Protective Efficacy of Two Novel DNA Vaccine Candidates Encoding TgGRA28 and TgGRA83 with an IL-28B Molecular Adjuvant Against Acute and Chronic Toxoplasmosis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cells, Parasites and Antigens
2.3. Construction of the Eukaryotic Expression Plasmids
2.4. In Vitro Expression Analysis of Recombinant Plasmids
2.5. Longitudinal Analysis of Antigen-Specific Humoral Immunity
2.6. Evaluation of Cellular Immune Responses by Lymphoproliferation Assay
2.7. Quantification of Antigen-Specific Cytotoxic T Lymphocyte Responses
2.8. Flow Cytometric Analysis of Splenic T Cell Subpopulations and DC Activations
2.9. Quantification of Antigen-Specific Cytokine Production and Cytokine-Related Transcription Factors
2.10. Experimental Design and Immunological Evaluation of DNA Vaccination Against T. gondii
2.11. Statistical Analysis
3. Results
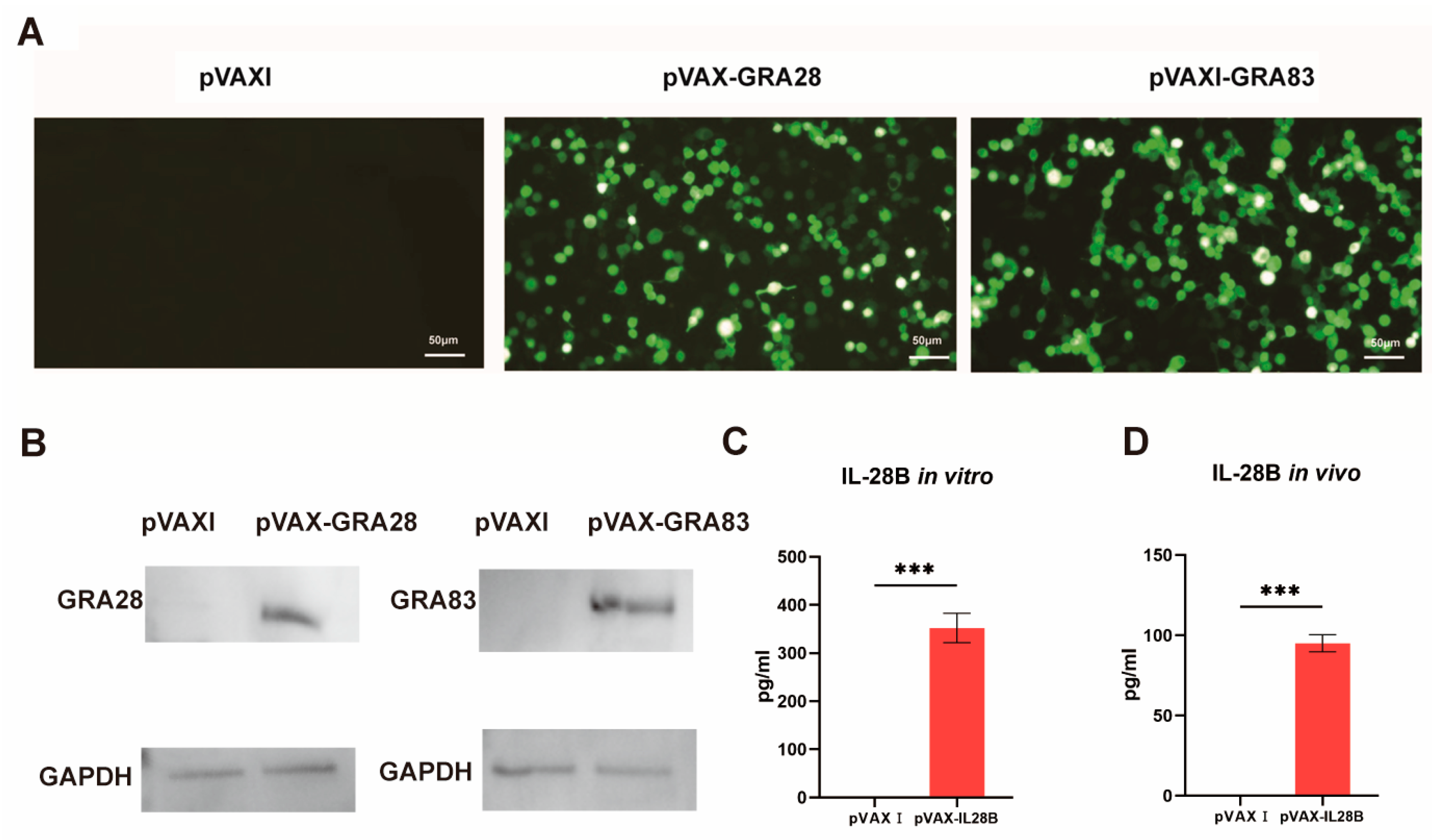
3.1. Identification of Plasmids
3.2. Humoral Immune Responses Induced by DNA Immunization
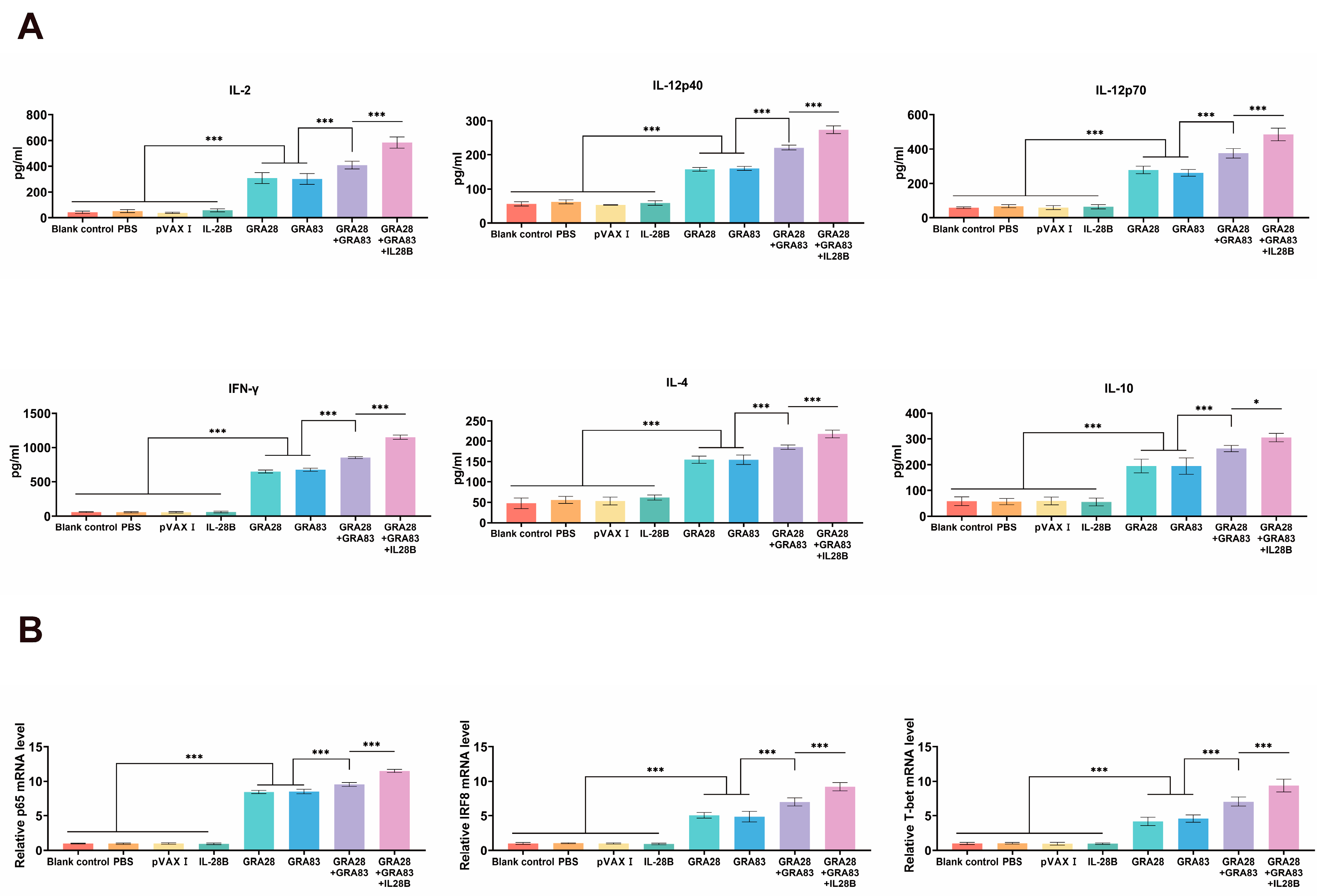
3.3. Cytokine Production and Cytokine-Related Transcription Factors
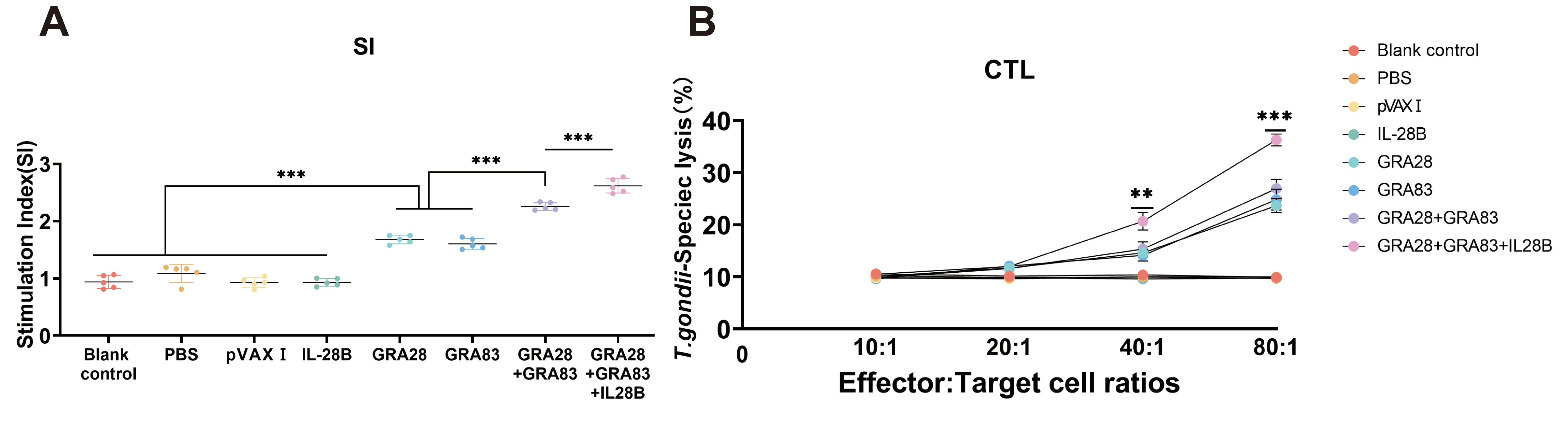
3.4. Cellular Immune Responses Induced by DNA Immunizations
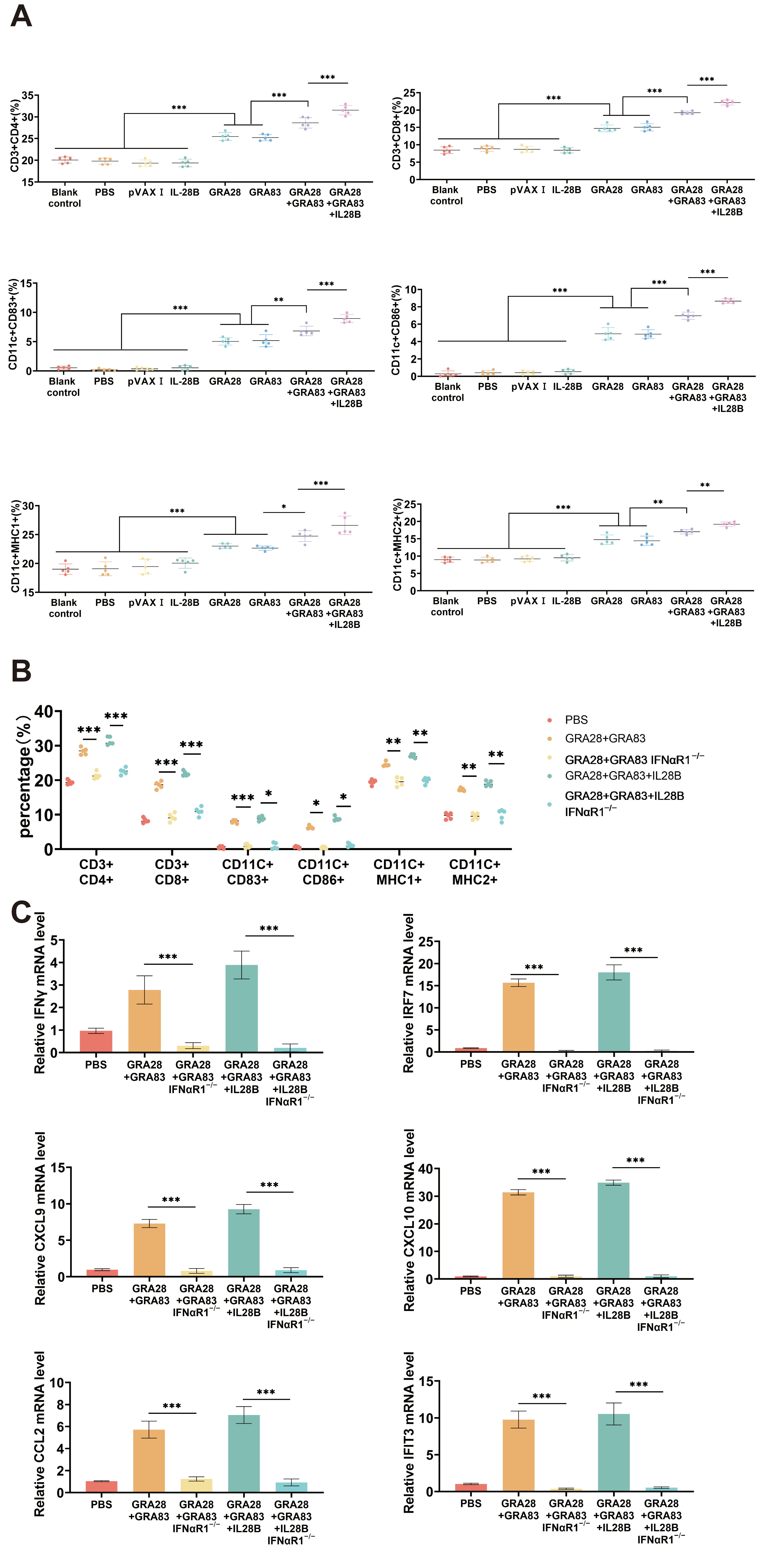
3.5. DNA Vaccinations Activate Immune Responses Through the I-IFN Signaling Pathway
3.6. IFNαR1 Knockout Impacts DNA Vaccination-Mediated Protective Efficacies in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar] [CrossRef]
- Wang, Z.D.; Wang, S.C.; Liu, H.H.; Ma, H.Y.; Li, Z.Y.; Wei, F.; Zhu, X.Q.; Liu, Q. Prevalence and burden of Toxoplasma gondii infection in HIV-infected people: A systematic review and meta-analysis. Lancet HIV 2017, 4, e177–e188. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Marra, C.M.; Zhu, X.Q. Epidemiology, Pathophysiology, Diagnosis, and Management of Cerebral Toxoplasmosis. Clin. Microbiol. Rev. 2021, 34, e00115-19. [Google Scholar] [CrossRef]
- Milne, G.; Webster, J.P.; Walker, M. Toxoplasma gondii: An Underestimated Threat? Trends Parasitol. 2020, 36, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Dard, C.; Marty, P.; Brenier-Pinchart, M.P.; Garnaud, C.; Fricker-Hidalgo, H.; Pelloux, H.; Pomares, C. Management of toxoplasmosis in transplant recipients: An update. Expert Rev. Anti-Infect. Ther. 2018, 16, 447–460. [Google Scholar] [CrossRef]
- Kravetz, J.D.; Federman, D.G. Toxoplasmosis in pregnancy. Am. J. Med. 2005, 118, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Tenter, A.M.; Heckeroth, A.R.; Weiss, L.M. Toxoplasma gondii: From animals to humans. Int. J. Parasitol. 2000, 30, 1217–1258. [Google Scholar] [CrossRef] [PubMed]
- Konstantinovic, N.; Guegan, H.; Stäjner, T.; Belaz, S.; Robert-Gangneux, F. Treatment of toxoplasmosis: Current options and future perspectives. Food Waterborne Parasitol. 2019, 15, e00036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Z.; Wang, M.; Xu, Y.; Petersen, E.; Zhu, X.Q. Recent advances in developing vaccines against Toxoplasma gondii: An update. Expert Rev. Vaccines 2015, 14, 1609–1621. [Google Scholar] [CrossRef]
- Jongert, E.; Roberts, C.W.; Gargano, N.; Förster-Waldl, E.; Petersen, E. Vaccines against Toxoplasma gondii: Challenges and opportunities. Mem. Inst. Oswaldo Cruz 2009, 104, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zhang, N.Z.; Li, T.T.; He, J.J.; Elsheikha, H.M.; Zhu, X.Q. Advances in the Development of Anti-Toxoplasma gondii Vaccines: Challenges, Opportunities, and Perspectives. Trends Parasitol. 2019, 35, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Goulart, C.; Hayward, J.A.; Kupz, A.; Miller, C.M.; van Dooren, G.G. Control of human toxoplasmosis. Int. J. Parasitol. 2021, 51, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Z.; Chen, J.; Wang, M.; Petersen, E.; Zhu, X.Q. Vaccines against Toxoplasma gondii: New developments and perspectives. Expert Rev. Vaccines 2013, 12, 1287–1299. [Google Scholar] [CrossRef]
- Ten Hoeve, A.L.; Rodriguez, M.E.; Säflund, M.; Michel, V.; Magimel, L.; Ripoll, A.; Yu, T.; Hakimi, M.A.; Saeij, J.P.J.; Ozata, D.M.; et al. Hypermigration of macrophages through the concerted action of GRA effectors on NF-κB/p38 signaling and host chromatin accessibility potentiates Toxoplasma dissemination. mBio 2024, 15, e02140-24. [Google Scholar] [CrossRef] [PubMed]
- Thind, A.C.; Mota, C.M.; Gonçalves, A.P.N.; Sha, J.; Wohlschlegel, J.A.; Mineo, T.W.P.; Bradley, P.J. The Toxoplasma gondii effector GRA83 modulates the host’s innate immune response to regulate parasite infection. mSphere 2023, 8, e00263-23. [Google Scholar] [CrossRef]
- Schijns, V.E.; Lavelle, E.C. Trends in vaccine adjuvants. Expert Rev. Vaccines 2011, 10, 539–550. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Tsounis, E.P.; Triantos, C. Molecular Mechanisms Underlying IL-33-Mediated Inflammation in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2022, 24, 623. [Google Scholar] [CrossRef]
- Möller, A.M.; Vettermann, S.; Baumann, F.; Pütter, M.; Müller, D. Trifunctional antibody-cytokine fusion protein formats for tumor-targeted combination of IL-15 with IL-7 or IL-21. Front. Immunol. 2025, 16, 1498697. [Google Scholar] [CrossRef]
- Li, Z.Y.; Chen, J.; Petersen, E.; Zhou, D.H.; Huang, S.Y.; Song, H.Q.; Zhu, X.Q. Synergy of mIL-21 and mIL-15 in enhancing DNA vaccine efficacy against acute and chronic Toxoplasma gondii infection in mice. Vaccine 2014, 32, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Z.Y.; Petersen, E.; Liu, W.G.; Zhu, X.Q. Co-administration of interleukins 7 and 15 with DNA vaccine improves protective immunity against Toxoplasma gondii. Exp. Parasitol. 2016, 162, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; He, Y.; Liu, J.F.; Chen, J. Adjuvantic cytokine IL-33 improves the protective immunity of cocktailed DNA vaccine of ROP5 and ROP18 against Toxoplasma gondii infection in mice. Parasite 2020, 27, 26. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, X.; Wang, Y.; Chen, J. IL-24 Is a Promising Molecular Adjuvant for Enhancing Protective Immunity Induced by DNA Vaccination Against Toxoplasma gondii. Microorganisms 2025, 13, 1661. [Google Scholar] [CrossRef]
- Tang, B.; Liu, Z.; Xiong, H.; Zhang, J.; Dai, J. IFN-λ: Unleashing Its Potential in Disease Therapies From Acute Inflammation Regulation to Cancer Immunotherapy. Immunology 2025, 176, 197–214. [Google Scholar] [CrossRef]
- Morrow, M.P.; Pankhong, P.; Laddy, D.J.; Schoenly, K.A.; Yan, J.; Cisper, N.; Weiner, D.B. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood 2009, 113, 5868–5877. [Google Scholar] [CrossRef]
- Luo, Y.; Ma, X.; Liu, X.; Lu, X.; Niu, H.; Yu, H.; Bai, C.; Peng, J.; Xian, Q.; Wang, Y.; et al. IL-28B down-regulates regulatory T cells but does not improve the protective immunity following tuberculosis subunit vaccine immunization. Int. Immunol. 2016, 28, 77–85. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Yang, W.; Chen, J. Protective immunity induced by DNA vaccine containing TgGRA35, TgGRA42, and TgGRA43 against Toxoplasma gondii infection in Kunming mice. Front. Cell. Infect. Microbiol. 2023, 13, 1236130. [Google Scholar] [CrossRef] [PubMed]
- García-Arriaza, J.; Garaigorta, U.; Pérez, P.; Lázaro-Frías, A.; Zamora, C.; Gastaminza, P.; Del Fresno, C.; Casasnovas, J.M.; Sorzano CÓ, S.; Sancho, D.; et al. COVID-19 vaccine candidates based on modified vaccinia virus Ankara expressing the SARS-CoV-2 spike induce robust T- and B-cell immune responses and full efficacy in mice. J. Virol. 2021, 95, e02260-20. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.M.; Babuadze, G.G.; Plourde-Campagna, M.A.; Azizi, H.; Berger, A.; Kozak, R.; de La Vega, M.A.; Xiii, A.; Naghibosadat, M.; Nepveu-Traversy, M.E.; et al. A novel intradermal tattoo-based injection device enhances the immunogenicity of plasmid DNA vaccines. npj Vaccines 2022, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Li, H.; Song, X.; Ma, Z.; Lu, H.; Liu, S.; Zhao, Y.; Tan, M.; Wang, S.; et al. Identification of Toxoplasma gondii Tyrosine Hydroxylase (TH) Activity and Molecular Immunoprotection against Toxoplasmosis. Vaccines 2020, 8, 158. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H. Moving towards improved vaccines for Toxoplasma gondii. Expert Opin. Biol. Ther. 2018, 18, 273–280. [Google Scholar] [CrossRef]
- Rezaei, F.; Sharif, M.; Sarvi, S.; Hejazi, S.H.; Aghayan, S.; Pagheh, A.S.; Dodangeh, S.; Daryani, A. A systematic review on the role of GRA proteins of Toxoplasma gondii in host immunization. J. Microbiol. Methods 2019, 165, 105696. [Google Scholar] [CrossRef]
- Sun, X.; Mei, M.; Zhang, X.; Han, F.; Jia, B.; Wei, X.; Chang, Z.; Lu, H.; Yin, J.; Chen, Q.; et al. The extracellular matrix protein mindin as a novel adjuvant elicits stronger immune responses for rBAG1, rSRS4 and rSRS9 antigens of Toxoplasma gondii in BALB/c mice. BMC Infect. Dis. 2014, 14, 429. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L. SAG4 DNA and Peptide Vaccination Provides Partial Protection against T. gondii Infection in BALB/c Mice. Front. Microbiol. 2017, 8, 1733. [Google Scholar] [CrossRef] [PubMed]
- Spunde, K.; Korotkaja, K.; Sominskaya, I.; Zajakina, A. Genetic adjuvants: A paradigm shift in vaccine development and immune modulation. Mol. Ther. Nucleic Acids 2025, 36, 102536. [Google Scholar] [CrossRef]
- Tan, Y.; Mu, J.; Chen, J. IL-36 Gamma: A Novel Adjuvant Cytokine Enhancing Protective Immunity Induced by DNA Immunization with TGIST and TGNSM Against Toxoplasma gondii Infection in Mice. Microorganisms 2024, 12, 2258. [Google Scholar] [CrossRef] [PubMed]
- Frickel, E.M.; Hunter, C.A. Lessons from Toxoplasma: Host responses that mediate parasite control and the microbial effectors that subvert them. J. Exp. Med. 2021, 218, e20201314. [Google Scholar] [CrossRef] [PubMed]
- Lüder, C.G.K. IFNs in host defence and parasite immune evasion during Toxoplasma gondii infections. Front. Immunol. 2024, 15, 1356216. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Cao, X. The immune potential and immunopathology of cytokine-producing B cell subsets: A comprehensive review. J. Autoimmun. 2014, 55, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Ding, J.; Lou, D.; Tong, Q.; Zhuo, X.; Ding, H.; Kong, Q.; Lu, S. The Virulence-Related MYR1 Protein of Toxoplasma gondii as a Novel DNA Vaccine Against Toxoplasmosis in Mice. Front. Microbiol. 2019, 10, 734. [Google Scholar] [CrossRef]
- Zheng, B.; Lou, D.; Ding, J.; Zhuo, X.; Ding, H.; Kong, Q.; Lu, S. GRA24-Based DNA Vaccine Prolongs Survival in Mice Challenged With a Virulent Toxoplasma gondii Strain. Front. Immunol. 2019, 10, 418. [Google Scholar] [CrossRef]
- Sun, H.C.; Huang, J.; Fu, Y.; Hao, L.L.; Liu, X.; Shi, T.Y. Enhancing Immune Responses to a DNA Vaccine Encoding Toxoplasma gondii GRA7 Using Calcium Phosphate Nanoparticles as an Adjuvant. Front. Cell. Infect. Microbiol. 2021, 11, 787635. [Google Scholar] [CrossRef] [PubMed]
- Molestina, R.E.; Payne, T.M.; Coppens, I.; Sinai, A.P. Activation of NF-kappaB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IkappaB to the parasitophorous vacuole membrane. J. Cell Sci. 2003, 116, 4359–4371. [Google Scholar] [CrossRef]
- Townsend, M.J.; Weinmann, A.S.; Matsuda, J.L.; Salomon, R.; Farnham, P.J.; Biron, C.A.; Gapin, L.; Glimcher, L.H. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity 2004, 20, 477–494. [Google Scholar] [CrossRef]
- Sichien, D.; Scott, C.L.; Martens, L.; Vanderkerken, M.; Van Gassen, S.; Plantinga, M.; Joeris, T.; De Prijck, S.; Vanhoutte, L.; Vanheerswynghels, M.; et al. IRF8 Transcription Factor Controls Survival and Function of Terminally Differentiated Conventional and Plasmacytoid Dendritic Cells, Respectively. Immunity 2016, 45, 626–640. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Shen, Y.; Li, S.; Lu, S.; Zheng, B. Immunization with a novel mRNA vaccine, TGGT1_216200 mRNA-LNP, prolongs survival time in BALB/c mice against acute toxoplasmosis. Front. Immunol. 2023, 14, 1161507. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Chu, H.; Li, J.; Lu, S.; Zheng, B. A novel mRNA vaccine, TGGT1_278620 mRNA-LNP, prolongs the survival time in BALB/c mice with acute toxoplasmosis. Microbiol. Spectr. 2024, 12, e02866-23. [Google Scholar] [CrossRef]
- Greenwald, R.J.; Freeman, G.J.; Sharpe, A.H. The B7 family revisited. Annu. Rev. Immunol. 2005, 23, 515–548. [Google Scholar] [CrossRef]
- Aosai, F.; Rodriguez Pena, M.S.; Mun, H.S.; Fang, H.; Mitsunaga, T.; Norose, K.; Kang, H.K.; Bae, Y.S.; Yano, A. Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via Toll-like receptor 4. Cell Stress Chaperones 2006, 11, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.E.; Anthony, J.P.; Ferguson, D.J.; Byrne, N.; Alexander, J.; Roberts, F.; Roberts, C.W. Immunological studies of chronic ocular toxoplasmosis: Up-regulation of major histocompatibility complex class I and transforming growth factor beta and a protective role for interleukin-6. Infect. Immun. 2001, 69, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Tubo, N.J.; Jenkins, M.K. CD4+ T Cells: Guardians of the phagosome. Clin. Microbiol. Rev. 2014, 27, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Halle, S.; Halle, O.; Förster, R. Mechanisms and Dynamics of T Cell-Mediated Cytotoxicity In Vivo. Trends Immunol. 2017, 38, 432–443. [Google Scholar] [CrossRef]
- Yu, Z.; He, K.; Cao, W.; Aleem, M.T.; Yan, R.; Xu, L.; Song, X.; Li, X. Nano vaccines for T. gondii Ribosomal P2 Protein With Nanomaterials as a Promising DNA Vaccine Against Toxoplasmosis. Front. Immunol. 2022, 13, 839489. [Google Scholar] [CrossRef]
- Pifer, R.; Yarovinsky, F. Innate responses to Toxoplasma gondii in mice and humans. Trends Parasitol. 2011, 27, 388–393. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2–Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Pollard, C.; Rejman, J.; De Haes, W.; Verrier, B.; Van Gulck, E.; Naessens, T.; De Smedt, S.; Bogaert, P.; Grooten, J.; Vanham, G.; et al. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 251–259. [Google Scholar] [CrossRef]
- Broos, K.; Van der Jeught, K.; Puttemans, J.; Goyvaerts, C.; Heirman, C.; Dewitte, H.; Verbeke, R.; Lentacker, I.; Thielemans, K.; Breckpot, K. Particle-mediated Intravenous Delivery of Antigen mRNA Results in Strong Antigen-specific T-cell Responses Despite the Induction of Type I Interferon. Mol. Therapy Nucleic Acids 2016, 5, e326. [Google Scholar] [CrossRef]
- Li, C.; Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Lindgren, G.; Lin, A.; Thompson, E.A.; Ols, S.; Röhss, J.; John, S.; Hassett, K.; Yuzhakov, O.; Bahl, K.; et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified mRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 2635–2647. [Google Scholar] [CrossRef] [PubMed]
- Kastenmüller, K.; Wille-Reece, U.; Lindsay, R.W.; Trager, L.R.; Darrah, P.A.; Flynn, B.J.; Becker, M.R.; Udey, M.C.; Clausen, B.E.; Igyarto, B.Z.; et al. Protective T cell immunity in mice following protein-TLR7/8 agonist-conjugate immunization requires aggregation, type I IFN, and multiple DC subsets. J. Clin. Investig. 2011, 121, 1782–1796. [Google Scholar] [CrossRef] [PubMed]
- Welsh, R.M.; Bahl, K.; Marshall, H.D.; Urban, S.L. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog. 2012, 8, e1002352. [Google Scholar] [CrossRef] [PubMed]
- Matta, S.K.; Olias, P.; Huang, Z.; Wang, Q.; Park, E.; Yokoyama, W.M.; Sibley, L.D. Toxoplasma gondii effector TgIST blocks type I interferon signaling to promote infection. Proc. Natl. Acad. Sci. USA 2019, 116, 17480–17491. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Wu, D.; Lu, J.; Zhang, Y.; Yu, S.M.; Xie, Y.; Li, H.; Yang, J.; Lai, D.H.; Zeng, K.; et al. Inflammasome Activation Dampens Type I IFN Signaling to Strengthen Anti-Toxoplasma Immunity. mBio 2022, 13, e02361-22. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.; Schiavoni, G.; Mattei, F.; Gresser, I.; Belardelli, F.; Borrow, P.; Tough, D.F. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 2002, 99, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Vialle-Castellano, A.; Nunes, J.A.; Isnardon, D.; Olive, D.; Gaugler, B. IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J. Immunol. 2003, 171, 3385–3393. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, M.B.; Kacha, A.K.; Kline, J.; Woo, S.R.; Kranz, D.M.; Murphy, K.M.; Gajewski, T.F. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J. Exp. Med. 2011, 208, 2005–2016. [Google Scholar] [CrossRef] [PubMed]
- Peperzak, V.; Veraar, E.A.; Xiao, Y.; Babala, N.; Thiadens, K.; Brugmans, M.; Borst, J. CD8+ T cells produce the chemokine CXCL10 in response to CD27/CD70 costimulation to promote generation of the CD8+ effector T cell pool. J. Immunol. 2013, 191, 3025–3036. [Google Scholar] [CrossRef]


| Primer Name | Sequence |
|---|---|
| B-Actin-F | 5′-GCTTCTAGGCGGACTGTTAC-3′ |
| B-Actin-R | 5′-CCATGCCAATGTTGTCTCTT-3′ |
| NF-KB p65-F | 5′-GAACCAGGGTGTGTCCATGT-3′ |
| NF-KB p65-R | 5′-TCCGCAATGGAGGAGAAGTC-3′ |
| T-bet-F | 5′-GCCAGGGAACCGCTTATATG-3′ |
| T-bet-R | 5′-TGGAGAGACTGCAGGACGAT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Mu, J.; Li, R.; Chen, J. Protective Efficacy of Two Novel DNA Vaccine Candidates Encoding TgGRA28 and TgGRA83 with an IL-28B Molecular Adjuvant Against Acute and Chronic Toxoplasmosis in Mice. Vaccines 2025, 13, 1180. https://doi.org/10.3390/vaccines13121180
Fang J, Mu J, Li R, Chen J. Protective Efficacy of Two Novel DNA Vaccine Candidates Encoding TgGRA28 and TgGRA83 with an IL-28B Molecular Adjuvant Against Acute and Chronic Toxoplasmosis in Mice. Vaccines. 2025; 13(12):1180. https://doi.org/10.3390/vaccines13121180
Chicago/Turabian StyleFang, Jun, Jingqi Mu, Rui Li, and Jia Chen. 2025. "Protective Efficacy of Two Novel DNA Vaccine Candidates Encoding TgGRA28 and TgGRA83 with an IL-28B Molecular Adjuvant Against Acute and Chronic Toxoplasmosis in Mice" Vaccines 13, no. 12: 1180. https://doi.org/10.3390/vaccines13121180
APA StyleFang, J., Mu, J., Li, R., & Chen, J. (2025). Protective Efficacy of Two Novel DNA Vaccine Candidates Encoding TgGRA28 and TgGRA83 with an IL-28B Molecular Adjuvant Against Acute and Chronic Toxoplasmosis in Mice. Vaccines, 13(12), 1180. https://doi.org/10.3390/vaccines13121180





