A Novel, Safe, Non-Adjuvanted Alphavirus RNA Particle Vaccine Expressing the Rabies Virus Glycoprotein Induces a Three-Year Duration of Immunity in Dogs and Cats After a Single Vaccine Dose
Abstract
1. Introduction
2. Materials and Methods
2.1. Vaccine Formulations
2.2. Blood Collection
2.3. Serology
2.4. Challenge Virus
2.5. Bovine Serum Albumin Assay
2.6. Feline Graded Dose Study Design
2.7. Canine Graded Dose Study Design
2.8. Feline Three-Year Duration of Immunity Study Design
2.9. Canine Three-Year Duration of Immunity Study Design
2.10. Feline Field Safety Study Design
2.11. Canine Field Safety Study Design
3. Results
3.1. Feline Graded Dose Study
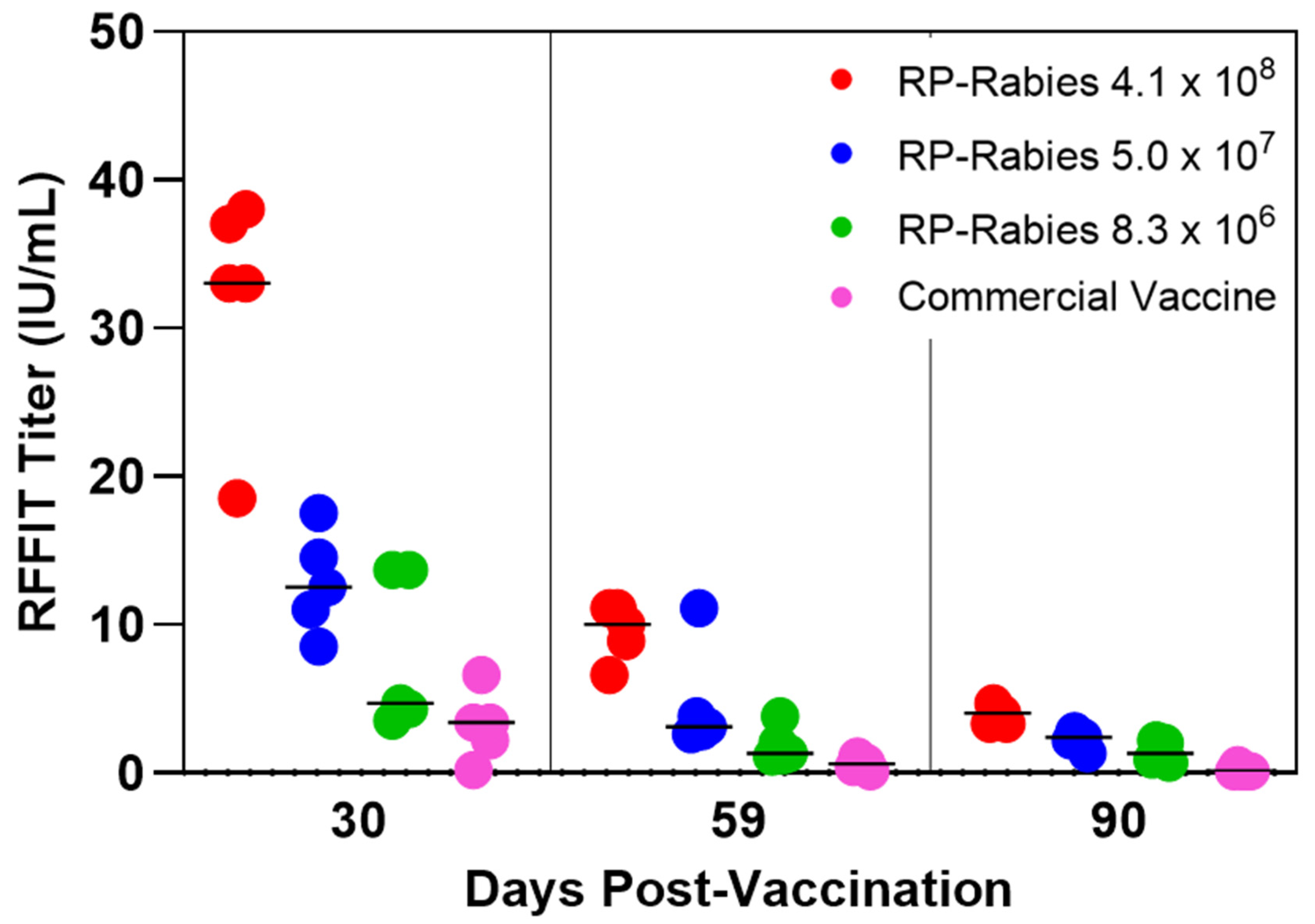
3.2. Canine Graded Dose Study
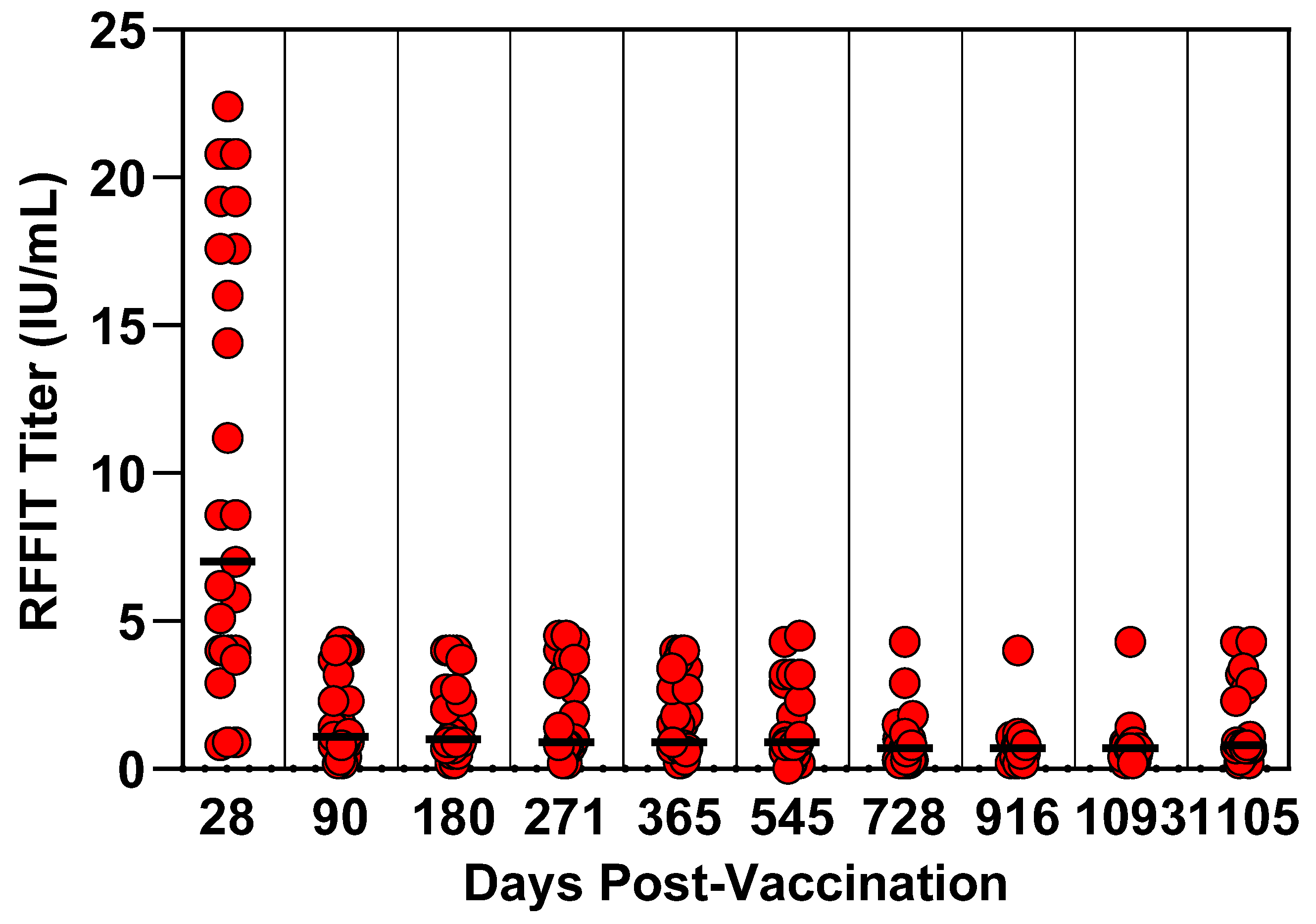
3.3. Feline Three-Year Duration of Immunity Study
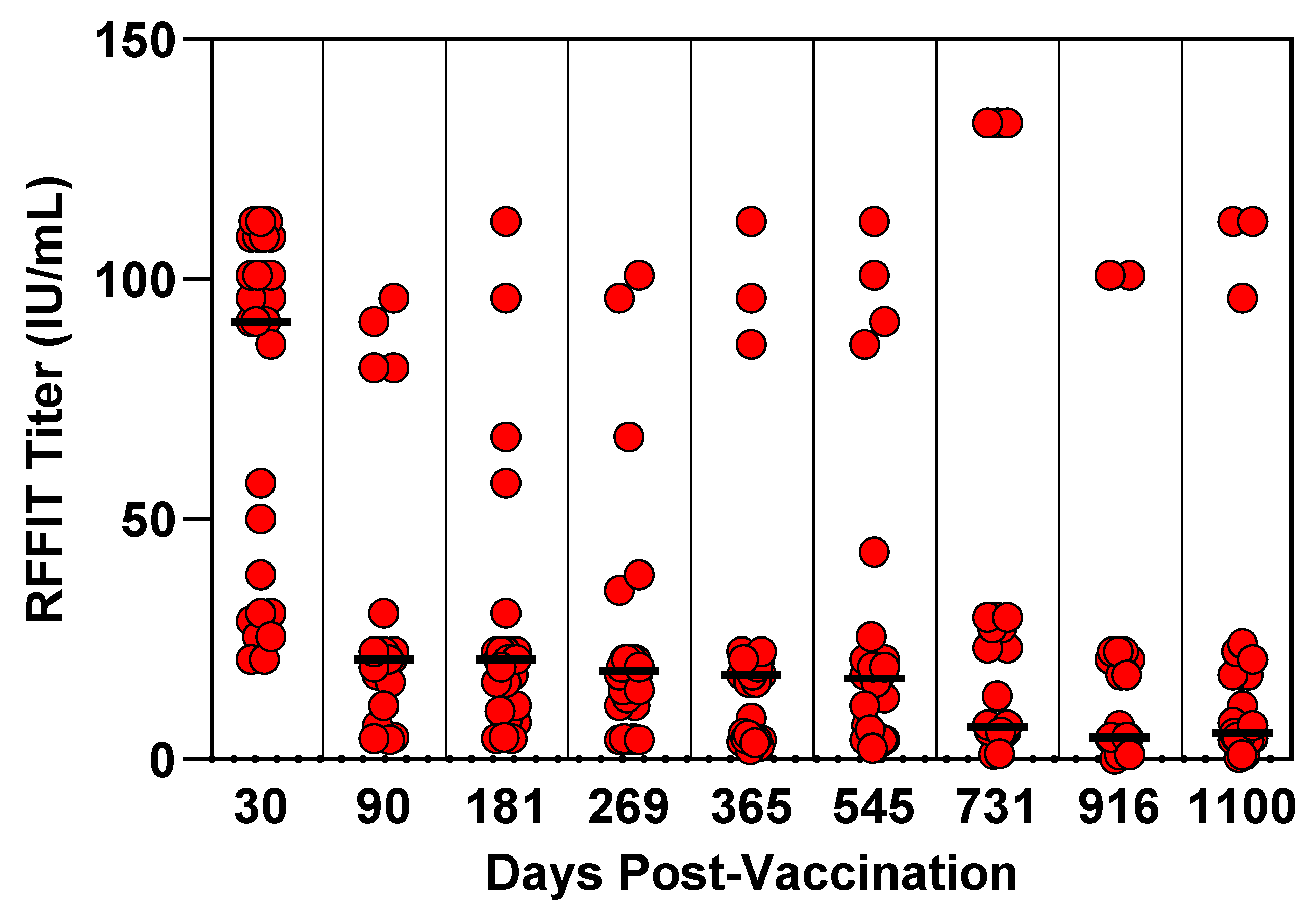
3.4. Canine Three-Year Duration of Immunity Study
3.5. Feline Field Safety Study
3.6. Canine Field Safety Study
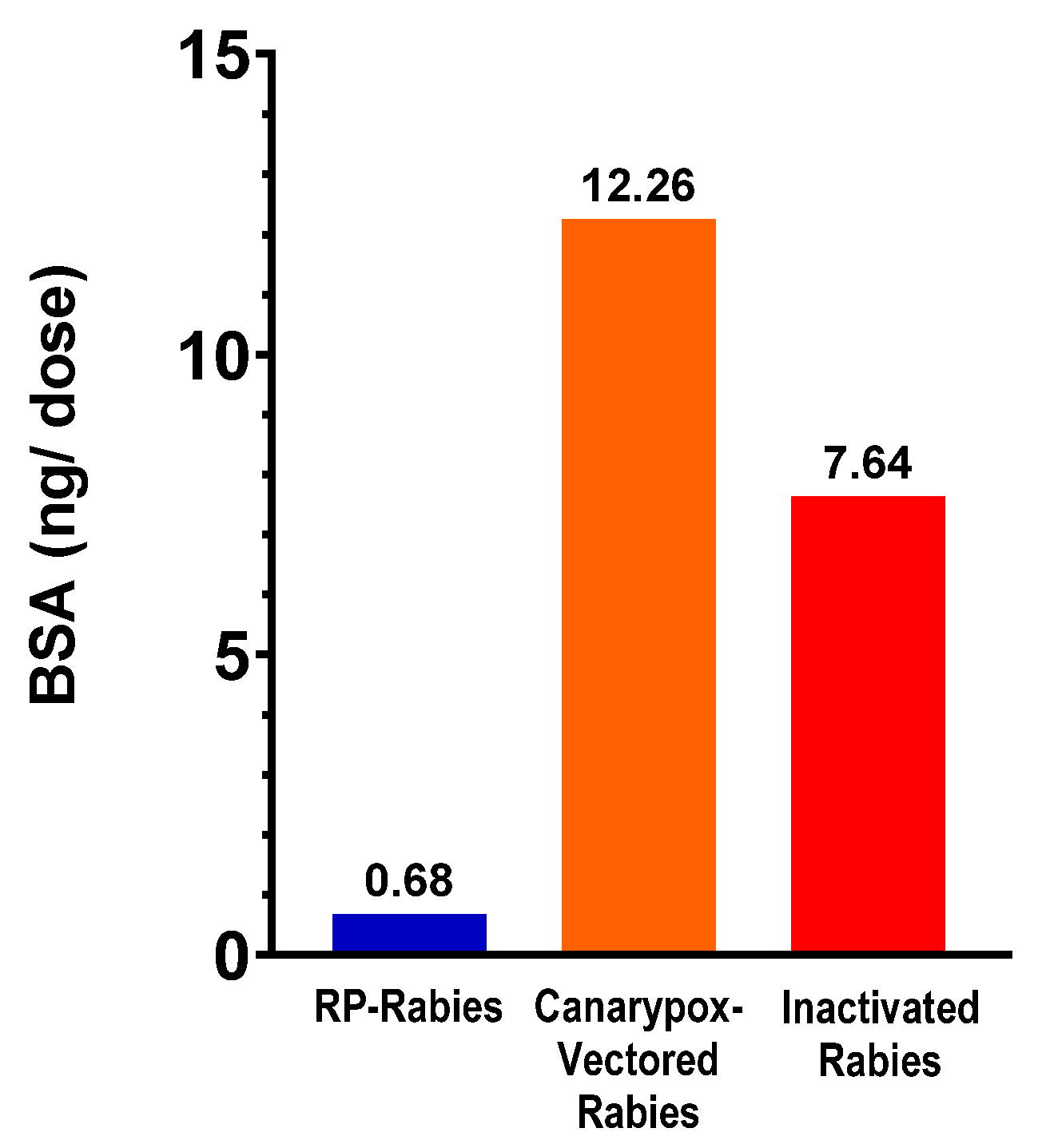
3.7. Bovine Serum Albumin Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/en/news-room/fact-sheets/detail/rabies (accessed on 7 July 2025).
- Rabies Bulletin Europe. Available online: https://www.who-rabies-bulletin.org/site-pate/epidemiology-rabies (accessed on 4 November 2025).
- Brunt, S.; Solomon, H.; Brown, K.; Davis, A. Feline and Canine Rabies in New York State, USA. Viruses 2021, 13, 450. [Google Scholar] [CrossRef]
- Roebling, A.D.; Johnson, D.; Blanton, J.D.; Levin, M.; Slate, D.; Fenwick, G.; Rupprecht, C.E. Rabies Prevention and Management of Cats in the Context of Trap–Neuter–Vaccinate–Release Programmes. Zoonoses Public Health 2014, 61, 290–296. [Google Scholar] [CrossRef]
- McVey, D.S.; Kennedy, M. Vaccines for Emerging and Re-Emerging Viral Diseases of Companion Animals. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 903–917. [Google Scholar] [CrossRef]
- Wilcock, B.P.; Yager, J.A. Focal Cutaneous Vasculitis and Alopecia at Sites of Rabies Vaccination in Dogs. J. Am. Vet. Med. Assoc. 1986, 188, 1174–1177. [Google Scholar] [CrossRef]
- Moore, G.E.; DeSantis-Kerr, A.C.; Guptill, L.F.; Glickman, N.W.; Lewis, H.B.; Glickman, L.T. Adverse Events After Vaccine Administration in Cats: 2560 Cases (2002–2005). J. Am. Vet. Med. Assoc. 2007, 231, 94–100. [Google Scholar] [CrossRef]
- Saba, C.F. Vaccine-Associated Feline Sarcoma: Current Perspectives. Vet. Med. Res. Rep. 2017, 8, 13–20. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Committee on Rabies [Meeting Held in Geneva from 24 to 30 September 1991]: Eighth Report; World Health Organization: Geneva, Switzerland, 1992; Available online: https://iris.who.int/handle/10665/39308 (accessed on 13 May 2025).
- Moore, S.M.; Wilkerson, M.J.; Davis, R.D.; Wyatt, C.R.; Briggs, D.J. Detection of Cellular Immunity to Rabies Antigens in Human Vaccinees. J. Clin. Immunol. 2006, 26, 533–545. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Sayers, R.; Fooks, A.R.; Burr, P.D.; Snodgrass, D. Factors Affecting the Serological Response of Dogs and Cats to Rabies Vaccination. Vet. Rec. 2004, 154, 423–426. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Lunt, M.; Barnes, A.; McElhinney, L.; Fooks, A.R.; Baxter, D.N.; Ollier, W.E.R. Factors Influencing the Antibody Response of Dogs Vaccinated Against Rabies. Vaccine 2007, 25, 8500–8507. [Google Scholar] [CrossRef]
- Minke, J.M.; Bouvet, J.; Cliquet, F.; Wasniewski, M.; Guiot, A.L.; Lemaitre, L.; Cariou, C.; Cozette, V.; Vergne, L.; Guigal, P.M. Comparison of Antibody Responses After Vaccination with Two Inactivated Rabies Vaccines. Vet. Microbiol. 2009, 133, 283–286. [Google Scholar] [CrossRef]
- Cliquet, F.; Verdier, Y.; Sagné, L.; Aubert, M.; Schereffer, J.L.; Selve, M.; Wasniewski, M.; Servat, A. Neutralising Antibody Titration in 25,000 Sera of Dogs and Cats Vaccinated Against Rabies in France, in the Framework of the New Regulations That Offer an Alternative to Quarantine. OIE Rev. Sci. Tech. 2003, 22, 857–866. [Google Scholar] [CrossRef]
- Amann, R.; Rohde, J.; Wulle, U.; Conlee, D.; Raue, R.; Martinon, O.; Rziha, H.-J. A New Rabies Vaccine Based on a Recombinant Orf Virus (Parapoxvirus) Expressing the Rabies Virus Glycoprotein. J. Virol. 2013, 87, 1618–1630. [Google Scholar] [CrossRef]
- Chen, T.; Zhou, X.; Qi, Y.; Mi, L.; Sun, X.; Zhang, S.; Liu, Y.; Olson, V.; Qiu, W.; Wu, X.; et al. Feline Herpesvirus Vectored-Rabies Vaccine in Cats: A Dual Protection. Vaccine 2019, 37, 2224–2231. [Google Scholar] [CrossRef]
- Ge, J.; Wang, X.; Tao, L.; Wen, Z.; Feng, N.; Yang, S.; Xia, X.; Yang, C.; Chen, H.; Bu, Z. Newcastle Disease Virus-Vectored Rabies Vaccine is Safe, Highly Immunogenic, and Provides Long-Lasting Protection in Dogs and Cats. J. Virol. 2011, 85, 8241–8252. [Google Scholar] [CrossRef]
- Hu, R.L.; Liu, Y.; Zhang, S.F.; Zhang, F.; Fooks, A.R. Experimental Immunization of Cats with a Recombinant Rabies-Canine Adenovirus Vaccine Elicits a Long-Lasting Neutralizing Antibody Response Against Rabies. Vaccine 2007, 25, 5301–5307. [Google Scholar] [CrossRef]
- Tesoro-Cruz, E.; Calderón-Rodríguez, R.; Hernández-González, R.; Blanco-Favéla, F.; Aguilar-Setién, A. Intradermal DNA Vaccination in Ear Pinnae is an Efficient Route to Protect Cats Against Rabies Virus. Vet. Res. 2008, 39, 16. [Google Scholar] [CrossRef]
- Taylor, J.; Trimarchi, C.; Weinberg, R.; Languet, B.; Guillermin, F.; Desmettre, P.; Paoletti, E. Efficacy Studies on a Canarypox-Rabies Recombinant Virus. Vaccine 1991, 9, 190–193. [Google Scholar] [CrossRef]
- Mogler, M.A.; Kamrud, K.I. RNA-Based Viral Vectors. Expert Rev. Vaccines 2014, 14, 283–312. [Google Scholar] [CrossRef]
- Vander Veen, R.L.; Harris, D.L.; Kamrud, K.I. Alphavirus Replicon Vaccines. Anim. Health Res. Rev. 2012, 13, 1–9. [Google Scholar] [CrossRef]
- Langereis, M.A.; Albulescu, I.C.; Stammen-Vogelzangs, J.; Lambregts, M.; Stachura, K.; Miller, S.; Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Allen, M.; et al. An Alphavirus Replicon-Based Vaccine Expressing a Stabilized Spike Antigen Induces Protective Immunity and Prevents Transmission of SARS-CoV-2 Between Cats. npj Vaccines 2021, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Classe, H.M.; Dant, J.C.; Mogler, M.; Stachura, K.A.; LaFleur, R.L.; Xu, Z.; Tarpey, I. Efficacy and Safety in Dogs Following Administration of an Alphavirus RNA Particle Canine Influenza H3N2 Vaccine. Vaccines 2024, 12, 1138. [Google Scholar] [CrossRef]
- Carritt, K.; Davis, R.; Stachura, K.; Crumley, P.; Mogler, M.; Stahl, M.; Deng, L.; Xu, Z.; Tarpey, I. A Novel, Safe, Non-Adjuvanted Alphavirus Replicon-Based Vaccine Expressing the Feline Leukemia Virus Envelope Protein Protects Against Virulent FeLV Challenge. Vaccines 2025, 13, 697. [Google Scholar] [CrossRef]
- Kitikoon, P.; Knetter, S.M.; Mogler, M.A.; Morgan, C.L.; Hoehn, A.; Puttamreddy, S.; Strait, E.L.; Segers, R.P.A.M. Quadrivalent Neuraminidase RNA Particle Vaccine Protects Pigs Against Homologous and Heterologous Strains of Swine Influenza Virus Infection. Vaccine 2023, 41, 6941–6951. [Google Scholar] [CrossRef]
- Vander Veen, R.L.; Loynachan, A.T.; Mogler, M.A.; Russell, B.J.; Harris, D.L.H.; Kamrud, K.I. Safety, Immunogenicity, and Efficacy of an Alphavirus Replicon-Based Swine Influenza Virus Hemagglutinin Vaccine. Vaccine 2012, 30, 1944–1950. [Google Scholar] [CrossRef]
- Pushko, P.; Parker, M.; Ludwig, G.V.; Davis, N.L.; Johnston, R.E.; Smith, J.F. Replicon-Helper Systems from Attenuated Venezuelan Equine Encephalitis Virus: Expression of Heterologous Genes in Vitro and Immunization Against Heterologous Pathogens in Vivo. Virology 1997, 239, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Kamrud, K.I.; Alterson, K.; Custer, M.; Dudek, J.; Goodman, C.; Owens, G.; Smith, J.F. Development and Characterization of Promoterless Helper RNAs for the Production of Alphavirus Replicon Particle. J. Gen. Virol. 2010, 91, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Yager, P.A.; Baer, G.M. A Rapid Fluorescent Focus Inhibition Test (RFFIT) for Determining Rabies Virus Neutralizing Antibody. In Laboratory Techniques in Rabies, 4th ed.; Meslin, F.X., Koprowsky, H., Kaplan, M.M., Eds.; WHO: Geneva, Switzerland, 1996; pp. 181–187. Available online: http://apps.who.int/iris/bitstream/10665/38286/1/9241544791_eng.pdf (accessed on 13 May 2025).
- United States Department of Agriculture, Center for Veterinary Biologics, Testing Protocol. Supplemental Assay Method for the Detection of Serum Neutralizing Antibodies to Rabies Virus using the Rapid Fluorescent Focus Inhibition Test (RFFIT), SAM 315.04. Available online: https://www.aphis.usda.gov/sites/default/files/sam315.pdf (accessed on 4 November 2025).
- Koprowski, H. Experimental Studies on Rabies Virus. Can. J. Public Health 1949, 40, 60–67. [Google Scholar] [PubMed]
- Ohmori, K.; Masuda, K.; Maeda, S.; Kaburagi, Y.; Kurata, K.; Ohno, K.; DeBoer, D.J.; Tsujimoto, H.; Sakaguchi, M. IgE Reactivity to Vaccine components in Dogs that Developed Immediate-Type Allergic Reactions after Vaccination. Vet. Immunol. Immunopathol. 2005, 104, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Mizukami, K.; Hisasue, M.; Imanishi, I.; Kurata, K.; Ochiai, M.; Itoh, M.; Nasukawa, T.; Uchiyama, J.; Tsujimoto, H.; et al. Anaphylaxis After Vaccination for Cats in Japan. J. Vet. Med. Sci. 2022, 84, 149–152. [Google Scholar] [CrossRef]
- Animal and Plant Health Inspection Service, United States Department of Agriculture. Veterinary Biologics, Product Summaries. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/veterinary-biologics/product-summaries/vet-label-data/6af73e8c-6ead-4448-a93c-8d21a7723b01 (accessed on 24 September 2024).

| Treatment Group | Number of Cats | Vaccine |
|---|---|---|
| 1 | 10 | RNA-particle Rabies 2.7 × 107 RNA particles/dose |
| 2 | 10 | RNA-particle Rabies 2.6 × 106 RNA particles/dose |
| 3 | 10 | RNA-particle Rabies 4.0 × 105 RNA particles/dose |
| 4 | 5 | Commercial Rabies Vaccine |
| Treatment Group | Number of Dogs | Vaccine |
|---|---|---|
| 1 | 5 | RNA-particle Rabies 4.0 × 108 RNA particles/dose |
| 2 | 5 | RNA-particle Rabies 5.0 × 107 RNA particles/dose |
| 3 | 5 | RNA-particle Rabies 8.3 × 106 RNA particles/dose |
| 4 | 5 | Commercial Rabies Vaccine |
| 5 | 5 | Placebo RNA particle Vaccine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stachura, K.; Davis, R.; Carritt, K.; Mogler, M.; Xu, Z.; Tarpey, I. A Novel, Safe, Non-Adjuvanted Alphavirus RNA Particle Vaccine Expressing the Rabies Virus Glycoprotein Induces a Three-Year Duration of Immunity in Dogs and Cats After a Single Vaccine Dose. Vaccines 2025, 13, 1176. https://doi.org/10.3390/vaccines13121176
Stachura K, Davis R, Carritt K, Mogler M, Xu Z, Tarpey I. A Novel, Safe, Non-Adjuvanted Alphavirus RNA Particle Vaccine Expressing the Rabies Virus Glycoprotein Induces a Three-Year Duration of Immunity in Dogs and Cats After a Single Vaccine Dose. Vaccines. 2025; 13(12):1176. https://doi.org/10.3390/vaccines13121176
Chicago/Turabian StyleStachura, Ken, Randall Davis, Kari Carritt, Mark Mogler, Zach Xu, and Ian Tarpey. 2025. "A Novel, Safe, Non-Adjuvanted Alphavirus RNA Particle Vaccine Expressing the Rabies Virus Glycoprotein Induces a Three-Year Duration of Immunity in Dogs and Cats After a Single Vaccine Dose" Vaccines 13, no. 12: 1176. https://doi.org/10.3390/vaccines13121176
APA StyleStachura, K., Davis, R., Carritt, K., Mogler, M., Xu, Z., & Tarpey, I. (2025). A Novel, Safe, Non-Adjuvanted Alphavirus RNA Particle Vaccine Expressing the Rabies Virus Glycoprotein Induces a Three-Year Duration of Immunity in Dogs and Cats After a Single Vaccine Dose. Vaccines, 13(12), 1176. https://doi.org/10.3390/vaccines13121176






