 | 5 (9.8%) | - | 3 (8.8%) | 0.773 |
| HPV, (%) | - | - | 9 (4.9%) | 0.806 | 5 (9.8%) | - | 5 (14.7%) | 0.367 |
| PNV, (%) | 7 (41.2%) | 8 (34.8%) | 66 (35.9%) | 0.900 | 9 (17.6%) | - | 9 (26.5%) | 0.094 |
| FLU, (%) | 11 (64.7%) | 15 (65.2%) | 109 (59.2%) | 0.796 | 23 (45.1%) | 5 (38.5%) | 12 (35.3%) | 0.655 |
| HBV, (%) | 4 (23.5%) | 3 (13.0%) | 28 (15.2%) | 0.635 | 18 (35.3%) | 5 (38.5%) | 10 (29.4%) | 0.791 |
| Not Offered N = 226 | Unaware N = 42 | Skeptical N = 47 | p Value | |
|---|---|---|---|---|
| Diagnosis | ||||
| RA | 161 (71.2%) | 24 (57.1%) | 34 (72.3%) | 0.171 |
| SLE | 65 (28.8%) | 18 (42.9%) | 13 (27.7%) | |
| Gender | ||||
| Males | 34 (15.0%) | 8 (19.0%) | 6 (12.8%) | 0.704 |
| Females | 192 (85.0%) | 34 (81.0%) | 41 (87.2%) | |
| Age (years) | ||||
| Median age [IQR] | 61.0 [49.2–73.0] | 56.5 [50.2–64.0] | 60.0 [52.5–67.0] | 0.224 |
| 18–49 | 57 (25.2%) | 9 (21.4%) | 9 (19.1%) | 0.017 |
| 50–64 | 78 (34.5%) | 25 (59.5%) | 23 (48.9%) | |
| ≥65 | 91 (40.3%) | 8 (19.0%) | 15 (31.9%) | |
| BMI (kg/m2) | ||||
| Median BMI [IQR] | 24.1 [22.3–27.3] | 23.6 [20.9–26.4] | 24.9 [23.3–26.7] | 0.088 |
| Education level | ||||
| Primary school | 81 (36.0%) | 13 (31.0%) | 22 (46.8%) | 0.509 |
| High School | 97 (43.1%) | 18 (42.9%) | 18 (38.3%) | |
| University | 47 (20.9%) | 11 (26.2%) | 7 (14.9%) | |
| Unknown | 1 | 0 | 0 | |
| Immunosuppression level | ||||
| Mild | 42 (18.8%) | 14 (33.3%) | 10 (21.3%) | 0.124 |
| Moderate | 22 (9.8%) | 5 (11.9%) | 8 (17.0%) | |
| High | 160 (71.4%) | 23 (54.8%) | 29 (61.7%) | |
| Unknown | 2 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellofatto, I.A.; Paci, V.; Conti, F.; Santoboni, G.; Sebastiani, G.D.; Cattaruzza, M.S.; Mazzanti, C.; Salemi, S.; Sesti, G.; Tesoriere, E.; et al. Coverage and Drivers of Vaccinations in Patients with Autoimmune Rheumatic Diseases: An Italian Multicentric Study. Vaccines 2025, 13, 1229. https://doi.org/10.3390/vaccines13121229
Bellofatto IA, Paci V, Conti F, Santoboni G, Sebastiani GD, Cattaruzza MS, Mazzanti C, Salemi S, Sesti G, Tesoriere E, et al. Coverage and Drivers of Vaccinations in Patients with Autoimmune Rheumatic Diseases: An Italian Multicentric Study. Vaccines. 2025; 13(12):1229. https://doi.org/10.3390/vaccines13121229
Chicago/Turabian StyleBellofatto, Ilaria Anna, Valentino Paci, Fabrizio Conti, Gianluca Santoboni, Gian Domenico Sebastiani, Maria Sofia Cattaruzza, Camilla Mazzanti, Simonetta Salemi, Giorgio Sesti, Emanuele Tesoriere, and et al. 2025. "Coverage and Drivers of Vaccinations in Patients with Autoimmune Rheumatic Diseases: An Italian Multicentric Study" Vaccines 13, no. 12: 1229. https://doi.org/10.3390/vaccines13121229
APA StyleBellofatto, I. A., Paci, V., Conti, F., Santoboni, G., Sebastiani, G. D., Cattaruzza, M. S., Mazzanti, C., Salemi, S., Sesti, G., Tesoriere, E., Fiorilli, V., Prevete, I., Spinelli, F. R., & Picchianti Diamanti, A. (2025). Coverage and Drivers of Vaccinations in Patients with Autoimmune Rheumatic Diseases: An Italian Multicentric Study. Vaccines, 13(12), 1229. https://doi.org/10.3390/vaccines13121229






