Building Vaccine Readiness for Future Pandemics: Insights from COVID-19 Vaccine Intent and Uptake
Abstract
1. Introduction
1.1. COVID-19 Vaccine Development and Uptake
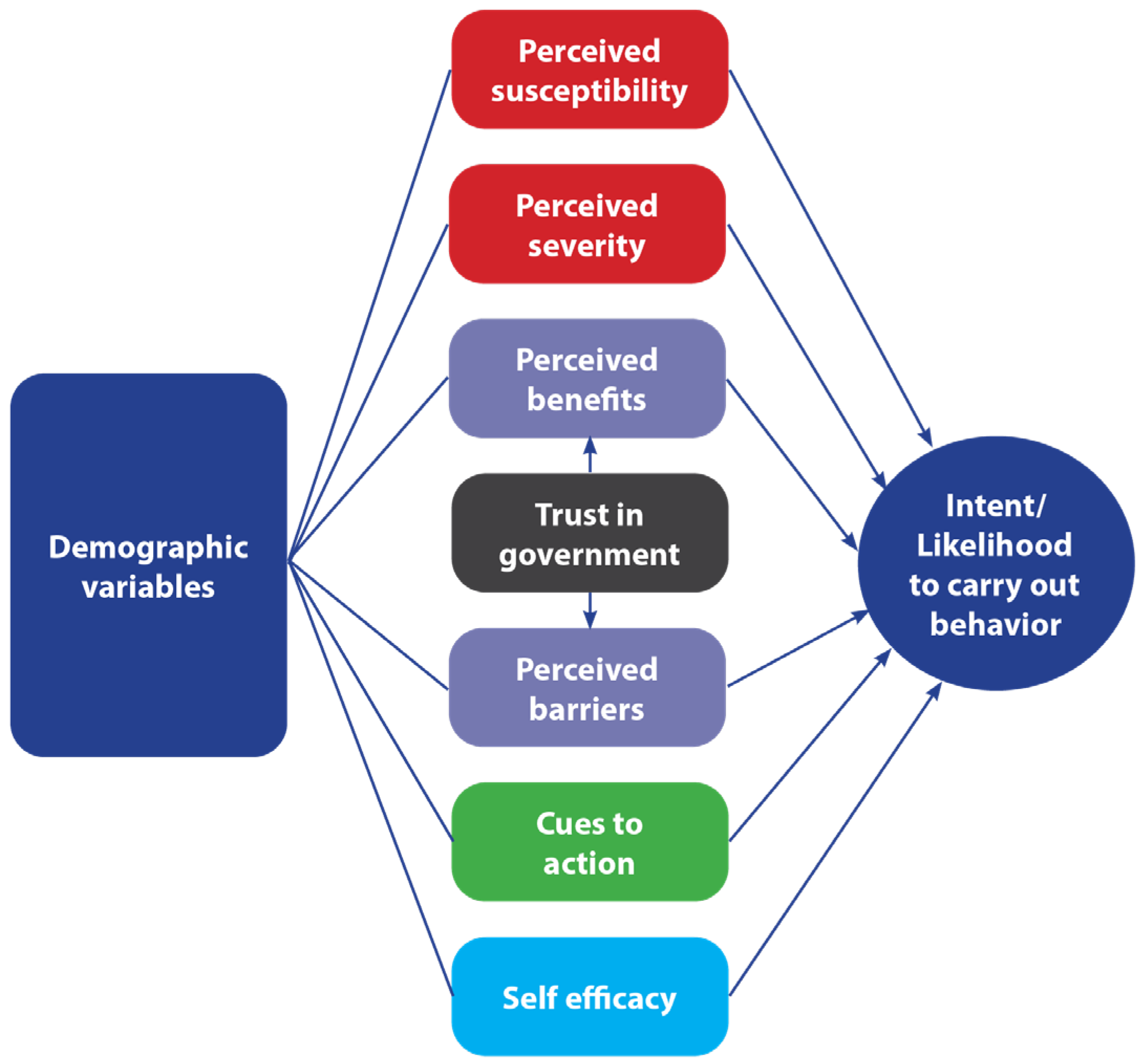
1.2. Theoretical Explanations for Vaccine Behavior
1.3. Study Hypotheses
2. Materials and Methods
2.1. Measures
2.2. Statistical Approach
3. Results
3.1. Bivariate Analyses
3.2. Psychosocial Predictors of Vaccine Intention/Uptake
3.3. Differences in HBM Constructs and Trust in Government over Time
4. Discussion
4.1. Implications
4.2. Strengths, Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention |
| HBM | Health Belief Model |
| WHO | World Health Organization |
References
- Marani, M.; Katul, G.G.; Pan, W.K.; Parolari, A.J. Intensity and frequency of extreme novel epidemics. Proc. Natl. Acad. Sci. USA 2021, 118, e2105482118. [Google Scholar] [CrossRef]
- Morens, D.M.; Fauci, A.S. Emerging pandemic diseases: How we got to COVID-19. Cell 2020, 182, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- Baumer-Mouradian, S.H.; Hofstetter, A.M.; O’Leary, S.T.; Opel, D.J. Vaccine Confidence as Critical to Pandemic Preparedness and Response. Pediatr. Clin. 2024, 71, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.C.; Monto, A.S.; Chan, P.K.; Szucs, T.D.; Nicholson, K.G. Stockpiling prepandemic influenza vaccines: A new cornerstone of pandemic preparedness plans. Lancet Infect. Dis. 2008, 8, 650–658. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 13 May 2022).
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar]
- McCarthy, T. Just Half of Americans Plan on Getting COVID-19 Vaccine, Poll Shows. Available online: https://www.theguardian.com/world/2020/may/27/americans-covid-19-vaccine-poll (accessed on 28 May 2020).
- Elbeshbishi, S.; King, L. Exclusive: Two-Thirds of Americans Say They Won’t Get COVID-19 Vaccine When It’s First Available, USA TODAY/Suffolk Poll Shows. Available online: https://www.usatoday.com/story/news/politics/2020/09/04/covid-19-two-thirds-us-wont-take-vaccine-right-away-poll-shows/5696982002/ (accessed on 5 September 2020).
- Neumann-Böhme, S.; Varghese, N.E.; Sabat, I.; Barros, P.P.; Brouwer, W.; van Exel, J.; Schreyögg, J.; Stargardt, T. Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. Eur. J. Health Econ. 2020, 21, 977. [Google Scholar] [CrossRef]
- Tiruneh, Y.M.; Cuccaro, P.M.; Elliott, K.S.; Xie, J.; Martinez, J.; Owens, M.; Alvarado, C.R.; Yamal, J.-M. Vaccine uptake and intentions: Insights from a texas survey on factors influencing COVID-19 vaccination decisions. Vaccines 2024, 12, 601. [Google Scholar] [CrossRef]
- Pijpers, J.; van Roon, A.; van Roekel, C.; Labuschagne, L.; Smagge, B.; Ferreira, J.A.; de Melker, H.; Hahné, S. Determinants of COVID-19 vaccine uptake in The Netherlands: A nationwide registry-based study. Vaccines 2023, 11, 1409. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Vaccination Coverage among Adults in the United States, National Health Interview Survey, 2021. Available online: https://www.cdc.gov/adultvaxview/publications-resources/vaccination-coverage-adults-2021.html (accessed on 24 July 2025).
- Daly, M.; Jones, A.; Robinson, E. Public trust and willingness to vaccinate against COVID-19 in the US from October 14, 2020, to March 29, 2021. Jama 2021, 325, 2397–2399. [Google Scholar] [CrossRef]
- Lazarus, J.V.; White, T.M.; Wyka, K.; Ratzan, S.C.; Rabin, K.; Larson, H.J.; Martinon-Torres, F.; Kuchar, E.; Abdool Karim, S.S.; Giles-Vernick, T. Influence of COVID-19 on trust in routine immunization, health information sources and pandemic preparedness in 23 countries in 2023. Nat. Med. 2024, 30, 1559–1563. [Google Scholar] [CrossRef]
- Vaughan, E.; Tinker, T. Effective health risk communication about pandemic influenza for vulnerable populations. Am. J. Public Health 2009, 99, S324–S332. [Google Scholar] [CrossRef]
- Quinn, S.C.; Parmer, J.; Freimuth, V.S.; Hilyard, K.M.; Musa, D.; Kim, K.H. Exploring communication, trust in government, and vaccination intention later in the 2009 H1N1 pandemic: Results of a national survey. Biosecur. Bioterror. Biodef. Strat. Pract. Sci. 2013, 11, 96–106. [Google Scholar] [CrossRef]
- Blendon, R.J.; Koonin, L.M.; Benson, J.M.; Cetron, M.S.; Pollard, W.E.; Mitchell, E.W.; Weldon, K.J.; Herrmann, M.J. Public response to community mitigation measures for pandemic influenza. Emerg. Infect. Dis. 2008, 14, 778. [Google Scholar] [CrossRef] [PubMed]
- Grygarová, D.; Kožený, J.; Tišanská, L.; Havlík, M.; Horáček, J. Trust in official information as a key predictor of COVID-19 vaccine acceptance: Evidence from a Czech longitudinal survey study. BMC Public Health 2025, 25, 770. [Google Scholar] [CrossRef] [PubMed]
- Zilinsky, J.; Theocharis, Y. Conspiracism and government distrust predict COVID-19 vaccine refusal. Humanit. Soc. Sci. Commun. 2025, 12, 1002. [Google Scholar] [CrossRef]
- Bajos, N.; Spire, A.; Silberzan, L.; Sireyjol, A.; Jusot, F.; Meyer, L.; Franck, J.-E.; Warszawski, J.; Group, E.S. When lack of trust in the government and in scientists reinforces social inequalities in vaccination against COVID-19. Front. Public Health 2022, 10, 908152. [Google Scholar] [CrossRef]
- Phillips, R.; Gillespie, D.; Hallingberg, B.; Evans, J.; Taiyari, K.; Torrens-Burton, A.; Cannings-John, R.; Williams, D.; Sheils, E.; Ashfield-Watt, P. Perceived threat of COVID-19, attitudes towards vaccination, and vaccine hesitancy: A prospective longitudinal study in the UK. Br. J. Health Psychol. 2022, 27, 1354–1381. [Google Scholar] [CrossRef]
- Myers, L.B.; Goodwin, R. Determinants of adults’ intention to vaccinate against pandemic swine flu. BMC Public Health 2011, 11, 15. [Google Scholar] [CrossRef] [PubMed]
- Gerend, M.A.; Shepherd, J.E. Predicting human papillomavirus vaccine uptake in young adult women: Comparing the health belief model and theory of planned behavior. Ann. Behav. Med. 2012, 44, 171–180. [Google Scholar] [CrossRef]
- Mercadante, A.R.; Law, A.V. Will they, or Won’t they? Examining patients’ vaccine intention for flu and COVID-19 using the Health Belief Model. Res. Soc. Adm. Pharm. 2021, 17, 1596–1605. [Google Scholar] [CrossRef]
- Smith, P.J.; Humiston, S.G.; Marcuse, E.K.; Zhao, Z.; Dorell, C.G.; Howes, C.; Hibbs, B. Parental delay or refusal of vaccine doses, childhood vaccination coverage at 24 months of age, and the Health Belief Model. Public Health Rep. 2011, 126, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Guidry, J.P.; Laestadius, L.I.; Vraga, E.K.; Miller, C.A.; Perrin, P.B.; Burton, C.W.; Ryan, M.; Fuemmeler, B.F.; Carlyle, K.E. Willingness to get the COVID-19 vaccine with and without emergency use authorization. Am. J. Infect. Control 2021, 49, 137–142. [Google Scholar] [CrossRef]
- Shmueli, L. Predicting intention to receive COVID-19 vaccine among the general population using the health belief model and the theory of planned behavior model. BMC Public Health 2021, 21, 804. [Google Scholar] [CrossRef]
- Limbu, Y.B.; Gautam, R.K.; Pham, L. The health belief model applied to COVID-19 vaccine hesitancy: A systematic review. Vaccines 2022, 10, 973. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Alam, M.Z.; Islam, M.S.; Sultan, S.; Faysal, M.M.; Rima, S.; Hossain, M.A.; Mamun, A.A. Health belief model, theory of planned behavior, or psychological antecedents: What predicts COVID-19 vaccine hesitancy better among the Bangladeshi adults? Front. Public Health 2021, 9, 711066. [Google Scholar] [CrossRef]
- Vírseda, S.; Restrepo, M.A.; Arranz, E.; Magán-Tapia, P.; Fernández-Ruiz, M.; de la Cámara, A.G.; Aguado, J.M.; López-Medrano, F. Seasonal and Pandemic A (H1N1) 2009 influenza vaccination coverage and attitudes among health-care workers in a Spanish University Hospital. Vaccine 2010, 28, 4751–4757. [Google Scholar] [CrossRef]
- Rodas, J.; Lau, C.; Zhang, Z.; Griffiths, S.; Luk, W.; Kim, J. Exploring predictors influencing intended and actual acceptability of the A/H1N1 pandemic vaccine: A cohort study of university students in Hong Kong. Public Health 2012, 126, 1007–1012. [Google Scholar] [CrossRef]
- To, K.-W.; Lee, S.; Chan, T.-O.; Lee, S.-S. Exploring determinants of acceptance of the pandemic influenza A (H1N1) 2009 vaccination in nurses. Am. J. Infect. Control 2010, 38, 623–630. [Google Scholar] [CrossRef]
- Peters, R.G.; Covello, V.T.; McCallum, D.B. The determinants of trust and credibility in environmental risk communication: An empirical study. Risk Anal. 1997, 17, 43–54. [Google Scholar] [CrossRef]
- Quinn, S.C.; Kumar, S.; Freimuth, V.S.; Kidwell, K.; Musa, D. Public Willingness to Take a Vaccine or Drug Under Emergency Use Authorization during the 2009 H1N1 pandemic. Biosecur. Bioterror. Biodef. Strat. Pract. Sci. 2009, 7, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Coe, A.B.; Gatewood, S.B.; Moczygemba, L.R. The use of the health belief model to assess predictors of intent to receive the novel (2009) H1N1 influenza vaccine. Innov. Pharm. 2012, 3, 1. [Google Scholar] [CrossRef]
- Dailey, H. Can Transparency Save Trust? The State of U.S. Public Confidence in 2024. Available online: https://publicsectornetwork.com/insight/can-transparency-save-trust-the-state-of-us-public-confidence-in-2024 (accessed on 22 July 2025).
- Yaghi, A. Longitudinal examination of trust in public administration during the COVID-19 pandemic. Public Integr. 2024, 26, 156–173. [Google Scholar] [CrossRef]
- Jennings, W.; Valgarðsson, V.; McKay, L.; Stoker, G.; Mello, E.; Baniamin, H.M. Trust and vaccine hesitancy during the COVID-19 pandemic: A cross-national analysis. Vaccine X 2023, 14, 100299. [Google Scholar] [CrossRef]
- Karlsson, L.C.; Soveri, A.; Lewandowsky, S.; Karlsson, L.; Karlsson, H.; Nolvi, S.; Karukivi, M.; Lindfelt, M.; Antfolk, J. Fearing the disease or the vaccine: The case of COVID-19. Personal. Individ. Differ. 2021, 172, 110590. [Google Scholar] [CrossRef]
- Dubé, E.; Laberge, C.; Guay, M.; Bramadat, P.; Roy, R.; Bettinger, J.A. Vaccine hesitancy: An overview. Hum. Vaccines Immunother. 2013, 9, 1763–1773. [Google Scholar] [CrossRef]
- Larson, H.J.; Cooper, L.Z.; Eskola, J.; Katz, S.L.; Ratzan, S. Addressing the vaccine confidence gap. Lancet 2011, 378, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, M.; Zingg, A. The role of public trust during pandemics. Eur. Psychol. 2014, 19, 23–32. [Google Scholar] [CrossRef]
- Quinn, S.C.; Jamison, A.; Freimuth, V.S.; An, J.; Hancock, G.R.; Musa, D. Exploring racial influences on flu vaccine attitudes and behavior: Results of a national survey of White and African American adults. Vaccine 2017, 35, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Goldfinch, S.; Taplin, R.; Gauld, R. Trust in government increased during the COVID-19 pandemic in Australia and New Zealand. Aust. J. Public Adm. 2021, 80, 3–11. [Google Scholar] [CrossRef]
- Liu, J.; Shahab, Y.; Hoque, H. Government response measures and public trust during the COVID-19 pandemic: Evidence from around the world. Br. J. Manag. 2022, 33, 571–602. [Google Scholar] [CrossRef]
- Parsons, S.; Wiggins, R.D. Trust in Government and Others During the COVID-19 Pandemic–Initial Findings from the COVID-19 Survey in Five National Longitudinal Studies; UCL Centre for Longitudinal Studies: London, UK, 2020. [Google Scholar]
- Melchinger, H.; Omer, S.B.; Malik, A.A. Change in confidence in public health entities among US adults between 2020–2024. PLOS Glob. Public Health 2025, 5, e0004747. [Google Scholar] [CrossRef]
- Pew Research Center. Views of the Incoming Biden Administration. Available online: https://www.pewresearch.org/politics/2021/01/15/views-of-the-incoming-biden-administration/ (accessed on 22 July 2025).
- Yang, D.; Wagner, A.L.; Gorin, S.S. Perceived severity of COVID-19 in a longitudinal study in Detroit, Michigan. Ethn. Dis. 2022, 32, 231. [Google Scholar] [CrossRef]
- Jones, C.H.; Jenkins, M.P.; Adam Williams, B.; Welch, V.L.; True, J.M. Exploring the future adult vaccine landscape—Crowded schedules and new dynamics. npj Vaccines 2024, 9, 27. [Google Scholar] [CrossRef]
- Thaker, J.; Ganchoudhuri, S. The role of attitudes, norms, and efficacy on shifting COVID-19 vaccine intentions: A longitudinal study of COVID-19 vaccination intentions in New Zealand. Vaccines 2021, 9, 1132. [Google Scholar] [CrossRef]
- Grills, L.A.; Wagner, A.L. The impact of the COVID-19 pandemic on parental vaccine hesitancy: A cross-sectional survey. Vaccine 2023, 41, 6127–6133. [Google Scholar] [CrossRef]
- de Figueiredo, A.; Eagan, R.; Hendrickx, G.; Karafillakis, E.; Van Damme, P.; Larson, H. State of Vaccine Confidence in the European Union; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Bavel, J.J.V.; Baicker, K.; Boggio, P.S.; Capraro, V.; Cichocka, A.; Cikara, M.; Crockett, M.J.; Crum, A.J.; Douglas, K.M.; Druckman, J.N. Using social and behavioural science to support COVID-19 pandemic response. Nat. Hum. Behav. 2020, 4, 460–471. [Google Scholar] [CrossRef]
- Edelman Trust Institute. Edelman Trust Barometer 2025. Available online: https://www.edelman.com/sites/g/files/aatuss191/files/2025-01/2025%20Edelman%20Trust%20Barometer%20Global%20Report_01.23.25.pdf (accessed on 22 July 2025).
- Caputo, A. Social desirability bias in self-reported well-being measures: Evidence from an online survey. Univ. Psychol. 2017, 16, 245–255. [Google Scholar] [CrossRef]
- Van de Mortel, T.F. Faking it: Social desirability response bias in self-report research. Aust. J. Adv. Nurs. 2008, 25, 40–48. [Google Scholar]
- Fuller, H.; Dubbala, K.; Obiri, D.; Mallare, M.; Advani, S.; De Souza, S.; Stoby, K.; King-Okoye, M. Addressing vaccine hesitancy to reduce racial and ethnic disparities in COVID-19 vaccination uptake across the UK and US. Front. Public Health 2021, 9, 789753. [Google Scholar] [CrossRef] [PubMed]
- Tyson, A. 5 Years Later: America Looks Back at the Impact of COVID-19. Available online: https://www.pewresearch.org/politics/2025/02/12/5-years-later-america-looks-back-at-the-impact-of-covid-19/ (accessed on 20 August 2025).
| Characteristics | Frequency | p-Value Intent Time 1 | p-Value Uptake Time 2 |
|---|---|---|---|
| Gender | 0.046 * | 0.043 * | |
| Female | 42.3% (n = 60) | ||
| Male | 57.7% (n = 82) | ||
| Age, years | 0.027 * | <0.001 * | |
| Mean, SD | 53.3, 15.64 | ||
| Race/ethnicity | 0.835 | 0.864 | |
| White | 33.8% (n = 48) | ||
| Persons of Color | 66.2% (n = 94) | ||
| Education | 0.139 | 0.118 | |
| Less than bachelor’s | 51.4% (n = 73) | ||
| Bachelor’s or higher | 48.6% (n = 69) |
| Variable | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Age | 1.02 | 0.99, 1.05 | 0.078 | 1.02 | 0.99, 1.06 | 0.060 | 1.03 | 0.99, 1.07 | 0.188 |
| Gender: Female (Ref: Male) | 0.45 | 1.19, 1.09 | 0.545 | 0.49 | 0.20, 1.20 | 0.116 | 0.61 | 0.14, 2.73 | 0.522 |
| Race: White (Ref: POC) | 0.53 | 0.20, 1.36 | 0.185 | 0.48 | 0.18, 1.26 | 0.135 | 0.66 | 0.14, 3.10 | 0.599 |
| Education: Bachelor’s (Ref: Less than bachelor’s) | 1.59 | 0.76, 3.35 | 0.222 | 1.55 | 0.73, 3.29 | 0.249 | 1.73 | 0.53, 5.67 | 0.365 |
| Trust in government | 1.38 | 0.80, 2.37 | 0.249 | 0.87 | 0.30, 2.57 | 0.804 | |||
| HBM: perceived severity | 1.64 | 0.90, 3.00 | 0.107 | ||||||
| HBM: perceived susceptibility | 1.69 | 1.03, 2.76 | 0.038 * | ||||||
| HBM: perceived benefits | 3.12 | 1.62, 6.04 | <0.001 * | ||||||
| HBM: perceived barriers | 0.21 | 0.08, 0.56 | 0.002 * | ||||||
| HBM: self-efficacy | 1.41 | 0.90, 2.20 | 0.134 | ||||||
| HBM: cues to action | 3.13 | 0.88, 11.15 | 0.078 |
| Variable | OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Age | 1.04 | 1.02, 1.07 | 0.002 * | 1.02 | 0.99, 1.06 | 0.165 | 0.99 | 0.95, 1.04 | 0.688 |
| Gender: Female (Ref: Male) | 0.41 | 0.16, 1.04 | 0.060 | 0.35 | 0.11, 1.05 | 0.061 | 0.21 | 0.35, 1.25 | 0.085 |
| Race: White (Ref: POC) | 0.37 | 0.13, 1.01 | 0.052 | 0.64 | 0.19, 2.13 | 0.469 | 0.69 | 0.10, 4.49 | 0.693 |
| Education: Bachelor’s (Ref: Less than bachelor’s) | 1.72 | 0.79, 3.74 | 0.170 | 1.21 | 0.48, 3.07 | 0.691 | 1.69 | 0.37, 7.85 | 0.501 |
| Trust in government | 5.18 | 2.73, 9.86 | <0.001 * | 2.82 | 1.01, 7.83 | 0.047 * | |||
| HBM: perceived severity | 1.22 | 0.64, 2.34 | 0.540 | ||||||
| HBM: perceived susceptibility | 1.51 | 0.81, 2.82 | 0.200 | ||||||
| HBM: perceived benefits | 3.27 | 1.71, 6.26 | <0.001 * | ||||||
| HBM: perceived barriers | 0.24 | 0.10, 0.59 | 0.002 * | ||||||
| HBM: self-efficacy | 0.67 | 0.42, 1.08 | 0.098 | ||||||
| HBM: cues to action | 3.46 | 0.75, 16.20 | 0.116 |
| HBM Construct | Time 1 | Time 2 | p-Value |
|---|---|---|---|
| Perceived severity | 5.5, 1.33 | 4.5, 1.68 | <0.001 * |
| Perceived susceptibility | 4.5, 1.57 | 4.2, 1.62 | 0.067 |
| Perceived benefits | 4.6, 1.30 | 4.6, 1.50 | 0.864 |
| Perceived barriers | 3.7, 1.00 | 3.1, 1.19 | <0.001 * |
| Self-efficacy | 5.0, 1.51 | 5.6, 1.5 | <0.001 * |
| Cues to action | 1.000 | ||
| Yes | n = 92 | n = 92 | |
| No | n = 50 | n = 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidry, J.P.D.; Laestadius, L.I.; Miller, C.A.; Stevens, M.P.; Burton, C.W.; Carlyle, K.E.; Perrin, P.B. Building Vaccine Readiness for Future Pandemics: Insights from COVID-19 Vaccine Intent and Uptake. Vaccines 2025, 13, 1201. https://doi.org/10.3390/vaccines13121201
Guidry JPD, Laestadius LI, Miller CA, Stevens MP, Burton CW, Carlyle KE, Perrin PB. Building Vaccine Readiness for Future Pandemics: Insights from COVID-19 Vaccine Intent and Uptake. Vaccines. 2025; 13(12):1201. https://doi.org/10.3390/vaccines13121201
Chicago/Turabian StyleGuidry, Jeanine P. D., Linnea I. Laestadius, Carrie A. Miller, Michael P. Stevens, Candace W. Burton, Kellie E. Carlyle, and Paul B. Perrin. 2025. "Building Vaccine Readiness for Future Pandemics: Insights from COVID-19 Vaccine Intent and Uptake" Vaccines 13, no. 12: 1201. https://doi.org/10.3390/vaccines13121201
APA StyleGuidry, J. P. D., Laestadius, L. I., Miller, C. A., Stevens, M. P., Burton, C. W., Carlyle, K. E., & Perrin, P. B. (2025). Building Vaccine Readiness for Future Pandemics: Insights from COVID-19 Vaccine Intent and Uptake. Vaccines, 13(12), 1201. https://doi.org/10.3390/vaccines13121201







